INTRODUCTION
Stroke is the major cause of long-term disability worldwide (Kolominsky-Rabas et al., 2001) and recovery of motor function is often incomplete (Roger et al., 2011). Six months after the ictal event, two-thirds of stroke survivors are unable to carry out independently activities of daily living with their paretic hand to the extent they could before (Kolominsky-Rabas et al., 2001), and only a few are able to return to their previous job (Lai et al., 2002).
Patients with motor deficits resulting from stroke must confront the need to generate compensatory strategies or to relearn the motor programs utilized to accomplish a particular goal before the lesion (Frey et al., 2011; Pomeroy et al., 2011; Sathian et al., 2011). From a behavioral point of view, there are different ways to accomplish the same goal in neurorehabilitation (e.g., grasp a glass of water). One possibility is to implement a motor strategy different from that utilized before the stroke (i.e., compensation) (Levin et al., 2009). An alternative way is to relearn to perform the task in the same way it was done before the lesion. Clearly, both processes are likely to involve fundamentally different pathophysiological mechanisms, even when the goals and outcomes are the same. Different motor training strategies have been tested in neurorehabilitation of motor function. Examples include constraint-induced movement therapy, bilateral arm training, mirror therapy, randomized training schedules, robotic-based approaches, virtual reality, electromyogram-triggered stimulation, action observation, motor imagery, and brain–computer interfaces between others (Cauraugh and Kim, 2003; Wittenberg et al., 2003; Bolton et al., 2004; Deutsch et al., 2004; Luft et al., 2004; Krakauer, 2006; Sharma et al., 2006; Birbaumer and Cohen, 2007; Ertelt et al., 2007; Page et al., 2007; Buch et al., 2008, 2012; Celnik et al., 2008; Cramer, 2008b; Cheeran et al., 2009a; Lo et al., 2009; Dimyan and Cohen, 2011). Noninvasive somatosensory (Conforto et al., 2007, 2010), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) (Hallett, 2000; Kaelin-Lang et al., 2002; Nitsche et al., 2008; Wassermann et al., 2008; Sandrini et al., 2011; Tanaka et al., 2011; Brunoni et al., 2012) have been proposed as adjuvant ways to improve the beneficial effects of training protocols on functional recovery.
In this chapter, we discuss the use of noninvasive brain stimulation (NIBS) in the setting of stroke rehabilitation. Interest in NIBS developed after the observation of long-term effects on cortical excitability that occur after repeated stimulation (Huang et al., 2005; Fitzgerald et al., 2006; Nitsche et al., 2008). Depending on the stimulation parameters, motor cortical excitability can be reduced (inhibition) by means of low-frequency repetitive TMS (rTMS), continuous theta-burst stimulation (cTBS), and cathodal tDCS, or enhanced (facilitation) by means of high-frequency rTMS, intermittent theta-burst stimulation (iTBS), and anodal stimulation. There is evidence that links the effects of these NIBS techniques to long-term potentiation (LTP)-like and long-term depression (LTD)-like mechanisms (Cooke and Bliss, 2006; Thickbroom, 2007; Wagner et al., 2007; Ziemann et al., 2008; Fritsch et al., 2010). After stroke, NIBS has been studied as a tool to modulate cortical excitability in the affected and intact hemispheres, predominantly with the goal of correcting hypothesized imbalances in interhemispheric interactions (Kinsbourne, 1974; Hummel and Cohen, 2006).
POSTSTROKE REORGANIZATION
The magnitude and type of motor impairments that follow stroke are influenced by multiple factors such as lesion site, size, and time after stroke (Rossini et al., 2003; Ward and Cohen, 2004; Frey et al., 2011; Pomeroy et al., 2011; Sathian et al., 2011).
Recovery of motor function after the first few days poststroke is likely related to the resolution of edema as well as to reperfusion of the ischemic penumbra through collateral circulation (Furlan et al., 1996). At later stages after stroke, other mechanisms such as recruitment of functionally homologous pathways, disinhibition of previously inactive neuronal connections, resolution of diaschisis, and the formation of new neural connections may take over the role of the damaged area, contributing to recovery of function (Rossini et al., 2007; Cramer, 2008a). Both pyramidal tract and alternate motor fibers, including the corticorubrospinal and co-rticoreticulospinal tracts (Fries et al., 1991; Lang and Schieber, 2003), may contribute to functional recovery (Lindenberg et al., 2010a). In the chronic stage after stroke, damage to corticospinal tract white-matter fibers seems to correlate with motor impairment (Talelli et al., 2006; Schaechter et al., 2009; Zhu et al., 2010). For example, indices of microstructural integrity (e.g., fractional anisotropy, FA) of the posterior limb of the internal capsule correlate with motor function. It has been proposed that FA in this white-matter region may predict the ability of TMS interventions to modulate motor function positively (Stinear et al., 2007). In addition to the corticospinal tract, a recent study (Lindenberg et al., 2012a) showed that FA in interhemispheric white-matter fibers connecting both primary motor cortices (M1) predicted functional gains in an experimental rehabilitation trial (Lindenberg et al., 2010b). Another factor that influences functional recovery is the ability of the lesioned brain to reorganize. Plasticity, defined as “experience-dependent enduring changes in neuronal or network properties either morphological or functional” (Donoghue et al., 1996), is an important contributor to functional recovery after stroke (Nudo et al., 1996).
