Abstract
The aim of this present investigation was to evaluate the clinicopathologic characteristics, oncogenic drivers, and prognosis of former smokers with non‐small‐cell lung cancer (NSCLC), and to compare them with those of the current and never smokers. This investigation was a single‐institution retrospective study of 2289 NSCLC patients, who were classified as former, current, or never smokers. A collection was made of the clinicopathological characteristics, spectra of well‐identified driver genes and survival rates. The survival rates were compared using log‐rank test, and independent prognostic factors, identified using Cox regression analysis. Of 2289 NSCLC patients, 257 (11.2%) were former smokers; 868 (37.9%), current smokers; and 1164 (50.9%), never smokers. Compared with the current, the former were characterized by older age at diagnosis (64.3y vs. 59.9y; P < 0.001), earlier TNM stage (stage I, 47.9% vs. 39.5%; P = 0.017), fewer solid predominance in adenocarcinomas (16.2% vs. 29.5%; P = 0.005), and more EGFR mutation (33.2% vs. 20.7%; P < 0.001) but less KRAS mutation (6.7% vs. 11.9%, P = 0.041). No statistically significant survival differences were observed between the former and current. However, the light former smokers presented favorable overall survival when compared with the light current and heavy former or current (the light former vs. the heavy former, P = 0.028; the light former vs. the light current, P = 0.048; and the light former vs. the heavy current, P = 0.048). Our findings suggest that the former smokers with NSCLCs can have distinctive clinicopathologic characteristics, oncogenic drivers, and prognosis, and they, especially the light former, can benefit from smoking cessation.
Keywords: EGFR, former smoker, non‐small‐cell lung cancer, prognosis, smoking cessation
Introduction
Lung cancer is the leading cause of cancer‐related death worldwide, with more than one million people dying from it each year 1. Decades of epidemiologic studies have established cigarette smoking as the most important risk factor for the development of lung cancer 2. These studies indicated that 85–90% of lung cancer cases are associated with cigarette smoking 3.
The association of smoking with lung cancer and subsequent death is irrefutable. Tobacco smoke contains over 60 known carcinogens 4. The relative risk of lung cancer development is estimated to be 10‐ to 30‐fold in long‐term smokers when compared with never smokers 5. For lung cancer patients, smoking history is associated with diagnosis at a later stage, increased operative mortality, higher rate of local recurrence, and worse long‐term survival 6, 7. Furthermore, those who quit smoking develop fewer radiation pneumonitis and infections during radiotherapy, and better response to chemotherapy and targeted therapy 8, 9. Smoking cessation is associated with improved pulmonary function, weight gain, and better quality of life 10.
Although previous studies have already demonstrated the benefits of smoking cessation for lung cancer patients, the reports are limited on associations between detailed smoking statuses and clinical outcomes in former smokers with non‐small‐cell lung cancer (NSCLC). Moreover, few studies have described the clinical and pathologic features and well‐identified driver mutations in the former with NSCLC in comparison with the current and never with NSCLC. In this study, we undertook a comprehensive analysis to further investigate the clinicopathologic features, oncogenic drivers of former smokers and associations between detailed smoking statuses and clinical outcomes.
Materials and Methods
Patients
We consecutively enrolled the patients with newly diagnosed non‐small‐cell lung cancer between October 2007 and May 2013 at the Department of Thoracic Surgery of Fudan University Shanghai Cancer Center, Shanghai, China. The history of cigarette smoking were obtained at the patient interview by professional doctors, and the patients were categorized into never smokers, former smokers, and current smokers according to their smoking statuses. The never were defined as those who had smoked less than 100 cigarettes; of the smokers, the former, as those who had quitted smoking 1 year ago or more before diagnosis; and the current, as those who continued their smoking habit at diagnosis or had quitted smoking less than 1 year ago. Pack‐years of smoking were calculated by multiplying the number of packs (one pack containing 20 cigarettes) smoked per day by the number of smoking years. This study was approved by the Institutional Review Board of Fudan University Shanghai Cancer Center, with written informed consent obtained from all patients. Disease recurrence and survival were observed in the follow‐up clinic or obtained by telephone.
Clinical and pathological variables
The clinical and pathological data collected for analyses covered sex, age at diagnosis, detailed information regarding cigarette smoking such as daily cigarette use, duration of smoking history and quit date if applicable, pathological TNM stage, tumor differentiation, and histological types according to the new IASLC/ATS/ERS multidisciplinary classification 11. Pathological TNM stages were evaluated in accordance with the seventh edition of the lung cancer staging classification system 12.
