Abstract
Few epidemiological studies have investigated the role of hypertensive disorders of pregnancy in the aetiology of childhood respiratory and atopic outcomes.
In the Avon Longitudinal Study of Parents and Children we examined associations of maternal gestational hypertension, hypertension before pregnancy and preeclampsia with wheezing at 18 months, wheezing and asthma at 7 years, and lung function at 8-9 years, after controlling for potential confounders (N=5322-8734, depending on outcome).
Gestational hypertension was not associated with any outcome. There was weak evidence for a positive association between preeclampsia and early wheezing (adjusted odds ratio compared to normotensive pregnancies 1.31 (95% CI: 0.94 to 1.82)) and for negative associations between preeclampsia and Forced Expiratory Volume in one second (adjusted mean difference in standard deviation score -0.14 (-0.33 to 0.06)) and Maximal Mid-Expiratory Flow (-0.15 (-0.34 to 0.04)). Hypertension before pregnancy was positively associated with wheezing (OR 1.63 (1.16 to 2.31) and asthma (OR 1.34 (1.00 to 1.79)).
Gestational hypertension is unlikely to be a risk factor for childhood respiratory disorders; hypertension before pregnancy may be a risk factor for childhood wheezing and asthma, but this finding needs replication. Larger studies are needed to confirm whether preeclampsia is associated with impaired childhood lung function.
Keywords: ALSPAC, hypertension, preeclampsia, pregnancy, asthma, lung function
Background
There is a growing body of epidemiological evidence implicating the prenatal environment in the aetiology of childhood respiratory disorders such as asthma1. Risk factors which deserve investigation include the hypertensive disorders of pregnancy (HDP), namely gestational hypertension, preeclampsia, and existing (chronic) hypertension. Hypertension in pregnancy has been associated with impaired infant lung function in an Australian cohort2. There is a paucity of data on the relation between HDP and lung function later in childhood, although one small Norwegian historical cohort study found no association between preeclampsia and childhood lung function3. A recent pooled analysis of 14 European birth cohorts reported positive associations between preeclampsia and ever/recurrent wheezing at 12-24 months of age4. Maternal hypertension in pregnancy or preeclampsia, analysed in combination, were positively associated with an increased risk of early, late onset and persistent wheezing in the first 6-7 years of life in a large Italian cross-sectional study, although information about HDP was based on maternal recall5. In contrast, a Norwegian birth cohort found no association between pregnancy complications (hypertension, preeclampsia or hyperemesis combined) and bronchial obstruction under two years of age or asthma at 4 years6. Two other Norwegian studies found no relation between preeclampsia and asthma in the offspring3;7, and a British birth cohort study found no association between maternal hypertension in pregnancy and offspring asthma8. A potential limitation of studies to date is that few have distinguished associations with gestational hypertension, hypertension before pregnancy and preeclampsia separately. Whilst gestational hypertension and preeclampsia are currently thought to represent a spectrum of severity of a single condition, with preeclampsia at the severe end9, guidelines for clinical management are different for each of the hypertensive disorders10, and thus it would seem sensible to analyse the disorders separately if possible.
One small Finnish study (N=378) also found that women who experienced either preeclampsia or placental abruption in pregnancy had offspring with an increased risk of severe atopy during adolescence, an association which was limited to males11. A larger Norwegian study reported that severe (but not mild/moderate) preeclampsia was also associated with a high level of allergic sensitisation3.
In the Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort we have investigated whether HDP are associated with an increased risk of early and later wheezing, asthma, impaired lung function and atopy in childhood.
Methods
Participants
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a population-based birth cohort that recruited 14,541 predominantly white pregnant women resident in Avon, UK with expected dates of delivery 1st April 1991 to 31st December 1992. Of these pregnancies there were 14,676 fetuses, resulting in 14,062 live births and 13,978 singleton or twin children who were alive at one year of age. Of these, 13,758 had maternal obstetric information abstracted from medical records. The cohort has been followed since birth with annual questionnaires and, since age 7 years, with objective measures in annual research clinics. The study protocol has been described previously12;13 and further information can be found at: http://www.alspac.bris.ac.uk. Please note that the study website contains details of all the data that are available through a fully searchable data dictionary: http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary/. Ethics approval for all aspects of data collection was obtained from the ALSPAC Ethics and Law Committee (IRB 00003312) and the Local NHS Research Ethics Committees.
Hypertensive disorders of pregnancy
All blood pressure and proteinuria measurements, which were taken as part of routine antenatal care by midwives or obstetricians, were abstracted from the women’s obstetric records by six trained research midwives. There was no between-midwife variation in mean values of the data abstracted and error rates were consistently <1% in repeated data entry checks. These data were used to derive three hypertensive disorders of pregnancy (HDP) using the widely used International Society for the Study of Hypertension in Pregnancy (ISSHP) criteria14, which are used clinically in the UK. Gestational hypertension was defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg on two occasions after 20 weeks gestation in a woman who did not report having hypertension prior to pregnancy. Preeclampsia was defined by the same blood pressure thresholds with proteinuria of at least 1+ on urine dipstick testing occurring at the same time as the elevated blood pressure. Women who responded to a questionnaire administered during pregnancy that they had previously had hypertension outside of pregnancy were classified as having hypertension before pregnancy.
Outcomes
When the children were 18 months old, their mothers were asked whether the child had wheezed in the previous 12 months and whether their child had “wheezing with whistling on the chest when (s)he breathed”. When the children were 7.5 years old, mothers were asked: ‘Has your child had any of the following in the past 12 months: wheezing; asthma?’. Children were defined as having current doctor-diagnosed asthma at 7.5 years (primary outcome of interest) if mothers responded positively to the question ‘Has a doctor ever actually said that your study child has asthma?’ and positively to one or both of the questions on wheezing and asthma in the past 12 months.