Local reorganization
In the case of focal cortical lesions, perilesional regions can be recruited to control the function of the damaged motor area through somatotopic reorganization, a phenomenon referred to as vicariation (Merzenich et al., 1984; Donoghue et al., 1990; Sanes et al., 1990). Studies in animals suggest that preservation of the peri-infarct penumbra may provide a substrate for functional recovery after cortical lesions by unmasking redundant motor representations adjacent to the site of injury (Nudo and Milliken, 1996; Nudo et al., 1996). This form of perilesional reorganization has been demonstrated in healthy human subjects undergoing ischemic nerve block (Brasil-Neto et al., 1993) and in chronic stroke patients (Cramer et al., 1997, 2000). In particular, representational plasticity within M1 may contribute to functional gains after a limited ischemic lesion to this area (Jaillard et al., 2005).
Reorganization of ipsilesional secondary motor areas
Regions distant to the lesion do reorganize as well (Ward, 2011). There is evidence that well recovered stroke patients continue to have reduced ipsilesional motor cortical excitability, as evidenced by reduced motor evoked potential (MEP) amplitudes (Byrnes et al., 2001), suggesting that alternative mechanisms such as cortical reorganization and increased activity of ipsilesional secondary motor areas may have contributed to their motor recovery. For example, the premotor cortex (PMC) and the supplementary motor area (SMA) may contribute to motor function (Ward et al., 2006; Swayne et al., 2008). TMS applied over the ipsilesional dorsal PMC disrupted performance of paretic hand motions in stroke patients with mild to moderate motor deficits, in whom corticospinal fibers originated in M1 were lesioned (Fridman et al., 2004). Functional magnetic resonance imaging (fMRI) studies also suggest the involvement of nonprimary motor areas in recovery of motor function after stroke (Nowak et al., 2010). With respect to the activity in the SMA, a study in acute stroke patients showed that decreased blood oxygen level-dependent (BOLD) signal during movements of a paretic hand relative to controls was predictive of slower recovery, whereas higher BOLD activity was associated with faster motor recovery (Loubinoux et al., 2003).
Bihemispheric reorganization
Longitudinal neuroimaging studies have shown initially high bihemispheric BOLD signal activity associated with movements of the paretic hand 2 weeks poststroke. Over time, bihemispheric activation decreases progressively toward levels observed in healthy subjects concomitant to motor recovery (Ward et al., 2003; Tombari et al., 2004; Rehme et al., 2011). In opposition to this pattern, patients with incomplete motor functional recovery in the chronic phase (i.e., more than 6–12 months post-stroke) typically maintain increased bihemispheric BOLD signal activity (Chollet et al., 1991; Weiller et al., 1992; Ward and Cohen, 2004; Gerloff et al., 2006; Grefkes et al., 2008b). Consistent with these findings, it has been reported that lateralized fMRI activation towards ipsilesional regions, similar to that seen in age-matched controls, is associated with more successful functional recovery (Ward et al., 2003; Gerloff et al., 2006; Nair et al., 2007).
Studies using TMS have attempted to discern the functional role of these neuroimaging findings. It is now known that the presence of ipsilateral MEPs to TMS applied over the contralesional M1 is associated with poor clinical outcome (Turton et al., 1996; Netz et al., 1997; Feydy et al., 2002). In addition to M1, TMS applied to the contralesional dorsal PMC in poorly recovered patients disrupted motor performance of the paretic hand (Johansen-Berg et al., 2002). A neuroimaging study carried out during the first 2 weeks after stroke also showed that functional recovery in severely affected patients was associated with gradually increasing activity in contralesional M1 and PMC (Rehme et al., 2011). In line with this, Lotze and colleagues (2006) showed that disruption of activity in contralesional regions (i.e., M1, dorsal PMC, and superior parietal lobe, SPL) active during movements of the paretic hand affected motor performance. While interference with the dorsal PMC and M1 induced timing errors only, SPL stimulation caused both timing and accuracy deficits.
Overall, these results are consistent with the view that patients with more severe motor impairments may engage more actively regions of the contralesional hemisphere to carry out a task with the paretic hand (Johansen-Berg et al., 2002; Ward et al., 2003; Fridman et al., 2004; Buch et al., 2012).