Mutation analyses
Comprehensive mutational analyses of EGFR, KRAS, HER2, BRAF, ALK, ROS1, RET, and FGFR were performed in the patients with NSCLC. Tumor samples resected with curative intent were snap‐frozen in liquid nitrogen at the time of resection and stored in liquid nitrogen. RNA was extracted from tumors or distant histological normal lung as per standard protocols after frozen specimens were dissected into TRIZOL (Life Technologies, Carlsbad, CA). Total RNA samples were reverse transcribed into complementary DNA (cDNA) using RevertAid First Strand cDNA Synthesis Kit (Fermentas, St Leon‐Rot, Germany). EGFR (exons 18–22), KRAS (exons 2–3), HER2 (exons 18–21), and BRAF (exons 11–15) were amplified by polymerase chain reaction (PCR) using cDNA. Amplified products were analyzed by direct dideoxynucleotide sequencing. ALK, RET, ROS1, and FGFR fusions were analyzed by qRT‐PCR plus RT‐PCR and confirmed by FISH as we had previously reported 13, 14, 15, 16. PCR products were directly sequenced in forward and reverse directions. All mutations were verified by analysis of an independent PCR isolate.
Statistical analysis
Difference in proportions was analyzed using Pearson's chi‐squared test, when no cell of a contingency table had an expected count less than five, or Fisher's exact test, when any cell of a contingency table had an expected count less than five. Relapse‐free survival (RFS) and overall survival (OS) of patients with smoking statuses was estimated through the Kaplan–Meier method. The survival differences between groups were determined via the log‐rank test. Independent prognostic factors were identified through the Cox proportional hazards regression (forward likelihood ratio model). All tests were two tailed, and statistical significance was set as P < 0.05. All data were analyzed using SPSS Version 19.0 Software (SPSS Inc., Chicago, IL).
Results
Patient characteristics
A total of 2289 NSCLC patients were enrolled in this study, all patients of East Asians. As listed in Table 1, the mean age at diagnosis was 60.0 years (ranging 22–88), and 1374 (60.0%) were male. Of 2289, 257 (11.2%) were former smokers; 1164 (50.9%), never smokers; and 868 (37.9%), current smokers. The mean smoking dosage of smokers was 40 pack‐years, ranging from 0.25 to 240. The patients in the stages I‐IV numbered 1167 (51.0%), 383 (16.8%), 676 (29.6%), and 63 (2.8%), respectively. A diagnosis was made of adenocarcinoma in 1492 (65.2%), of squamous cell carcinoma in 630 (27.5%), of adenosquamous carcinoma in 68 (3.0%) and, of large cell carcinoma in 43 (1.9%). Not otherwise specified (NOS) were 41(1.8%), and a combination of different histology was observed in 15 (0.7%).
Table 1.
Patient clinicopathologic characteristics and mutation profile
| Character | Total | Former smoker | Current smoker | Never smoker | ||
|---|---|---|---|---|---|---|
| No. (n = 2289) | No.(n = 257) | No.(n = 868) | P(former vs. current) | No.(n = 1164) | P(former vs. never) | |
| Age at diagnosis | 2289 | |||||
| Mean (range), y | 60.0 (22‐88) | 64.3 (32‐87) | 59.9 (34‐82) | <0.001 | 59.1 (22‐84) | <0.001 |
| ≤60 years | 1133 (49.5%) | 81 (31.5%) | 447 (51.5%) | <0.001 | 605 (52.0%) | <0.001 |
| >60 years | 1156 (50.5%) | 176 (68.5%) | 421 (48.5%) | 559 (48.0%) | ||
| Sex | 2289 | |||||
| Male | 1374 (60.0%) | 247 (96.1%) | 853 (98.3%) | 0.039 | 274 (23.5%) | <0.001 |
| Female | 915 (40.0%) | 10 (3.9%) | 15 (1.7%) | 890 (76.5%) | ||
| Initial stage | 2289 | |||||
| I | 1166 (50.9%) | 123 (47.9%) | 343 (39.5%) | 0.017 | 700 (60.1%) | <0.001 |
| II/III/IV | 1123 (49.1%) | 134 (52.1%) | 525 (60.5%) | 464 (39.9%) | ||
| Histology | 2289 | |||||
| Adenocarcinoma | 1492 (65.2%) | 117 (45.5%) | 359 (41.4%) | 0.333 | 1016 (87.3%) | <0.001 |
| Squamous cell | 630 (27.5%) | 118 (45.9%) | 412 (47.5%) | 100 (8.6%) | ||
| AS/L/NOS/other | 167 (7.3%) | 22 (8.6%) | 97 (11.2%) | 48 (4.1%) | ||
| Histologic subtypes | 1492 | |||||
| AIS | 44 (2.9%) | 1 (0.9%) | 5 (1.4%) | 1.000 | 38 (3.7%) | 0.173 |
| MIA | 47 (3.2%) | 3 (2.6%) | 2 (0.6%) | 0.098 | 42 (4.1%) | 0.616 |
| Lepidic | 154 (10.3%) | 9 (7.7%) | 24 (6.7%) | 0.710 | 121 (11.9%) | 0.173 |