Lung function was measured by spirometry (Vitalograph 2120) at age 8½ years after withholding short-acting bronchodilators for at least 6 hours and long-acting bronchodilators and theophyllines for at least 24 hours. The best of three reproducible flow-volume curves was used to measure FEV1, FVC and maximal mid-expiratory flow (FEF25-75). Lung function measurements were transformed to age, height and gender adjusted standard deviation units15. The tests adhered to American Thoracic Society (ATS) criteria for standardisation and reproducibility of flow-volume measurement16, with the exception of ATS recommendations for duration of expiration17; as many children did not fulfil forced expiratory time >6 seconds end of test criteria, a minimal volume change over the final 1 second was used.
Atopy at 7 years was defined as a positive reaction (any detectable weal) to D. pteronyssinus, cat or grass (after subtracting positive saline reactions from histamine and allergen weals, and excluding children unreactive to 1% histamine). (Atopy defined in this way identified 96% of children sensitised to 26 other allergens in this cohort18).
Maternal covariables
Maternal age at delivery was abstracted from obstetric records. Maternal asthma, pre-pregnancy weight and height, parity, smoking status, highest educational qualification and ethnicity were obtained from questionnaires administered during pregnancy. Maternal asthma was defined as a positive response to having had asthma either recently or in the past. Pre-pregnancy BMI was calculated as weight(kg)/ height(m)2 and classed according to World Health Organisation definitions of underweight (<18.5kg/m2), normal (18.5-24.9kg/m2), overweight (25.0-29.9kg/m2) and obese (≥30.0kg/m2). Smoking status was categorised as the maximum maternal exposure during pregnancy (never, passive smoking only, 1-9 cigarettes per day, 10-19 cigarettes per day, ≥20 cigarettes per day). Maternal ethnicity was categorised as white or non-white.
Statistical analyses
We compared the distributions of child and maternal variables across HDP categories using f-statistics for differences in the means of continuous variables and chi-squared tests for differences in the categorical variables.
We used logistic regression to relate maternal HDP to binary offspring outcomes: wheezing, asthma, and atopy. We present odds ratios for these outcomes comparing children whose mothers had gestational hypertension and preeclampsia (separately) with children of normotensive pregnancies (the reference category). Linear regression was used to relate maternal HDP to the continuous lung function outcomes: FEV1, FVC and FEF25-75. Mean differences in the age, sex and height-standardised SD scores15 for these outcomes associated with maternal gestational hypertension and preeclampsia compared with normotensive pregnancies are presented.
For both the logistic and linear regression analyses, four stages of adjustment were used. We selected potential confounding factors which are known (from existing literature) to be associated with HDP and one or more of our outcomes of interest. In Model 1 we adjusted for offspring sex only. In Model 2 we adjusted additionally for the potential maternal confounders of maternal asthma, pre-pregnancy BMI, age, parity, highest educational qualification, twin pregnancy and ethnicity. In Model 3 we adjusted additionally for maternal smoking during pregnancy. This was included in a separate stage of adjustment since maternal smoking during pregnancy has been shown to be negatively associated with HDP19 but also associated with increased risk of asthma in the child, meaning that it had the potential to negatively confound and mask, rather than inflate, any effect of maternal HDP on these outcomes. By way of subsidiary analyses, we additionally adjusted for gestational age at delivery and birth weight to explore whether prematurity or impaired fetal growth might mediate associations between HDP and childhood outcomes. We have made some key assumptions which are necessary for mediation analysis, namely that there is no interaction between the exposures and mediators20 and no unmeasured confounding of the mediator-outcome associations21. Figure E1 in the online supplement shows a directed acyclic graph to illustrate potential confounders and mediators of the associations between HDP and childhood outcomes. All statistical analyses were completed using Stata version 12.1 (StataCorp LP, USA).
Results
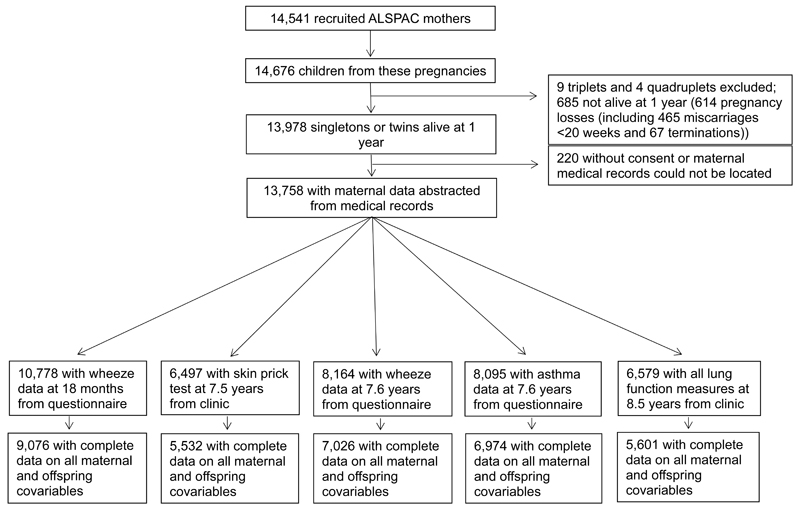
Figure 1 shows the participant flow in the ALSPAC birth cohort, and numbers of mother/child pairs with complete data according to childhood outcomes. Characteristics of mothers and offspring who were included in the analyses of each childhood outcome and who were excluded from all analyses because of incomplete data are shown in supplementary table E1. Mothers with incomplete data were more likely to be smokers, less well educated, multiparous, younger and non-white than mothers who were included. Excluded offspring had lower lung function and lower birth weight and a higher prevalence of early and later wheezing and asthma than those who were included, though differences were small.
Figure 1. Participant flow.