PHYSIOLOGICAL MECHANISMS OF REORGANIZATION
It is possible to study physiological features of cortical organization after stroke using TMS. Paired-pulse TMS delivered through the same magnetic coil over M1, where a suprathreshold test stimulus (TS) is preceded by a subthreshold or suprathreshold conditioning stimulus (CS), can be used to gain insight into the relative contribution of local inhibitory and excitatory inputs to M1 pyramidal tract cells (Reis et al., 2008). Inter-stimulus intervals of approximately 1–5 ms cause attenuation of MEP amplitudes or short intracortical inhibition (SICI) (Kujirai et al., 1993), whereas longer intervals (~6–10 ms) elicit a facilitatory effect referred to as intracortical facilitation. Decreased SICI has been reported in both affected and unaffected hemispheres of chronic stroke patients (Liepert et al., 2000a, b; Butefisch et al., 2003, 2008). It is not only resting SICI that is abnormal after stroke, but also movement-related SICI. For example, in stroke patients who exhibit relatively good functional recovery, SICI measured immediately preceding performance of a voluntary movement by the paretic hand was abnormal compared with that in age-matched controls (Hummel et al., 2009).
TMS has been also used to study interhemispheric interactions. Early studies in stroke patients examined the excitability of bilateral motor cortices evaluated by differences in MEP amplitudes and in cortical areas (motor maps) producing MEPs in the contralateral hand muscles (Cicinelli et al., 1997; Traversa et al., 1997). These investigations found relative hyperexcitability of the contralesional in comparison with the ipsilesional M1 that normalized with time after stroke. A paired-pulse TMS technique has been used to study interhemispheric inhibition (IHI) between the two M1s, where a suprathreshold CS is applied over the conditioning M1 about 10 ms prior to the TS applied to the opposite conditioned M1 (Ferbert et al., 1992). Abnormalities in resting IHI after stroke may depend on the location of the lesion (Boroojerdi et al., 1996). Other studies started to evaluate movement-related changes in IHI after stroke. In relatively well recovered chronic stroke patients, initially normal levels of IHI from the contralesional to the ipsilesional M1 remain present at the onset of a paretic hand movement, in contrast to the disinhibition that accompanies nonparetic hand movement and motions in age-matched controls (Murase et al., 2004; Duque et al., 2005).
It is also important to consider the interactions between IHI and SICI. Examining whether changes in IHI and SICI after stroke may be related, Butefisch and coworkers (2008) showed that the attenuation of SICI in ipsilesional M1 was not accompanied by a change in resting IHI from contralesional to ipsilesional M1. In contrast, disinhibition of contralesional M1 was accompanied but did not correlate with a decrease in IHI from ipsilesional to contralesional M1s. Altogether these findings were interpreted as indicating that, at rest, local modulation of inhibition within ipsilesional M1 is prominent. However, a more accurate investigation of the resting interactions between SICI and IHI using TMS paired-pulse techniques, as recently implemented in healthy individuals (Daskalakis et al., 2002; Perez and Cohen, 2008), remains to be done after stroke. Additionally, an understanding of IHI–SICI interactions in the process of generation of a voluntary movement by the paretic hand will be important to determine the mechanisms underlying deficits in motor function after stroke. For example, the phenomenon of facilitation of M1 excitability by ipsilateral limb movement has been explored in the healthy brain (Muellbacher et al., 2000; Hortobagyi et al., 2003; Perez and Cohen, 2008), but remains to be evaluated thoroughly after stroke. It has been proposed that, with isometric force production, nonparetic arm activity in stroke patients does not lead to as much ipsilateral M1 facilitation as seen in healthy controls (Woldag et al., 2004; Renner et al., 2005).
Overall, these findings suggest that motor function of the paretic hand may be influenced by abnormalities in interhemispheric inhibitory interactions. This view has been documented extensively in recent literature using TMS measures in healthy subjects (Tinazzi and Zanette, 1998; Schambra et al., 2003; Kim et al., 2004; Kobayashi et al., 2004, 2009; Dafotakis et al., 2008; Dimyan and Cohen, 2010; Williams et al., 2010) and fMRI (Grefkes et al., 2008a, b; Wang et al., 2010). It was shown that stroke patients experience an increased inhibitory influence from contralesional to ipsilesional M1, which is not present in healthy subjects or when the same patients moved their unaffected hand. Importantly, the strength of this inhibition from contralesional M1 correlated with the motor impairment of the paretic hand (Grefkes et al., 2008b). Similar abnormalities in interhemispheric interactions relating to other cognitive functions have been reported in the fields of language and spatial attention (Kinsbourne, 1980; Chrysikou and Hamilton, 2011; Oliveri, 2011).
NONINVASIVE BRAIN STIMULATION IN NEUROREHABILITATION
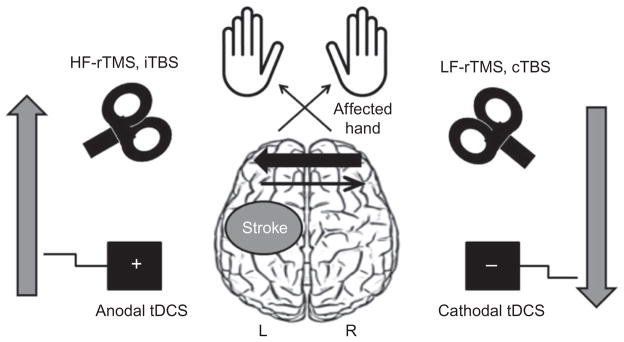
Based on the hypothesis of abnormal interhemispheric inhibitory interactions after stroke, two strategies have been proposed to ameliorate paretic hand function: (1) to downregulate excitability of contralesional M1, and (2) to upregulate excitability of ipsilesional M1 (Hummel and Cohen, 2006; Webster et al., 2006) (Fig. 40.1). Depending on the stimulus type and stimulation parameters, NIBS can facilitate cortical excitability in the ipsilesional M1 through direct stimulation of this region or indirectly via inhibition of the contralesional M1. To target simultaneously both components of this imbalance, it is possible to use bihemispheric modulatory approaches (Takeuchi et al., 2009; Lindenberg et al., 2010b).