| Acinar | 653 (43.8%) | 53 (45.3%) | 132 (36.8%) | 0.100 | 468 (46.2%) | 0.861 |
| Papillary | 186 (12.4%) | 20 (17.1%) | 45 (12.5%) | 0.212 | 121 (11.9%) | 0.110 |
| Solid | 238 (16.0%) | 19 (16.2%) | 106 (29.5%) | 0.005 | 113 (11.1%) | 0.104 |
| Micropapillary | 23 (1.5%) | 2 (1.7%) | 5 (1.4%) | 0.683 | 16 (1.6%) | 0.709 |
| IMA | 77 (5.2%) | 4 (3.4%) | 19 (5.3%) | 0.412 | 54 (5.3%) | 0.378 |
| EGFR | 1894 | |||||
| Mutant | 902 (47.6%) | 65 (33.2%) | 139 (20.7%) | <0.001 | 698 (67.9%) | <0.001 |
| KRAS | 1881 | |||||
| Mutant | 123 (6.5%) | 13 (6.7%) | 79 (11.9%) | 0.041 | 34 (3.3%) | 0.024 |
| HER2 | 1878 | |||||
| Mutant | 40 (2.1%) | 1 (0.5%) | 2 (0.3%) | 0.538 | 37 (3.6%) | 0.023 |
| BRAF | 1865 | |||||
| Mutant | 21 (1.1%) | 1 (0.5%) | 12 (1.8%) | 0.318 | 8 (0.8%) | 1.000 |
| ALK | 1862 | |||||
| Fusion | 76 (4.1%) | 5 (2.6%) | 14 (2.2%) | 0.782 | 57 (5.6%) | 0.085 |
| RET | 1863 | |||||
| Fusion | 18 (1.0%) | 0 (0.0%) | 4 (0.6%) | 0.579 | 14 (1.4%) | 0.144 |
| ROS1 | 1863 | |||||
| Fusion | 11 (0.6%) | 0 (0.0%) | 4 (0.6%) | 0.579 | 7 (0.7%) | 0.605 |
| FGFR | 1863 | |||||
| Fusion | 19 (1.0%) | 4 (2.1%) | 12 (1.8%) | 0.769 | 3 (0.3%) | 0.014 |
AS, adenosquamous carcinoma; L, large cell carcinoma; NOS, non‐small‐cell lung cancer not otherwise specified; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; IMA, invasive mucinous adenocarcinoma.
In lung adenocarcinoma, the most common histologic subtype was acinar predominant (43.8%), followed by solid predominant (16.0%), papillary predominant (12.4%), lepidic predominant (10.3%), and micropapillary predominant (1.5%). Other types included adenocarcinoma in situ (AIS; 2.9%), minimally invasive adenocarcinoma (MIA; 3.2%), and invasive mucinous adenocarcinoma (IMA; 5.2%).
Differences in clinicopathological features and driver mutations between former, never, and current smokers
A collection was made of the clinicopathological features of the former smokers, which were compared with those of the current and never. The former were diagnosed at an older age than the current and never (64.3year vs. 59.9year, P < 0.001; 64.3year vs. 59.1year, P < 0.001, respectively). When compared with the current, fewer male patients were defined as the former (96.1% as former vs. 98.3% as current; P = 0.039). The former produced an earlier TNM stage than the current (stage I: 47.9% as former vs. 39.5% as current; P = 0.017). The former were more likely to develop squamous cell carcinoma than the never, as in the same case of the current.
An analysis was made of the associations between smoking states and histologic subtypes in adenocarcinoma. Fewer solid predominant adenocarcinomas were detected in the former than in the current (16.2% as former vs. 29.5% as current, P = 0.005; 16.2% as former vs. 11.1% as never, P < 0.104). No differences were observed in other histologic subtypes between the former and the never or the current.
Mutational analyses were performed in approximately 1800 patients (Fig. 1). Of the former, 33.2% had EGFR kinase domain mutations (including 32 exon 19 deletions, 31 L858R, and two other mutations); 6.7%, KRAS (including two G12A, three G12C, one G12D, three G12V, one L19F, one R41M, and two Q61H); 0.5%, HER2 (one exon 20 insertion mutation); and 0.5%, BRAF mutations (one L485S). ALK rearrangement was detected in the patients by 2.6% (five EML4‐ALK fusions), and FGFR fusion, in those by 2.1% (four FGFR3‐TACC3 fusions), whereas RET and ROS1 fusion were not found in the former. Interestingly for EGFR and KRAS, the most common mutations in NSCLC, the mutation profile of the former fell just between the never and the current (EGFR: 33.2% as former vs. 20.7% as current, P < 0.001; 33.2% as former vs. 67.9% as never, P < 0.001; KRAS: 6.7% as former vs. 11.9% as current, P = 0.041; 6.7% as former vs. 3.3% as never, P = 0.024). As in the same case of the current, the former carried fewer HER2 mutations, but more FGFR fusions than the never. As to BRAF, ALK, RET, and ROS1, no significant differences were found between the former and the current or the never.
Figure 1.