Table 1 shows the distribution of maternal and offspring characteristics, including respiratory and atopic outcomes, according to maternal hypertensive disorders of pregnancy (HDP). The prevalences of gestational hypertension, preeclampsia and hypertension before pregnancy were 14%, 2% and 4% respectively. Children of mothers who had preeclampsia had lower FEF25-75 and lower gestational age and birth weight than children of other mothers. The prevalence of wheeze at 7 years was higher in children of mothers who had hypertension before pregnancy, but the prevalence of early wheezing and later asthma and atopy did not differ according to HDP. Mothers who had gestational hypertension and preeclampsia were more likely than normotensive mothers to be carrying twin pregnancies, and mothers with HDP were more likely to be nulliparous and to have higher pre-pregnancy BMI, and less likely to have actively smoked during pregnancy. Mothers with gestational hypertension and hypertension before pregnancy were more likely than normotensive mothers to have asthma.
Table 1. Characteristics of mothers and offspring who had information on at least one set of outcome variables (wheeze, asthma, lung function or atopy) by maternal hypertensive disorders of pregnancy (total N=11,601).
| Mother and offspring characteristics | N | Normotensive | Gestational hypertension | Preeclampsia | Hypertension before pregnancy | P for difference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N=9,268 |
N=1,675 |
N=238 |
N= 420 |
||||||||
| N | Mean (SD)/% | N | Mean (SD)/% | N | Mean (SD)/% | N | Mean (SD)/% | ||||
| Wheeze at 18 m | No | 7815 | 6218 | 72.3 | 1154 | 74.0 | 158 | 70.9 | 285 | 71.3 | |
| Yes | 2963 | 2378 | 27.7 | 405 | 26.0 | 65 | 29.1 | 115 | 28.8 | 0.47 | |
| Wheeze at 7 years | No | 7285 | 5861 | 89.6 | 1020 | 88.4 | 144 | 88.9 | 260 | 84.1 | |
| Yes | 879 | 678 | 10.4 | 134 | 11.6 | 18 | 11.1 | 49 | 15.9 | 0.02 | |
| Asthma | No | 6440 | 5185 | 79.9 | 902 | 79.1 | 120 | 74.5 | 233 | 75.6 | |
| Yes | 1655 | 1301 | 20.1 | 238 | 20.9 | 41 | 25.5 | 75 | 24.4 | 0.11 | |
| FEV1 SD score* | 6579 | 5238 | −0.01 (0.99) | 957 | 0.03 (1.03) | 138 | −0.09 (1.01) | 246 | 0.08 (0.98) | 0.27 | |
| FVC SD score* | 6676 | 5323 | −0.01 (0.99) | 964 | 0.06 (1.03) | 138 | −0.01 (1.01) | 251 | 0.03 (1.03) | 0.23 | |
| FEF25-75 SD score* | 6676 | 5323 | 0.00 (1.01) | 964 | −0.03 (0.99) | 138 | −0.18 (0.91) | 251 | 0.10 (1.02) | 0.04 | |
| Atopy | No | 5158 | 4114 | 79.5 | 743 | 79.1 | 107 | 81.1 | 194 | 77.9 | |
| Yes | 1339 | 1063 | 20.5 | 196 | 20.9 | 25 | 18.9 | 55 | 22.1 | 0.89 | |
| Sex | Male | 5980 | 4729 | 51.0 | 899 | 53.7 | 125 | 52.5 | 227 | 54.0 | |
| Female | 5621 | 4539 | 49.0 | 776 | 46.3 | 113 | 47.5 | 193 | 46.0 | 0.16 | |
| Multiple pregnancy | Singleton | 11297 | 9058 | 97.7 | 1597 | 95.3 | 224 | 94.1 | 418 | 99.5 | |
| Twin | 304 | 210 | 2.3 | 78 | 4.7 | 14 | 5.9 | 2 | 0.5 | <0.001 | |
| Maternal ethnicity | White | 10742 | 8562 | 97.6 | 1569 | 98.8 | 214 | 97.3 | 397 | 98.8 | |
| Non-white | 241 | 211 | 2.4 | 19 | 1.2 | 6 | 2.7 | 5 | 1.2 | 0.01 | |
| Maternal pre-pregnancy BMI (kg/m2) | 10312 | 8271 | 22.50 (3.33) | 1450 | 24.67 (4.69) | 208 | 24.64 (5.28) | 383 | 24.55 (4.91) | <0.001 | |
| Maternal age (years) | 11601 | 9268 | 28.50 (4.74) | 1675 | 28.31 (4.86) | 238 | 27.82 (5.45) | 420 | 28.91 (4.74) | 0.02 | |
| Parity | Nulliparous | 5009 | 3724 | 41.9 | 910 | 57.0 | 160 | 71.7 | 215 | 53.1 | |
| Multiparous | 6099 | 5160 | 58.1 | 686 | 43.0 | 63 | 28.3 | 190 | 46.9 | <0.001 | |