Fig. 40.1.
Noninvasive brain stimulation (NIBS) strategies tested to promote recovery of motor function after stroke. Motor deficits following stroke (in this diagram involving the left hemisphere) are associated with disinhibition of the contralesional primary motor cortex and increased interhemispheric inhibition from contralesional to ipsilesional M1. This imbalance of interhemispheric inhibition may be reduced by inhibitory NIBS (low-frequency (LF) repetitive transcranial magnetic stimulation (rTMS), continuous theta-burst stimulation (cTBS) and cathodal transcranial direct current stimulation (tDCS)) applied to the contralesional hemisphere, or facilitatory NIBS (high-frequency (HF) rTMS, intermittent TBS (iTBS), and anodal tDCS) of the ipsilesional M1.
Studies investigating the effects of NIBS on motor function after stroke are summarized in Table 40.1.
Table 40.1.
Summary of previous studies investigating the effectiveness of inhibitory rTMS or tDCS over primary motor cortex of unaffected hemisphere on motor behavior
| Technique | Reference | Area of stimulation | No. of patients, mean age, lesion location, and time since stroke | Study design | Control condition | Stimulation parameters | Main results |
|---|---|---|---|---|---|---|---|
| rTMS, low frequency | Mansur et al., 2005 | Hand area of M1 and dorsal PMC, unaffected hemisphere | 10 adults, 54 years, 10 subcortical, <12 months | Single-blind, crossover, sham- controlled | Real TMS, sham TMS (sham coil), real TMS over PMC | 1 Hz, 100% rMT, 10 min | Decrease in simple and choice RTs and improved performance of Purdue Pegboard Test with affected hand after real rTMS |
| rTMS, low frequency | Takeuchi et al., 2005 | Hand area of M1, unaffected hemisphere | 20 adults, 59 years, 20 subcortical, 6–60 months | Double-blind, randomized, sham- controlled | 20 patients, 10 real TMS, 10 sham TMS (perpendicular to the scalp ) | 1 Hz, 90% rMT, 25 min | Real rTMS reduced MEP amplitude in contralesional M1 and abnormal transcallosal inhibition. Moreover rTMS induced improvement in pinch acceleration |
| rTMS, low frequency | Fregni et al., 2006 | Hand area of M1, unaffected hemisphere | 15 adults, 56 years, 2 cortical, 13 subcortical, 1–11 years | Single-blind, longitudinal, randomized, sham- controlled, Phase II | 15 patients, 10 real TMS, 5 patients sham TMS (sham coil) | 1 Hz, 100% MT, 20 min, repeated daily over 5 days | Real TMS resulted in improvement of motor function performance (Jebsen–Taylor Hand Function Test, simple and choice RTs, Purdue Pegboard Test) in affected hand that lasted for 2 weeks. Corticospinal excitability decreased in stimulated unaffected hemisphere and increased in affected hemisphere |
| rTMS, low frequency | Boggio et al., 2006 | Hand area of M1, unaffected hemisphere | 1 adult, 74-year- old woman, subcortical, 23–107 months | Double-blind, crossover, single-case study | Real TMS, sham TMS (sham coil) over M1 | 1 Hz, 100% rMT, 20 min | rTMS improved motor function (thumb and finger movements) |
| rTMS, low frequency | Liepert et al., 2007 | Hand area of M1, unaffected hemisphere | 12 adults, 64 years, 12 subcortical, <14 days | Double-blind, crossover, sham- controlled | Real TMS, sham TMS (sham coil) | 1 Hz, 90% rMT, 20 min | Real rTMS improved Nine Hole Peg Test results but not grip strength in affected hand |
| rTMS, low frequency | Dafotakis et al., 2008 | Hand area of M1, unaffected hemisphere | 12 adults, 45 years, 12 subcortical, 1–15 months | Double-blind, crossover, sham- controlled | Real TMS, sham TMS over vertex | 1 Hz, 100% rMT, 10 min | Real rTMS improved efficiency and timing of grasping and lifting with affected hand |
| rTMS, low frequency | Nowak et al., 2008 | Hand area of M1, unaffected hemisphere | 15 adults, 46 years, 15 subcortical, 1–4 months | Double-blind, crossover, sham- controlled | Real TMS, sham TMS over vertex | 1 Hz, 100 rMT, 10 min | Real rTMS improved kinematics of finger and grasp movements in affected hand and reduced overactivity in contralesional M1 and nonprimary motor areas. Overactivity of contralesional dorsal PMC, contralesional parietal operculum, and ipsilesional mesial frontal cortex at baseline predicted improvement of movement |