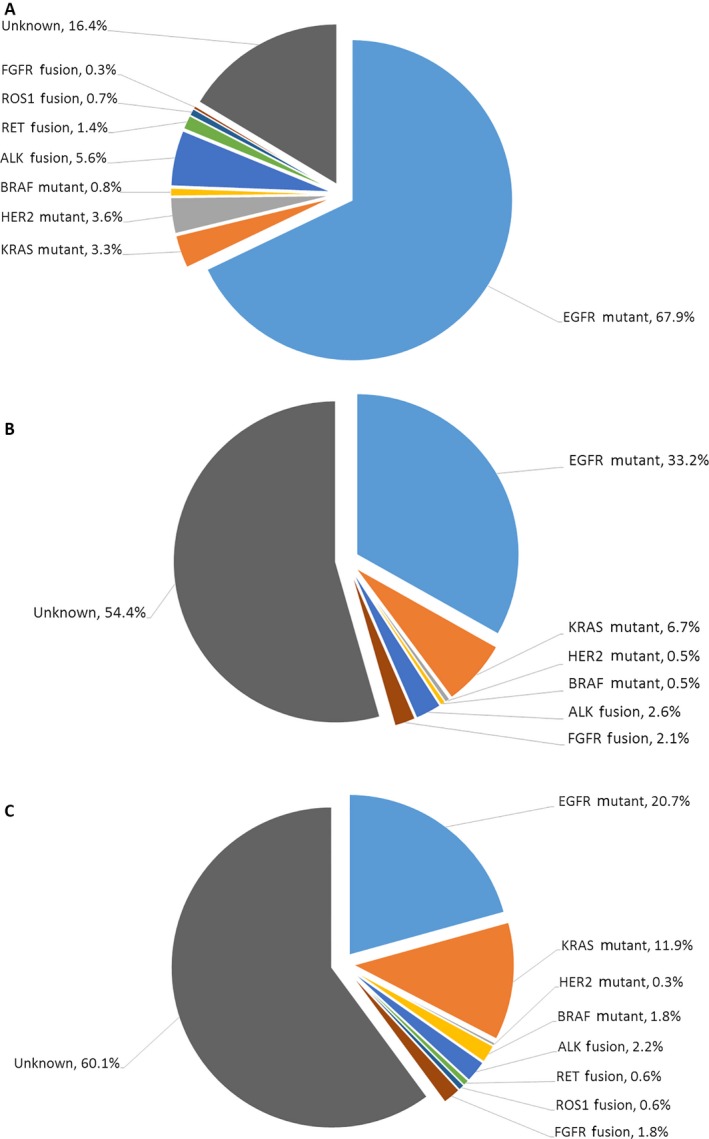
Mutation profiles for never smokers, former smokers, and current smokers with non‐small‐cell lung cancers (NSCLCs). (A) Never smokers. (B) Former smokers. (C) Current smokers.
Univariate analysis of the clinicopathologic variables on survival outcomes
The median follow‐up period was 29.5 months (ranging 1–103 months). The log‐rank test on the Kaplan–Meier survival analysis demonstrated that although there were differences between the former and current in terms of the median RFS and OS, they were not statistically significant (Fig. 2A and B, RFS; median survival: 40 vs. 38 months; log rank: P = 0.348; OS: median survival not reached, log rank: P = 0.168). Additionally, the never were found to have significantly longer RFS and OS than the current (Fig. 2A and B, RFS; median survival: 67 vs. 38 months; log rank: P < 0.001; OS: median survival not reached, P < 0.001).
Figure 2.
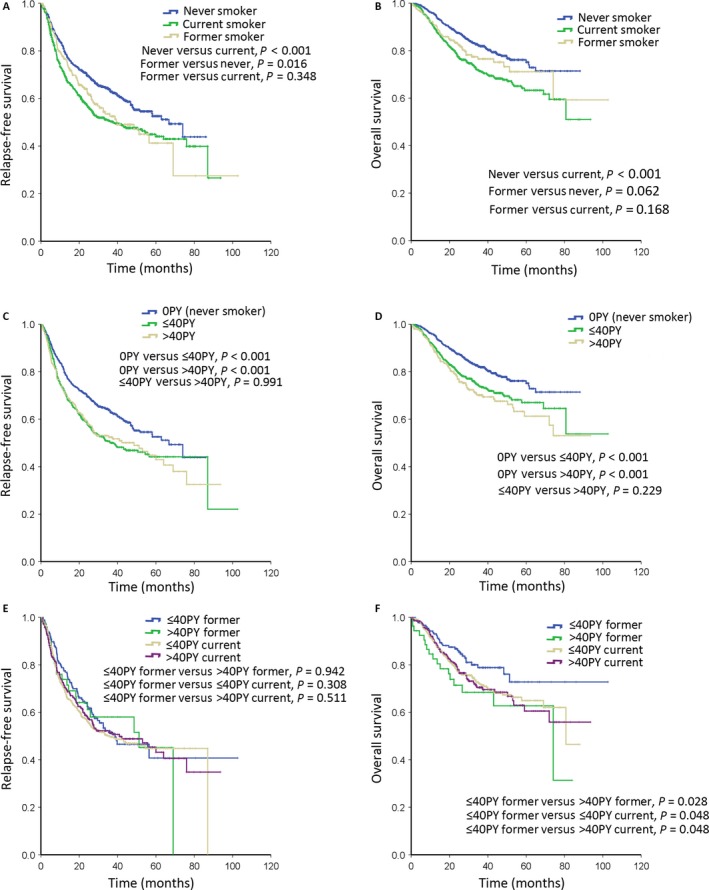
Kaplan–Meier survival curves for relapse‐free survival (RFS) and overall survival (OS) in NSCLC patients. (A) RFS according to smoking status. (B) OS according to smoking status. (C) RFS according to smoking dosage. (D) OS according to smoking dosage. (E) RFS according to combined parameters of smoking status and dosage. (F) OS according to combined parameters of smoking status and dosage. NSCLC, non‐small‐cell lung cancer.