| Maternal smoking in pregnancy | None | 4902 | 3893 | 42.8 | 710 | 43.5 | 113 | 49.3 | 186 | 44.8 | |
| Passive | 3711 | 2884 | 31.7 | 603 | 36.9 | 79 | 34.5 | 145 | 34.9 | ||
| Mother 1-9 | 987 | 819 | 9.0 | 124 | 7.6 | 16 | 7.0 | 28 | 6.7 | ||
| Mother 10-19 | 1214 | 1027 | 11.3 | 132 | 8.1 | 16 | 7.0 | 39 | 9.4 | ||
| Mother 20+ | 553 | 467 | 5.1 | 64 | 3.9 | 5 | 2.2 | 17 | 4.1 | <0.001 | |
| Maternal education | Certificate of Secondary Education/vocational | 3003 | 2432 | 27.6 | 411 | 25.8 | 62 | 27.9 | 98 | 24.3 | |
| Ordinary level | 3906 | 3048 | 34.6 | 600 | 37.7 | 84 | 37.8 | 174 | 43.1 | ||
| Advanced level | 2601 | 2119 | 24.0 | 354 | 22.2 | 50 | 22.5 | 78 | 19.3 | ||
| Degree | 1520 | 1212 | 13.8 | 228 | 14.3 | 26 | 11.7 | 54 | 13.4 | 0.02 | |
| Maternal asthma | No | 9797 | 7851 | 89.1 | 1387 | 87.0 | 202 | 90.2 | 357 | 85.0 | |
| Yes | 1256 | 963 | 10.9 | 208 | 13.0 | 22 | 9.8 | 63 | 15.0 | 0.007 | |
| Gestational age (weeks)† | 11601 | 9268 | 40 (39, 41) | 1675 | 40 (39, 41) | 238 | 38 (36, 40) | 420 | 40 (38, 40) | <0.001 | |
| Birth weight (g) | 11460 | 9150 | 3421 (531) | 1658 | 3420 (587) | 234 | 2964 (849) | 418 | 3368 (619) | <0.001 | |
Lung function SD scores are standardised by age, sex and height
Median (inter-quartile range) is presented as this variable has a skewed distribution
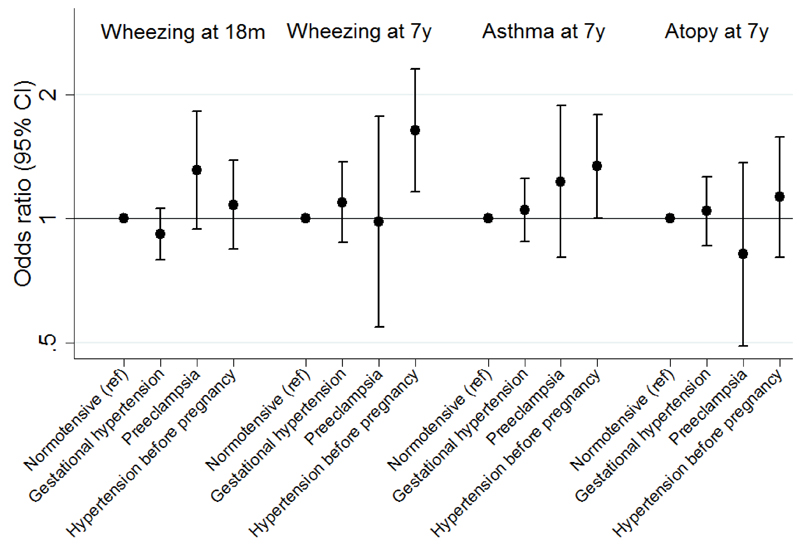
After controlling for confounders (model 3), gestational hypertension was not associated with wheezing at 18 months or 7 years, however, there was weak evidence for a positive association between preeclampsia and early wheezing and stronger evidence for a positive association between hypertension before pregnancy and wheezing at 7 years (Table 2 and Figure 2). After controlling for confounders (model 3), there was evidence for a positive association between hypertension before pregnancy and asthma at 7 years, but neither gestational hypertension nor preeclampsia was associated with asthma; none of the HDP were associated with atopy at 7 years (Tables 3 and 4 and Figure 2).
Table 2. Odds ratios (95% confidence interval) for wheezing at 18 months and 7 years associated with maternal hypertensive disorders of pregnancy*.
| Model 1 |
Model 2 |
Model 3 |
|
|---|---|---|---|
| Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | |
| Wheezing at age 18 months (N=9,076) | |||
| Normotensive (reference) | 1 | 1 | 1 |
| Gestational hypertension | 0.89 (0.78, 1.02) | 0.90 (0.78, 1.04) | 0.92 (0.79, 1.06) |
| Preeclampsia | 1.16 (0.84, 1.60) | 1.27 (0.91, 1.76) | 1.31 (0.94, 1.82) |
| Hypertension before pregnancy | 1.06 (0.84, 1.35) | 1.07 (0.84, 1.37) | 1.08 (0.84, 1.38) |
| Wheezing at age 7 years (N=7,026) | |||
| Normotensive (reference) | 1 | 1 | 1 |
| Gestational hypertension | 1.10 (0.89, 1.37) | 1.08 (0.87, 1.35) | 1.09 (0.87, 1.37) |
| Preeclampsia | 0.96 (0.54, 1.71) | 0.98 (0.54, 1.76) | 0.98 (0.55, 1.77) |
| Hypertension before pregnancy | 1.69 (1.21, 2.36) | 1.62 (1.15, 2.28) | 1.63 (1.16, 2.31) |
Model 1 is adjusted for offspring sex;
Model 2 is additionally adjusted for maternal asthma, maternal pre-pregnancy BMI, maternal age, maternal parity, maternal education, multiple pregnancy and maternal ethnicity;
Model 3 is additionally adjusted for maternal smoking during pregnancy
Figure 2. Odds ratios (95% confidence interval) for early childhood wheezing and later childhood wheezing, asthma and atopy associated with maternal hypertensive disorders of pregnancy in the confounder-adjusted model*.