| rTMS, low frequency | Kirton et al., 2008 | Hand area of M1, unaffected hemisphere | 10 children, 14 years, 10 subcortical, 3–13 years | Single-blind, longitudinal, randomized, sham controlled | 10 patients, 5 real TMS, 5 sham TMS (perpendicular to scalp) | 1 Hz, 100% rMT, 20 min repeated daily for 8 days | Real rTMS improved grip strength and Melbourne assessment of upper extremity function |
| rTMS, low frequency | Takeuchi et al., 2008 | Hand area of M1, unaffected hemisphere | 20 adults, 62 years, 20 subcortical, 7–121 months | Double-blind, randomized, sham- controlled | 20 patients, 10 real TMS, 10 sham TMS (perpendicular to scalp) | 1 Hz, 90% rMT, 25 min +motor training | Real rTMS induced an increase in excitability in affected hemisphere and improvement in acceleration of affected hand. Improvements in motor function lasted for 1 week |
| rTMS, priming stimulation | Carey et al., 2008 | Hand area of M1, unaffected hemisphere | 10 adults, 66 years, 8 cortical, 2 subcortical, 16–192 months | No sham rTMS, sham- controlled | 10 patients real TMS | 10 min of 6 Hz (600 pulses), 90% rMT. Immediately after, 10 min of 1 Hz (600 pulses), 90% rMT | Safety of treatment. No seizures and impairment in National Institutes of Health Stroke Scale, Wechsler Adult Intelligence Scale (3rd edition), Hopkins Verbal Learning Test–Revised, Beck Depression Inventory (2nd edition) |
| rTMS, priming stimulation | Carey et al., 2010 | Hand area of M1, unaffected hemisphere | 2 adults, with middle cerebral artery stroke, 71-year-old male, 52-year-old female, >10 years | Longitudinal, no sham rTMS, sham- controlled | 2 patients real TMS | 10 min of 6 Hz (600 pulses), 90% rMT. Immediately after, 10 min of 1 Hz (600 pulses), 90% rMT, 5 sessions | fMRI showed that intervention disrupted cortical activation at contralesional M1. Behavioral results (Box and Block Test, Motor Activity Log) were mixed |
| rTMS, priming stimulation | Kakuda et al., 2011 | Hand area of M1, unaffected hemisphere | 11 adults, 61 years, 11 subcortical, average 70.2 months | Longitudinal, no sham rTMS, sham- controlled | 5 patients real TMS | 10 min of 6 Hz (600 pulses), 90% rMT. Immediately after, 20 min of 1 Hz (600 pulses), 90% rMT+OT, 15-day protocol, 22 sessions | rTMS improved Fugl–Meyer score and shortened log performance time of Wolf Motor Function Test |
| rTMS, low frequency | Kakuda et al., 2010a | Hand area of M1, unaffected hemisphere | 5 adults, 66.8 years, 5 subcortical, 14.6 months | Longitudinal, no sham rTMS, sham- controlled | 5 patients real TMS | 1 Hz (20 min, 90% rMT)+OT, 10 sessions over 6 consecutive days | Improvements in scores of Fugl– Meyer Assessment, Wolf Motor Function Test, and Ten-Second Test. No deterioration of improved upper limb function observed at 4 weeks after treatment |
| rTMS, low frequency | Kakuda et al., 2010b | Hand area of M1, unaffected hemisphere | 15 adults, 55 years, 15 subcortical, 57±55 months | Longitudinal | Real TMS | 1 Hz, 1200 pulses, 90% MT, 22 sessions in 2-week period combined with OT | Fugl–Meyer Assessment score increased in all 15 patients. Shortening of performance time on Wolf Motor Function Test noted in 12 patients. Modified Ashworth Scale score for some flexor muscles decreased in 12 patients |
| rTMS, low frequency | Grefkes et al., 2010 | Hand area of M1, unaffected hemisphere | 11 adults, 46 years, 11 subcortical, 1–3 months | Single-blind, crossover, sham- controlled | Real TMS, sham TMS over vertex | 1 Hz, 100% rMT, 10 min | Real rTMS improved motor performance of paretic hand. Connectivity analysis (Dynamic Causal Modeling) revealed that behavioral improvements were significantly correlated with a reduction of negative influences originating from contralesional M1 during paretic hand movements. Concurrently, endogenous coupling between ipsilesional SMA and M1 was significantly enhanced only after rTMS |
| rTMS, low frequency | Conforto et al., 2012 | Hand area of M1, unaffected hemisphere | 30 adults, 55.75 years, 14 corticosub- cortical, 16 subcortical, average 27.65 days | Double-blind, randomized, longitudinal, sham- controlled | 30 patients, 15 real TMS, 15 sham TMS (perpendicular to vertex) | 1 Hz, 90% rMT, 25 min, 10 sessions | Real rTMS improved performance in Jebsen–Taylor Test (1 month after treatment) and pinch force |