To determine what patients could benefit more from smoking cessation, we focused on detailed smoking information of the former, intending to identify the risk factors for recurrence and mortality. According to the age of smoking cessation before or after 57 as the median, the years of smoking cessation lasting longer or shorter than 5 years as the median duration, and the smoking dosage over or less than 40 pack‐years as the median dosage, the former were divided into different subgroups. The univariate analysis identified that the more advanced initial stage was associated with recurrence, and that smoking dosage over 40 pack‐years, advanced initial stage, EGFR wildtype and FGFR fusion were associated with mortality (Table 2).
Table 2.
Univariate analysis of the clinicopathologic variables on survival outcomes in former smokers
| Variable | Category | RFS | OS | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐Value | HR | 95% CI | P‐Value | ||
| Clinicopathologic variables | |||||||
| Age | >60 years vs. ≤60 years (Ref.) | 1.059 | 0.710–1.577 | 0.780 | 1.324 | 0.765–2.291 | 0.316 |
| Sex | Female vs. male (Ref.) | 0.557 | 0.176–1.764 | 0.320 | 0.359 | 0.050–2.603 | 0.311 |
| Age of smoking cessation | >57 years vs. ≤57 years (Ref.) | 0.761 | 0.510–1.135 | 0.180 | 1.067 | 0.619–1.838 | 0.816 |
| Years of smoking cessation | Quit≤5 years vs. > 5 years (Ref.) | 0.765 | 0.512–1.144 | 0.192 | 1.024 | 0.593–1.766 | 0.933 |
| Smoking dosage | PY>40 vs.PY≤40 (Ref.) | 1.018 | 0.628–1.651 | 0.942 | 1.778 | 1.054–3.001 | 0.031 |
| Initial stage | II/III/IV vs. I (Ref.) | 3.180 | 2.503–4.927 | <0.001 | 4.040 | 2.073–7.872 | <0.001 |
| Histology | Adenocarcinoma (Ref.) | ||||||
| Squamous cell | 1.988 | 0.770–5.129 | 0.155 | 0.967 | 0.284–3.286 | 0.957 | |
| AS/L/NOS/other | 1.580 | 0.609–4.099 | 0.347 | 1.768 | 0.538–5.817 | 0.348 | |
| Driver genes | |||||||
| EGFR | Mutant vs. wild type (Ref.) | 1.206 | 0.781–1.861 | 0.399 | 0.288 | 0.129–0.641 | 0.002 |
| KRAS | Mutant vs. wild type (Ref.) | 0.705 | 0.286–1.741 | 0.449 | 0.503 | 0.121–2.095 | 0.346 |
| HER2 | Mutant vs. wild type (Ref.) | 5.692 | 0.774–41.839 | 0.088 | 3.123 | 0.492–22.713 | 0.261 |
| BRAF | Mutant vs. wild type (Ref.) | — | — | — | — | — | — |
| ALK | Fusion vs. wild type (Ref.) | 1.603 | 0.393–6.535 | 0.510 | 1.069 | 0.147–7.769 | 0.947 |
| RET | Fusion vs. wild type (Ref.) | — | — | — | — | — | — |
| ROS1 | Fusion vs. wild type (Ref.) | — | — | — | — | — | — |
| FGFR | Fusion vs. wild type (Ref.) | 0.963 | 0.134–6.950 | 0.970 | 4.774 | 1.137–20.046 | 0.033 |
AS, adenosquamous carcinoma; L, large cell carcinoma; OS, overall survival; NOS, non‐small‐cell lung cancer not otherwise specified.
Multivariate analysis of the clinicopathologic variables on survival outcomes in former smokers
As indicated by the Cox proportional hazards models (Table 3), the multivariate analysis was adjusted for age, initial stage, age of smoking cessation, years of smoking cessation, smoking dosage, EGFR, and FGFR. It was found that the late initial stage was a significant and independent risk factor for relapse, while such a stage and EGFR wild‐type status were significant and independent risk factors for worse overall survival.