* Adjusted for offspring sex, maternal asthma, maternal pre-pregnancy BMI, maternal age, maternal parity, maternal education, multiple pregnancy, maternal ethnicity and maternal smoking during pregnancy (Model 3)
Table 3. Odds ratios (95% confidence interval) for asthma at age 7 years associated with maternal hypertensive disorders of pregnancy (N=6,974)*.
| Model 1 |
Model 2 |
Model 3 |
|
|---|---|---|---|
| Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | |
| Normotensive (reference) | 1 | 1 | 1 |
| Gestational hypertension | 1.08 (0.91, 1.28) | 1.04 (0.87, 1.24) | 1.05 (0.88, 1.25) |
| Preeclampsia | 1.23 (0.81, 1.86) | 1.20 (0.78, 1.83) | 1.23 (0.80, 1.88) |
| Hypertension before pregnancy | 1.39 (1.05, 1.85) | 1.33 (1.00, 1.78) | 1.34 (1.00, 1.79) |
Model 1 is adjusted for offspring sex;
Model 2 is additionally adjusted for maternal asthma, maternal pre-pregnancy BMI, maternal age, maternal parity, maternal education, multiple pregnancy and maternal ethnicity;
Model 3 is additionally adjusted for maternal smoking during pregnancy
Table 4. Odds ratios (95% confidence interval) for atopy at age 7 years associated with maternal hypertensive disorders of pregnancy (N=5,532)*.
| Model 1 |
Model 2 |
Model 3 |
|
|---|---|---|---|
| Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | |
| Normotensive (reference) | 1 | 1 | 1 |
| Gestational hypertension | 1.04 (0.86, 1.25) | 1.05 (0.87, 1.28) | 1.04 (0.86, 1.26) |
| Preeclampsia | 0.81 (0.49, 1.33) | 0.83 (0.50, 1.39) | 0.82 (0.49, 1.37) |
| Hypertension before pregnancy | 1.14 (0.82, 1.58) | 1.13 (0.81, 1.58) | 1.13 (0.81, 1.58) |
Model 1 is adjusted for offspring sex;
Model 2 is additionally adjusted for maternal asthma, maternal pre-pregnancy BMI, maternal age, maternal parity, maternal education, multiple pregnancy and maternal ethnicity;
Model 3 is additionally adjusted for maternal smoking during pregnancy
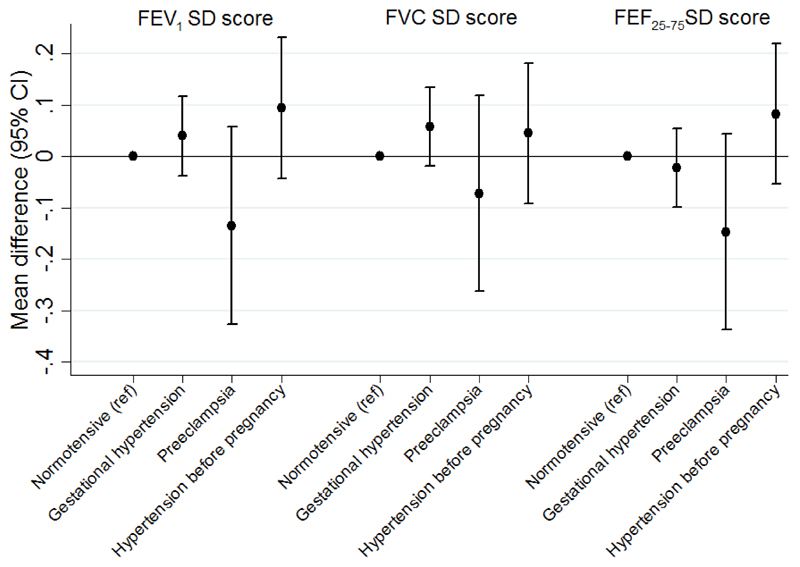
Table 5 and Figure 3 show the associations between HDP and childhood lung function. After controlling for potential confounders (model 3), there was weak evidence to suggest that, compared to children of normotensive mothers, FEV1 and FEF25-75 were lower in children whose mothers had had preeclampsia.
Table 5. Mean differences (95% confidence interval) in lung function outcomes at age 8 years associated with maternal hypertensive disorders of pregnancy (N=5,601)*.
| Model 1 |
Model 2 |
Model 3 |
|
|---|---|---|---|
| Mean difference (95% CI) | Mean difference (95% CI) | Mean difference (95% CI) | |
| FEV1 SD score† | |||
| Normotensive (reference) | 0 | 0 | 0 |
| Gestational hypertension | 0.04 (−0.03, 0.12) | 0.04 (−0.04, 0.12) | 0.04 (−0.04, 0.12) |
| Preeclampsia | −0.14 (−0.33, 0.05) | −0.13 (−0.32, 0.06) | −0.14 (−0.33, 0.06) |
| Hypertension before pregnancy | 0.09 (-0.05, 0.23) | 0.10 (-0.04, 0.23) | 0.09 (-0.04, 0.23) |
| FVC SD score† | |||
| Normotensive (reference) | 0 | 0 | 0 |
| Gestational hypertension | 0.06 (−0.02, 0.13) | 0.05 (−0.02, 0.13) | 0.06 (−0.02, 0.13) |
| Preeclampsia | −0.07 (−0.26, 0.12) | −0.08 (−0.27, 0.11) | −0.07 (−0.26, 0.12) |
| Hypertension before pregnancy | 0.04 (−0.09, 0.18) | 0.04 (−0.09, 0.18) | 0.04 (−0.09, 0.18) |
| FEF25-75 SD score† | |||
| Normotensive (reference) | 0 | 0 | 0 |
| Gestational hypertension | −0.02 (−0.10, 0.05) | −0.01 (−0.09, 0.06) | −0.02 (−0.10, 0.05) |
| Preeclampsia | −0.15 (−0.34, 0.04) | −0.13 (−0.32, 0.06) | −0.15 (−0.34, 0.04) |
| Hypertension before pregnancy | 0.08 (−0.05, 0.22) | 0.09 (−0.05, 0.22) | 0.08 (−0.05, 0.22) |
Model 1 is adjusted for offspring sex;
Model 2 is additionally adjusted for maternal asthma, maternal pre-pregnancy BMI, maternal age, maternal parity, maternal education, multiple pregnancy and maternal ethnicity;
Model 3 is additionally adjusted for maternal smoking during pregnancy
SD scores are adjusted for age, sex and height
Figure 3. Mean differences (95% confidence interval) in lung function outcomes at age 8 years associated with maternal hypertensive disorders of pregnancy in the confounder-adjusted model*.