| rTMS, low frequency | Kakuda et al., 2011 | Hand area of M1, unaffected hemisphere | 204 adults, 58.4 years, 107 intracerebral hemorrhage, 27 cerebral cortical infarction, 70 lacunar infarction, 5.0±4.5 years | Multi-center, longitudinal, no sham rTMS, sham- controlled | 204 patients real TMS | 1 Hz, 90% rMT, 20 min, 120-min intensive OT daily, 22 sessions during 15-day hospitali-zation | Fugl–Meyer Assessment score and Wolf Motor Function Test log performance time decreased significantly at discharge. Changes seen persistently up to 4 weeks after discharge in 79 patients |
| rTMS, low frequency | Avenanti et al., 2012 | Hand area of M1, unaffected hemisphere | 30 adults, 60.9 years, 29 subcortical, 1 cortical, average 31 months | Double-blind, randomized, parallel, factorial design, longitudinal, sham- controlled, Phase II | 30 patients, 8 real TMS+PT, 8 PT+real TMS, 7 sham TMS+PT, 7PT+sham TMS | 1 Hz, 90% rMT, 25 min, 45 min physical training daily, 10 sessions | Behavioral improvement (Jebsen– Taylor Hand Function Test, Nine- Hole Peg Test) and reduction of interhemispheric inhibition found after real rTMS, with the group receiving real TMS+PT showing robust and stable improvements, and the other group (PT+real TMS) showing a slight improvement decline over time |
| tDCS, cathodal | Nair et al., 2011 | Hand area of M1, unaffected hemisphere | 14 adults, 58.5 years, 9 cortical and 5 subcortical, average 30.5 months | Double-blind, randomized, longitudinal, sham- controlled | 14 patients, 7 cathodal tDCS, 7 sham tDCS | 60 min of OT and 30 min of tDCS (1 mA) each day for 5 days in a row | Cathodal tDCS+OT results in more improvement in range-of-motion in multiple joints of paretic upper extremity and in upper extremity Fugl–Meyer score than sham tDCS+OT |
fMRI, functional magnetic resonance imaging; M1, primary motor cortex; MEP, motor evoked potential; OT, occupational therapy; PMC, premotor cortex; PT, physical training; rMT, resting motor threshold; RT, reaction time; rTMS, repetitive transcranial magnetic stimulation; SMA, supplementary motor area; tDCS, transcranial direct current stimulation.
Downregulation of excitability in the contralesional M1
It has been reported that downregulation of cortical excitability in the contralesional M1 with NIBS (i.e., low-frequency rTMS, cTBS, and cathodal tDCS) may contribute to improve recovery of function of the affected hand after stroke. For example, 1-Hz TMS applied over the contralesional M1 after stroke induces a reduction in motor cortical excitability in the unaffected hemisphere (Takeuchi et al., 2005; Fregni et al., 2006), increased cortical excitability in the affected hemisphere (Fregni et al., 2006; Takeuchi et al., 2008), and reduced interhemispheric inhibition (Takeuchi et al., 2005; Avenanti et al., 2012).
Repeated application over several days (Fregni et al., 2006; Boggio et al., 2007; Kirton et al., 2008; Khedr et al., 2009; Emara et al., 2010; Kakuda et al., 2010a, b, 2012; Nair et al., 2011; Sasaki et al., 2011; Avenanti et al., 2012; Conforto et al., 2012), alone or in combination with motor training protocols (Takeuchi et al., 2008, 2009; Emara et al., 2010; Kakuda et al., 2010a, b, 2012; Nair et al., 2011; Avenanti et al., 2012), produces lasting effects on hand motor function. Some of these studies reported improvements maintained for up to 1–2 weeks (Fregni et al., 2006; Boggio et al., 2007; Takeuchi et al., 2008), 1 month (Conforto et al., 2012; Kakuda et al., 2012), and 3 months (Khedr et al., 2009; Emara et al., 2010) after the end of interventions. In one report, however, Talelli and colleagues (2007) did not find beneficial effects of application of cTBS. Direct comparison between these individual trials has been difficult due to substantial differences in stimulation protocols, endpoint measures, and the relationship of stimulation with rehabilitative treatments and environments, as well as patient characteristics.
Although the majority of these studies tested chronic stroke patients (>6 months after stroke), inhibitory 1-Hz rTMS also appears to influence motor function in patients tested less than 6 months after stroke (Liepert et al., 2007; Di Lazzaro et al., 2008; Nowak et al., 2008; Khedr et al., 2009; Grefkes et al., 2010; Conforto et al., 2012). In patients with moderate to severe motor impairment of the affected hand, Conforto and associates (2012) showed improvement of the Jebsen–Taylor test (mean 12.3% 1 month after treatment) and pinch force (mean 0.5 N) in the real NIBS group, but not in the sham group.