Table 3.
Multivariate analysis of the clinicopathologic variables on survival outcomes in former smokers
| Variable | Category | RFS | OS | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐Value | HR | 95% CI | P‐Value | ||
| Age | >60 years vs. ≤ 60 years (Ref.) | 1.587 | 0.871–2.890 | 0.131 | 2.026 | 0.852–4.819 | 0.110 |
| Age of smoking cessation | >57 years vs. ≤ 57 years (Ref.) | 0.816 | 0.471–1.413 | 0.468 | 0.783 | 0.362–1.693 | 0.534 |
| Years of smoking cessation | Quit≤5 years vs. > 5 years (Ref.) | 0.838 | 0.511–1.374 | 0.483 | 0.843 | 0.437–1.626 | 0.609 |
| Smoking dosage | PY>40 vs.PY≤40 (Ref.) | 1.000 | 0.585–1.708 | 0.999 | 1.542 | 0.852–4.819 | 0.110 |
| Initial stage | II/III/IV vs. I (Ref.) | 3.432 | 2.133–5.525 | <0.001 | 4.643 | 2.211–9.749 | <0.001 |
| EGFR | Mutant vs. wild type (Ref.) | 1.207 | 0.749–1.947 | 0.439 | 0.294 | 0.127–0.680 | 0.004 |
| FGFR | Fusion vs. wild type (Ref.) | 0.814 | 0.109–6.093 | 0.841 | 3.595 | 0.778–16.608 | 0.101 |
RFS, Relapse‐free survival; OS, overall survival.
Survival outcomes of cigarette smoking status and dosage
As smoking dosage was an important prognosis factor in the former, we further analyzed the association between smoking dosage and outcomes in all patients, who were divided into three groups: never smokers (0 pack‐years), light smokers (≤40 pack‐years), and heavy smokers (>40 pack‐years). It was found that the never presented more significant RFS and OS than the light and heavy (Fig. 2C & D; RFS: never vs. light, P < 0.001; never vs. heavy, P < 0.001; OS: never vs. light, P < 0.001; never vs. heavy, P < 0.001). However, the light did not show significant differences when compared with the heavy (RFS: light vs. heavy, P = 0.991; OS: light vs. heavy, P = 0.229).
Combining the two important groups of smoking information: smoking status and smoking dosage, we found out that the light former had favorable overall survival than the heavy former, the light current, and the heavy current (Fig. 2F; light former vs. heavy former, P = 0.028; light former vs. light current, P = 0.048; light former vs. heavy current, P = 0.048).
Discussion
Over the past decades, studies have proved that patients with lung cancer can benefit from smoking cessation; however, there is a death of literature on the clinicopathological characteristics of the former smokers with NSCLC. Previous studies have identified a number of molecularly distinct subsets of lung cancer characterized by different oncogenes 17, 18, 19. Researchers have found tumor mutational frequencies and spectra differences between smokers and nonsmokers, as indicated by the reported differences in the frequency of somatic mutations of EGFR and KRAS observed between smoking and nonsmoking lung cancer patients 20. However, the mutational spectra of the former smokers with NSCLC have not been reported.
To our knowledge, this study pioneered the comprehensive analysis of well‐identified driver mutations, clinical characteristics, and survival analysis in an Asian cohort of non‐small‐cell lung cancer patients who had quitted smoking. While previous studies have examined the characteristics and outcomes between never and ever smokers, our investigations have focused on NSCLC in former smokers. In this study, we demonstrated that the former smokers were older at diagnosis, showing an earlier TNM stage and harboring more EGFR but less KRAS mutations than the current. Additionally, we proved that the light former presented favorable overall survival when compared with the light current and the heavy former or the current.
The former with NSCLC were found to benefit from cessation according to the analyses of clinicopathological characteristics, as indicated by the mean age of four years older than that of the current, suggesting a longer lifetime. The findings were in agreement with the data from the NCCN network that former smokers had older age than current smokers 21. When the former had an earlier TNM stage than the current, this was an independent prognosis factor for RFS and OS. In adenocarcinomas, former smokers have been reported to be associated with a lower proportion of solid predominant histology subtype, which is indicative of more aggressive biological features and predicts worse outcomes 22, 23, 24. These clinicopathological characteristics of the former may partly explain their clinical outcomes.
We now understand that NSCLCs are subdivided into different molecular subtypes according to the primary driver genes identified 25. Driver mutations in smoking NSCLCs are different from those in nonsmoking NSCLCs 26, 27. We performed a comprehensive analysis of well‐identified driver genes in the former smokers with NSCLCs, and compared the results with those of the current and never with NSCLCs, demonstrating that the former harbored more EGFR but less KRAS mutations than the current. It has been reported that NSCLCs with EGFR mutations are associated with better outcomes, whereas KRAS mutations, with worse outcome 28, 29. Moreover, lung cancer of EGFR mutation can be treated with EGFR‐TKI and prolong PFS overall than treated with chemotherapy, especially in those with exon 19 deletions, never smokers and women 30. Our results supported the conclusion that former smokers with NSCLCs may benefit from their specific mutational profiles.