* Adjusted for offspring sex, maternal asthma, maternal pre-pregnancy BMI, maternal age, maternal parity, maternal education, multiple pregnancy, maternal ethnicity and maternal smoking during pregnancy (Model 3). Lung function SD scores are standardised by age, sex and height
In order to explore potential mediation of associations by fetal growth retardation and prematurity, we controlled additionally for birth weight and gestational age (online supplement Table E2). This attenuated the effect estimates for the relations between preeclampsia and early wheezing, FEV1 and FEF25-75, however, the associations between hypertension before pregnancy and asthma and wheezing at 7 years were not attenuated.
Discussion
In this population-based birth cohort study we have found weak evidence for an association between preeclampsia and wheezing at 18 months and impaired ventilatory function later in childhood. Our findings for preeclampsia and early wheezing are in keeping with recent results from a large meta-analysis4. In that study effect estimates were stronger for recurrent wheezing than for ever wheezing. We must await new data from other large studies to see whether our findings for lung function are robustly replicated; to date, only one, relatively small, study reported no association between preeclampsia and childhood lung function3. We also found that hypertension before pregnancy in the mother, but not gestational hypertension, was positively associated with wheezing and asthma at 7 years in the offspring. To our knowledge this has not been reported previously and needs replicating. We know of no previous studies that have considered the specific association of maternal hypertension before pregnancy with offspring wheezing and asthma. A previous study of maternal hypertension (including all forms of HDP) and childhood asthma was negative8, and results of two studies of the relation between maternal hypertension and preeclampsia (analysed in combination) and childhood wheezing and asthma were conflicting5;6. However, in these studies gestational hypertension and hypertension before pregnancy were not distinguished and analysed separately. In our study, preeclampsia was not associated with childhood asthma, which is in keeping with two previous studies3;7, although, given the confidence limits around the effect estimates in our study, we cannot rule out a positive association; despite ALSPAC’s size, the number of women with preeclampsia was still small, and therefore confidence intervals were wide.
We did not find an association between preeclampsia and atopy. Whilst one small study reported a positive association between preeclampsia or placental abruption and severe atopy in the adolescent offspring, the study was limited to Caesarean section deliveries, and preeclampsia and placental abruption were not analysed separately11. The only other study to examine the relation between preeclampsia and atopy reported a positive association between severe preeclampsia and severe atopy only3. We did not attempt to sub-analyse our data further with respect to severity of preeclampsia or atopy because statistical power would have been compromised. The lack of a positive association between preeclampsia and atopy in our study suggests that preeclampsia is unlikely to explain any of the well-established relation between parity (birth order) and atopy in the offspring, even though preeclampsia is more common in first pregnancies and atopy is more common in firstborns22;23.
Potential mechanisms
As preeclampsia is associated with low birth weight and prematurity24;25, and given that low birth weight and prematurity have been associated independently with wheezing, asthma and impaired lung function in children26–30, it is plausible that a relation between preeclampsia and adverse childhood respiratory outcomes, if causal, may be mediated through detrimental effects on fetal growth or gestational age at birth. In support of this hypothesis, and making key assumptions of mediation analysis20;21, we found that controlling for birth weight and gestational age attenuated associations between preeclampsia and early childhood wheezing and lung function substantially. This has not been explored in previous studies, with the exception of a recent meta-analysis, which confirmed that such adjustment led to moderate change in the risk estimate for the relation between preeclampsia and early childhood wheezing, although the magnitude of change was not quantified4. In our study, deficits in lung function associated with preeclampsia were stronger for FEV1 and FEF25-75 than for FVC, suggesting detrimental effects on fetal airway growth. We speculate that the mechanism underlying such effects may involve overproduction of anti-angiogenic factors in amniotic fluid, which disrupts lung growth31; animal work has suggested that a similar mechanism may explain the link between preeclampsia and bronchopulmonary dysplasia in humans32. We have previously shown that preeclampsia is related to babies being small for gestational age, whilst gestational hypertension is not in this cohort25; this might explain why preeclampsia, but not gestational hypertension, was associated with early wheezing and reduced lung function. In contrast, the relations between hypertension before pregnancy and childhood wheezing and asthma were not attenuated by controlling for birth weight and gestational age; this suggests that, if these associations are causal, they are unlikely to be mediated through impaired fetal growth or prematurity. Nor are they likely to be explained by atopy, given that hypertension before pregnancy was unrelated to this outcome. We cannot offer an alternative mechanistic explanation for the associations with wheezing and asthma. A lack of a positive association between preeclampsia and atopy is perhaps unsurprising, given that preeclampsia is associated with a Th1 predominant immune profile33.
Strengths and limitations
Aside from ALSPAC’s size and population-based prospective design, a strength of our study is that we were able to define gestational hypertension and preeclampsia using data abstracted from the mothers’ obstetric records, applying a standard definition of HDP to the blood pressure and proteinuria measurements themselves, rather than reliance on self-report. We were also able to distinguish the three conditions of hypertension before pregnancy, gestational hypertension and preeclampsia, in contrast with some previous studies5;6; grouping the three hypertensive disorders for analysis may potentially dilute associations. Despite the size of ALSPAC, however, we lacked power to exclude a possible modest detrimental effect of preeclampsia on early wheezing and later lung function, because preeclampsia is not a common condition; more definitive results would require investigation in larger birth cohorts or through meta-analysis of multiple cohorts. Whilst we cannot rule out the possibility that the associations between hypertension before pregnancy and childhood wheezing/asthma, and the weak associations between preeclampsia and early wheezing and impaired lung function, might be explained by uncontrolled or residual confounding, we controlled for a large number of potential confounders. These included maternal smoking in pregnancy, which is associated with a lower incidence of preeclampsia19 and would therefore negatively confound the associations between preeclampsia and respiratory outcomes, such that adjustment for maternal smoking would strengthen, not weaken, these associations; better measurement of maternal smoking would therefore be expected to increase the magnitude of associations with lung function further as it is likely that maternal smoking is underreported. Another important potential confounder that we considered was maternal asthma, which not only increases the risk of asthma in the offspring, but is also associated with a higher incidence of HDP (and low birth weight and prematurity)34–36.