In a recent multicenter study (including 5 institutions and 204 patients), each patient received 22 treatment sessions of 20-min low-frequency 1-Hz rTMS and 120-min intensive occupational therapy (OT) daily (Kakuda et al., 2012). The intensive OT, consisting of 60-min one-to-one therapy and 60-min self-exercise, was administered after the application of low-frequency rTMS. Fugl–Meyer Assessment (FMA) and Wolf Motor Function Test (WMFT) were tested serially. The personnel involved in this study received training prior to the study to standardize the therapeutic protocol. All patients completed the protocol without any adverse effects. The FMA score increased and WMFT performance time decreased significantly at discharge, relative to baseline values. These improvements were reported up to 4 weeks following discharge in 79 patients. However, the study did not include a sham control group. It would be useful to compare these effects with those of a control group that received stimulation to another location or sham stimulation. An additional consideration is that the design of the study did not allow dissection of effects induced by each intervention (i.e., rTMS or OT) on motor function of the affected upper limb.
Another interesting technique proposed to modify motor function is the application of “priming” rTMS protocols over the unaffected hemisphere (i.e., priming trains of 6-Hz rTMS immediately before application of the more classic protocol of 1-Hz rTMS) (Carey et al., 2008, 2010; Kakuda et al., 2011). This design of the rTMS application is based on bench work showing that synaptic plasticity can be enhanced by pretreatment with stimulation in the 5–6-Hz range (Christie and Abraham, 1992; Abraham and Bear, 1996). A recent 15-day protocol of 6-Hz primed low-frequency rTMS combined with intensive OT seems safe and a potentially useful strategy to evaluate (Kakuda et al., 2011). Specifically, this protocol increased the FMA score and shortened the performance time of WMFT. However, as in the study described above, a limitation to interpret the results is the absence of a control group (i.e., sham stimulation). The clinical significance of this range of changes in FMA scores is still low.
At the neural level, 1-Hz rTMS-induced inhibition of the contralesional M1 may result in a normalization of interhemispheric functional connectivity patterns as assessed by fMRI (Grefkes et al., 2008a; Nowak et al., 2008). Nowak and colleagues (2008) showed that in subacute stroke patients the application of rTMS over the contralesional M1 improved the kinematics of finger (25%) and grasp (30%) movements in the affected hand and reduced overactivity in the contralesional M1 and nonprimary motor areas. There was no significant correlation between the rTMS-induced reduction in BOLD responses within the contralesional M1 and the degree of behavioral improvement of the affected hand. However, overactivity of the contralesional dorsal PMC, contralesional parietal operculum, and ipsilesional mesial frontal cortex at baseline predicted improvement of movement kinematics of the affected hand after rTMS of the contralesional M1.
A common feature of all correlative approaches to functional connectivity is that they do not provide any direct insight into how correlations are mediated. Therefore, functional integration within a distributed network is usually better described using measures of effective connectivity that refer explicitly to the influence that one neural system exerts over another (Friston, 1994; Grefkes and Fink, 2011; Venkatakrishnan and Sandrini, 2012). Interhemispheric inhibitory interactions after application of 1-Hz rTMS to the contralesional M1 were evaluated using fMRI and a model of effective connectivity (i.e., dynamic causal modeling) (Grefkes et al., 2010). Subacute stroke patients were scanned 1–3 months after stroke while performing whole-hand fist-closure movements. The authors reported that stimulation of contralesional M1 induced a small but significant increase of paretic hand performance compared with both baseline and vertex stimulation in almost every patient. Interestingly, the effective connectivity analysis showed that the behavioral improvements correlated significantly with a reduction of the influence of the stimulated contralesional M1 on the ipsilesional M1 during paretic hand movements. Concurrently, coupling between ipsilesional SMA and M1 was significantly enhanced.
Hence, a focal stimulation by means of TMS does not alter connectivity only of the region stimulated, but also of areas distant to the stimulation site. The authors implied that a more effective integration of ipsilesional M1 into the motor network architecture might constitute a key factor for improving motor performance of stroke patients by means of rTMS (Grefkes et al., 2010). Such a conclusion is in line with resting state fMRI data (Wang et al., 2010) showing that spontaneous recovery over time is associated with increased connectivity of ipsilesional M1 analyzed using graph theoretical approaches (Sporns, 2010).
Upregulation of excitability in the ipsilesional M1
It has been reported that upregulation of cortical excitability in the ipsilesional M1 with NIBS (i.e., high-frequency rTMS, iTBS, and anodal tDCS) may contribute to improve recovery of function of the affected hand after stroke. Repeated application over several days (Khedr et al., 2005, 2009, 2010; Boggio et al., 2007; Pomeroy et al., 2007; Celnik et al., 2009; Yozbatiran et al., 2009; Chang et al., 2010; Emara et al., 2010; Sasaki et al., 2011) alone or in combination with a motor training protocol (Kim et al., 2006; Chang et al., 2010; Emara et al., 2010) produce lasting effects on hand motor function. Some of these studies reported improvements maintained for 1–2 weeks (Khedr et al., 2005; Boggio et al., 2007), 3 months (Chang et al., 2010; Emara et al., 2010), or even at 1 year (Khedr et al., 2010) after the end of interventions.