A number of studies have investigated the relationship between smoking and outcome of lung cancer patients, but the results are diverse 7, 20, 21, 31, 32, 33, 34. The present results clearly supported the conclusion that smoking history can exert a negative influence on RFS and OS. Furthermore, the never had favorable RFS and OS rates when compared with those of the current. As to the former, those who had smoked no more than 40 pack‐years showed more significant OS than those who had continued smoking with the same dosage, although smoking dosage is not an independent prognostic factor. Such results emphasized the importance of smoking cessation, even for light smokers.
The present retrospective study had several limitations in the analyses. Smoking extent was reported by the patients themselves without biochemical confirmation; therefore, this could have bias. Additionally, second‐hand tobacco smoke, which has been established as an inducement of lung cancer, was too difficult to quantify exactly and include it in the analyses. Nonetheless, the large number of the patients enrolled for investigation may reduce error in different groups.
In conclusion, NSCLC patients who underwent surgical resection with smoking history can benefit from smoking cessation; former smokers with NSCLCs can age older at diagnosis, presenting an earlier TNM stage and more EGFR but less KRAS mutations than current ones with NSCLCs; and light former ones can have better long‐term overall survival than heavy former ones as well as light current ones.
Conflict of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81330056, 81401886, 81401891, 81422029, 81472173, 81572253, and 81372525), the Key Project of Science and Technology Commission of Shanghai Municipality (JGGG1302), Wu Jieping Medical Foundation (320.6750.14317) and Shen‐Kang Center Project (SKMB1201), the grant from Health and Family Planning Commission of Shanghai Municipality (No. 2013ZYJB0301), and the grant from Science and Technology Commission of Shanghai Municipality (No. 14495810800).
Cancer Medicine 2016; 5(8):2117–2125
References
- 1. Siegel, R. L. , Miller K. D., and Jemal A.. 2015. Cancer statistics, 2015. CA Cancer J. Clin. 65:5–29. [DOI] [PubMed] [Google Scholar]
- 2. Doll, R. 2004. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ 328:1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alberg, A. J. , Ford J. G., and Samet J. M.. 2007. Epidemiology of lung cancer: ACCP evidence‐based clinical practice guidelines (2nd edition). Chest 132(3 Suppl):29S–55S. [DOI] [PubMed] [Google Scholar]
- 4. Jiménez‐Ruiz, C. A. , et al. 2015. Statement on smoking cessation in COPD and other pulmonary diseases and in smokers with comorbidities who find it difficult to quit. Eur. Respir. J. 46:61–79. [DOI] [PubMed] [Google Scholar]
- 5. Samet, J. M. 1991. Health benefits of smoking cessation. Clin. Chest Med. 12:669–679. [PubMed] [Google Scholar]
- 6. Nordquist, L. T. , et al. 2004. Improved survival in never‐smokers vs current smokers with primary adenocarcinoma of the lung. Chest 126:347–351. [DOI] [PubMed] [Google Scholar]
- 7. Boyle, J. M. , et al. 2015. Smoking history predicts for increased risk of second primary lung cancer: a comprehensive analysis. Cancer 121:598–604. [DOI] [PubMed] [Google Scholar]
- 8. Duarte, R. L. , Luiz R. R., and Paschoal M. E.. 2008. The cigarette burden (measured by the number of pack‐years smoked) negatively impacts the response rate to platinum‐based chemotherapy in lung cancer patients. Lung Cancer 61:244–254. [DOI] [PubMed] [Google Scholar]
- 9. Hughes, A. N. , et al. 2009. Overcoming CYP1A1/1A2 mediated induction of metabolism by escalating erlotinib dose in current smokers. J. Clin. Oncol. 27:1220–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sloan, J. A. , et al. 2012. Relationship between deficits in overall quality of life and non‐small‐cell lung cancer survival. J. Clin. Oncol. 30:1498–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang, E. R. , Schreiner A. M., and Pua B. B.. 2014. Advances in lung adenocarcinoma classification: a summary of the new international multidisciplinary classification system (IASLC/ATS/ERS). J. Thorac. Dis. 6(Suppl 5):S489–S501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Detterbeck, F. C. , Boffa D. J., and Tanoue L. T.. 2009. The new lung cancer staging system. Chest 136:260–271. [DOI] [PubMed] [Google Scholar]
- 13. Wang, R. , et al. 2012. RET fusions define a unique molecular and clinicopathologic subtype of non‐small‐cell lung cancer. J. Clin. Oncol. 30:4352–4359. [DOI] [PubMed] [Google Scholar]