We acknowledge that there may have been some misclassification of maternally-reported outcomes, especially early childhood wheezing. Similarly, we cannot rule out the possibility of misclassification of the hypertensive disorders of pregnancy, especially hypertension before pregnancy, which relied on maternal self-report. However, as misclassification of outcome is likely to be non-differential with respect to the exposures of interest (and vice versa), this would be expected to bias effect estimates towards the null; in other words, the magnitude of associations would be underestimated. Furthermore, given that information on gestational hypertension and preeclampsia was obtained from obstetric records, we believe that any misclassification of these exposures will be less than in previous studies which have relied on maternal self-report of these disorders. As with any longitudinal study, data were not complete on exposures, outcomes, and confounders for the whole cohort. Therefore, we cannot rule out the possibility that exclusion of children without complete information might have biased our findings. However, we can see no reason why associations between HDP and the outcomes of interest would be different in those included and those excluded.
Conclusion
We conclude that gestational hypertension is unlikely to increase the risk of asthma, atopy or impaired lung function in the offspring, which is reassuring given that this disorder is not uncommon. Hypertension before pregnancy may be a risk factor for childhood wheezing and asthma although this observation requires replication. Determining whether preeclampsia is associated with impaired childhood lung function will require larger studies which are able to analyse preeclampsia separately from gestational hypertension and hypertension before pregnancy and adjust for a range of potential confounders.
Supplementary Material
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
The UK Medical Research Council, the Wellcome Trust (Grant ref: 092731) and the University of Bristol currently provide core support for ALSPAC. CM-W and DAL work in a Unit that receives funding from the MRC [grant number MC_UU_12013/5]. CM-W is funded by a UK MRC research fellowship [grant number MR/J011932/1].
Footnotes
Take home message: Gestational hypertension is unlikely to be a risk factor for childhood respiratory disorders; further studies are needed to clarify whether hypertension before pregnancy or preeclampsia are risk factors.
References
- 1.Henderson AJ, Warner JO. Fetal origins of asthma. Seminars in Fetal and Neonatal Medicine. 2012;17:82–91. doi: 10.1016/j.siny.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Stick SM, Burton PR, Gurrin L, Sly PD, Le Souef PN. Effects of maternal smoking during pregnancy and a family history of asthma on respiratory function in newborn infants. Lancet. 1996;348:1060–1064. doi: 10.1016/s0140-6736(96)04446-7. [DOI] [PubMed] [Google Scholar]
- 3.Byberg K, Ogland B, Eide G, Oymar K. Birth after preeclamptic pregnancies: association with allergic sensitization and allergic rhinoconjunctivitis in late childhood; a historically matched cohort study. BMC Pediatrics. 2014;14:101. doi: 10.1186/1471-2431-14-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zugna D, Galassi C, Annesi-Maesano I, Baiz N, Barros H, Basterrechea M, et al. Maternal complications in pregnancy and wheezing in early childhood: A pooled analysis of 14 birth cohorts. Int J Epidemiol. 2015 doi: 10.1093/ije/dyu260. [DOI] [PubMed] [Google Scholar]
- 5.Rusconi F, Galassi C, Forastiere F, Bellasio M, De Sario M, Ciccone G, et al. Maternal Complications and Procedures in Pregnancy and at Birth and Wheezing Phenotypes in Children. Am J Respir Crit Care Med. 2007;175:16–21. doi: 10.1164/rccm.200512-1978OC. [DOI] [PubMed] [Google Scholar]
- 6.Nafstad P, Magnus P, Jaakkola JJ. Risk of childhood asthma and allergic rhinitis in relation to pregnancy complications. J Allergy Clin Immunol. 2000;106:867–873. doi: 10.1067/mai.2000.110558. [DOI] [PubMed] [Google Scholar]
- 7.Nafstad P, Samuelsen SO, Irgens LM, Bjerkedal T. Pregnancy Complications and the Risk of Asthma among Norwegians Born between 1967 and 1993. Eur J Epidemiol. 2003;18:755–761. doi: 10.1023/a:1025395405101. [DOI] [PubMed] [Google Scholar]
- 8.Annesi-Maesano I, Moreau D, Strachan D. In utero and perinatal complications preceding asthma. Allergy. 2001;56:491–497. doi: 10.1034/j.1398-9995.2001.056006491.x. [DOI] [PubMed] [Google Scholar]
- 9.Roberts CL, Ford JB, Algert CS, Antonsen S, Chalmers J, Cnattingius S, et al. Population-based trends in pregnancy hypertension and pre-eclampsia: an international comparative study. BMJ Open. 2011;1(1) doi: 10.1136/bmjopen-2011-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visintin C, Mugglestone MA, Almerie MQ, Nherera LM, James D, Walkinshaw S. Management of hypertensive disorders during pregnancy: summary of NICE guidance. BMJ. 2010;341 doi: 10.1136/bmj.c2207. [DOI] [PubMed] [Google Scholar]
- 11.Keski-Nisula L, Heinonen S, Remes S, Pekkanen J. Pre-Eclampsia, Placental Abruption and Increased Risk of Atopic Sensitization in Male Adolescent Offspring. Am J Reprod Immunol. 2009;62:293–300. doi: 10.1111/j.1600-0897.2009.00738.x. [DOI] [PubMed] [Google Scholar]
- 12.Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. Cohort Profile: The 'Children of the 90s' - the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42:111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, et al. Cohort Profile: The Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42:97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutqui JM. The classification and diagnosis of the hypertensive disorders of pregnancy: Statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Hypertens Pregnancy. 2001;20:IX–XIV. doi: 10.1081/PRG-100104165. [DOI] [PubMed] [Google Scholar]
- 15.Chinn S, Rona RJ. Height and age adjustment for cross sectional studies of lung function in children aged 6-11 years. Thorax. 1992;47:707–714. doi: 10.1136/thx.47.9.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 17.Arets HGM, Brackel HJL, van der Ent CK. Forced expiratory manoeuvres in children: do they meet ATS and ERS criteria for spirometry? Eur Respir J. 2001;18:655–660. doi: 10.1183/09031936.01.00204301. [DOI] [PubMed] [Google Scholar]
- 18.Roberts G, Peckitt C, Northstone K, Strachan D, Lack G, Henderson J, et al. Relationship between aeroallergen and food allergen sensitization in childhood. Clin Exp Allergy. 2005;35:933–940. doi: 10.1111/j.1365-2222.2005.02280.x. [DOI] [PubMed] [Google Scholar]
- 19.England L, Zhang J. Smoking and risk of preeclampsia: a systematic review. Front Biosci. 2007;12:2471–2483. doi: 10.2741/2248. [DOI] [PubMed] [Google Scholar]
- 20.Robins JM, Greenland S. Identifiability and Exchangeability for Direct and Indirect Effects. Epidemiology. 1992;3:143–155. doi: 10.1097/00001648-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Cole SR, Hernan MA. Fallibility in estimating direct effects. Int J Epidemiol. 2002;31:163–165. doi: 10.1093/ije/31.1.163. [DOI] [PubMed] [Google Scholar]
- 22.Kirsten D, Deborah H. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong X, Mayes D, Demianczuk N, Olson DM, Davidge ST, Newburn-Cook C, et al. Impact of pregnancy-induced hypertension on fetal growth. Am J Obstet Gynecol. 1999;180:207–213. doi: 10.1016/s0002-9378(99)70176-6. [DOI] [PubMed] [Google Scholar]
- 25.Geelhoed JJM, Fraser A, Tilling K, Benfield L, Davey Smith G, Sattar N, et al. Preeclampsia and Gestational Hypertension Are Associated With Childhood Blood Pressure Independently of Family Adiposity Measures: The Avon Longitudinal Study of Parents and Children. Circulation. 2010;122:1192–1199. doi: 10.1161/CIRCULATIONAHA.110.936674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rona RJ, Gulliford MC, Chinn S. Effects of prematurity and intrauterine growth on respiratory health and lung function in childhood. BMJ. 1993;306:817–820. doi: 10.1136/bmj.306.6881.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaakkola JJK, Ahmed P, Ieromnimon A, Goepfert P, Laiou E, Quansah R, et al. Preterm delivery and asthma: A systematic review and meta-analysis. J Allergy Clin Immunol. 2006;118:823–830. doi: 10.1016/j.jaci.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 28.Kotecha SJ, Watkins WJ, Heron J, Henderson J, Dunstan FD, Kotecha S. Spirometric Lung Function in School-Age Children: Effect of Intrauterine Growth Retardation and Catch-up Growth. Am J Respir Crit Care Med. 2010;181:969–974. doi: 10.1164/rccm.200906-0897OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotecha SJ, Watkins WJ, Paranjothy S, Dunstan FD, Henderson AJ, Kotecha S. Effect of late preterm birth on longitudinal lung spirometry in school age children and adolescents. Thorax. 2012;67:54–61. doi: 10.1136/thoraxjnl-2011-200329. [DOI] [PubMed] [Google Scholar]
- 30.Sonnenschein-van der Voort A, Arends LR, de Jongste JC, Annesi-Maesano I, Arshad SH, Barros H, et al. Preterm birth, infant weight gain, and childhood asthma risk: A meta-analysis of 147,000 European children. J Allergy Clin Immunol. 2014;133:1317–1329. doi: 10.1016/j.jaci.2013.12.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang JR, Karumanchi SA, Seedorf G, Markham N, Abman SH. Excess soluble vascular endothelial growth factor receptor-1 in amniotic fluid impairs lung growth in rats: linking preeclampsia with bronchopulmonary dysplasia. Am J Physiol - Lung Cellular and Molecular Physiology. 2011;302:L36–L46. doi: 10.1152/ajplung.00294.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen AR, Barn+®s CM, Folkman J, McElrath TF. Maternal Preeclampsia Predicts the Development of Bronchopulmonary Dysplasia. J Pediatr. 2010;156:532–536. doi: 10.1016/j.jpeds.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 33.Saito S, Sakai M. Th1/Th2 balance in preeclampsia. J Reprod Immunol. 2003;59:161–173. doi: 10.1016/s0165-0378(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 34.Liu S, Wen SW, Demissie K, Marcoux S, Kramer MS. Maternal asthma and pregnancy outcomes: A retrospective cohort study. Am J Obstet Gynecol. 2001;184:90–96. doi: 10.1067/mob.2001.108073. [DOI] [PubMed] [Google Scholar]
- 35.Demissie K, Breckenridge MB, Rhoads GG. Infant and maternal outcomes in the pregnancies of asthmatic women. Am J Respir Crit Care Med. 1998;158:1091–1095. doi: 10.1164/ajrccm.158.4.9802053. [DOI] [PubMed] [Google Scholar]
- 36.Enriquez R, Griffin MR, Carroll KN, Wu P, Cooper WO, Gebretsadik T, et al. Effect of maternal asthma and asthma control on pregnancy and perinatal outcomes. J Allergy Clin Immunol. 2007;120:625–630. doi: 10.1016/j.jaci.2007.05.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.