- 14. Wang, R. , et al. 2014. FGFR1/3 tyrosine kinase fusions define a unique molecular subtype of non‐small cell lung cancer. Clin. Cancer Res. 20:4107–4114. [DOI] [PubMed] [Google Scholar]
- 15. Wang, R. , et al. 2012. The use of quantitative real‐time reverse transcriptase PCR for 5' and 3' portions of ALK transcripts to detect ALK rearrangements in lung cancers. Clin. Cancer Res. 18:4725–4732. [DOI] [PubMed] [Google Scholar]
- 16. Pan, Y. , et al. 2014. ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern‐specific clinicopathologic, histologic and cytologic features. Lung Cancer 84:121–126. [DOI] [PubMed] [Google Scholar]
- 17. Devarakonda, S. , Morgensztern D., and Govindan R.. 2015. Genomic alterations in lung adenocarcinoma. Lancet Oncol 16:e342–e351. [DOI] [PubMed] [Google Scholar]
- 18. Kohno, T. , et al. 2015. Beyond ALK‐RET, ROS1 and other oncogene fusions in lung cancer. Transl. Lung Cancer Res. 4:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Califano, R. , et al. 2015. Beyond EGFR and ALK inhibition: unravelling and exploiting novel genetic alterations in advanced non small‐cell lung cancer. Cancer Treat. Rev. 41:401–411. [DOI] [PubMed] [Google Scholar]
- 20. Varghese, A. M. , et al. 2013. Lungs don't forget: comparison of the KRAS and EGFR mutation profile and survival of collegiate smokers and never smokers with advanced lung cancers. J. Thorac. Oncol. 8:123–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferketich, A. K. , et al. 2013. Smoking status and survival in the national comprehensive cancer network non‐small cell lung cancer cohort. Cancer 119:847–853. [DOI] [PubMed] [Google Scholar]
- 22. Xu, S. , et al. 2015. Solid component and tumor size correlate with prognosis of stage IB lung adenocarcinoma. Ann. Thorac. Surg. 99:961–967. [DOI] [PubMed] [Google Scholar]
- 23. Morales‐Oyarvide, V. , and Mino‐Kenudson M.. 2014. High‐grade lung adenocarcinomas with micropapillary and/or solid patterns: a review. Curr. Opin. Pulm. Med. 20:317–323. [DOI] [PubMed] [Google Scholar]
- 24. Hung, J. J. , et al. 2014. Predictive value of the international association for the study of lung cancer/American Thoracic Society/European Respiratory Society classification of lung adenocarcinoma in tumor recurrence and patient survival. J. Clin. Oncol. 32:2357–2364. [DOI] [PubMed] [Google Scholar]
- 25. Ou, S. 1062. Republished: lung cancer in never‐smokers. Does smoking history matter in the era of molecular diagnostics and targeted therapy? Postgrad. Med. J. 2014:228–235. [DOI] [PubMed] [Google Scholar]
- 26. Sholl, L. M. , et al. 2015. Multi‐institutional Oncogenic Driver Mutation Analysis in Lung Adenocarcinoma: the Lung Cancer Mutation Consortium Experience. J. Thorac. Oncol. 10:768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gou, L. Y. , et al. 2015. Differences in driver genes between smoking‐related and non‐smoking‐related lung cancer in the Chinese population. Cancer 121(Suppl 17):3069–3079. [DOI] [PubMed] [Google Scholar]
- 28. Guan, J. L. , et al. 2013. KRAS mutation in patients with lung cancer: a predictor for poor prognosis but not for EGFR‐TKIs or chemotherapy. Ann. Surg. Oncol. 20:1381–1388. [DOI] [PubMed] [Google Scholar]
- 29. Zhang, Q. , et al. 2014. EGFR mutations and clinical outcomes of chemotherapy for advanced non‐small cell lung cancer: a meta‐analysis. Lung Cancer 85:339–345. [DOI] [PubMed] [Google Scholar]
- 30. Lee, C. K. , et al. 2015. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR‐mutant lung cancer: a meta‐analysis. J. Clin. Oncol. 33:1958–1965. [DOI] [PubMed] [Google Scholar]
- 31. Tammemagi, C. M. , et al. 2004. Smoking and lung cancer survival: the role of comorbidity and treatment. Chest 125:27–37. [DOI] [PubMed] [Google Scholar]
- 32. Meguid, R. A. , et al. 2010. Long‐term survival outcomes by smoking status in surgical and nonsurgical patients with non‐small cell lung cancer: comparing never smokers and current smokers. Chest 138:500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Okamoto, T. , et al. 2014. The prognostic impact of the amount of tobacco smoking in non‐small cell lung cancer—Differences between adenocarcinoma and squamous cell carcinoma. Lung Cancer 85:125–130. [DOI] [PubMed] [Google Scholar]
- 34. Kogure, Y. , et al. 2013. Histology and smoking status predict survival of patients with advanced non‐small‐cell lung cancer. Results of West Japan Oncology Group (WJOG) Study 3906L. J. Thorac. Oncol. 8:753–758. [DOI] [PubMed] [Google Scholar]