Somatosensory stimulation, in the form of peripheral nerve stimulation (PNS) applied to the paretic limb, has also been used to enhance motor cortical excitability (Kaelin-Lang et al., 2002; Celnik et al., 2009). PNS consists of bursts of electrical stimuli delivered to the skin overlying peripheral nerves at regular intervals. The mechanisms underlying the effects of PNS on motor function still remain unclear, but may include modulation of cortical excitability that outlasts the period of stimulation as well (Kaelin-Lang et al., 2002). A recent study demonstrated that combined PNS and tDCS can facilitate a beneficial effect of motor training in chronic cortical and subcortical stroke patients (Celnik et al., 2009). The authors demonstrated that the combination of PNS of the paretic hand with anodal tDCS over the ipsilesional M1 before a sequential finger movement training task induced greater improvement compared with the use of each intervention alone (e.g., 41.3% compared with sham conditions). This effect outlasted the stimulation and training periods by days.
In another study, Koganemaru et al. (2010) examined, in chronic subcortical stroke patients, the single intervention effect of repetitive wrist and finger extension exercises aided by PNS, the single intervention of 5-Hz rTMS over the affected M1, and the combined effect of the two interventions. The findings indicate that the combination of motor training, PNS, and rTMS can facilitate use-dependent plasticity (UDP) and improve motor performance to an extent that cannot be attained by either intervention alone. The authors proposed that these functional improvements in the range of movements, grip power and flexor hypertonia (i.e., Ashworth scale) may be brought about by enhanced UDP in the affected M1 area controlling the agonist muscles involved in the exercises. Moreover, performing this combination of interventions over 6 weeks induced a beneficial effect that remained present 2 weeks later.
Although the majority of studies have focused on chronic stroke patients, high-frequency rTMS has been also tested in more acute stages (Khedr et al., 2005, 2010; Pomeroy et al., 2007; Di Lazzaro et al., 2008; Chang et al., 2010; Sasaki et al., 2011). In patients with moderate to severe motor impairment of the affected hand within the first months after stroke, Khedr and colleagues (2005) showed that high-frequency rTMS compared with sham stimulation resulted in improvements in the Scandinavian Stroke Scale, National Institutes of Health (NIH) Stroke Scale, and Barthel Index (Khedr et al., 2005).
Moreover, Ameli and coworkers (2009) compared ipsilesional high-frequency rTMS in patients with subcortical lesions and in patients with both subcortical and cortical damage. After real rTMS, patients with subcortical lesions had improvement in movement kinematics (i.e., index finger and hand tapping movements) compared with vertex stimulation (control condition), whereas patients with additional cortical lesions showed either no changes or in some cases worsening in movement kinematics. rTMS over ipsilesional M1 reduced neural activity of the contralesional M1 in patients with subcortical stroke, but caused a widespread bilateral recruitment of primary and secondary motor areas in patients with additional cortical stroke. Activity in ipsilesional M1 at baseline correlated with improvement of movement kinematics induced by rTMS. These data suggest that neural activity in ipsilesional M1 may serve as a surrogate marker for the effectiveness of rTMS and may allow for an individual selection of patients most suited for rTMS procedures.
Altogether, these studies in stroke patients suggest that NIBS may represent a possible strategy, in combination with customary neurorehabilitative treatments, to facilitate training effects and improve motor function. However, it remains unclear which hemisphere is the optimal target for stimulation, or whether a combination of both contralesional and ipsilesional M1 stimulation sites might be more effective. Recent studies have begun to address this question by comparing different forms of NIBS in the affected and unaffected hemispheres in the same experimental design (Fregni et al., 2005; Boggio et al., 2007; Talelli et al., 2007; Di Lazzaro et al., 2008; Khedr et al., 2009; Takeuchi et al., 2009; Ackerley et al., 2010; Emara et al., 2010; Sasaki et al., 2011; Stagg et al., 2012).
Interestingly, a recent study explored functional changes associated with application of anodal tDCS over the ipsilesional M1 or cathodal tDCS over the contralesional M1 in patients with stable, chronic stroke (Stagg et al., 2012). The authors reported that anodal tDCS applied to ipsilesional M1 improved patients’ response time (mean 5–10%). Both ipsilesional anodal and contralesional cathodal tDCS were associated with some degree of performance improvement, consistent with previous reports (Fregni et al., 2005; Hummel et al., 2005, 2006). Anodal tDCS to ipsilesional M1 was associated with increased task-related activity in the (stimulated) M1, in the PMC and SMA. The magnitude of improvement immediately following tDCS correlated with the stimulation-induced changes in fMRI signal in the stimulated M1. These results are consistent with TMS neurophysiological evidence showing an increased cortical excitability following stimulation of the affected hemisphere (Hummel and Cohen, 2005; Hummel et al., 2005; Di Lazzaro et al., 2008; Khedr et al., 2010).
See Tables 40.2 and 40.3 for details of these studies.
Table 40.2.
Summary of previous studies investigating the effectiveness of facilitatory rTMS or tDCS over primary motor cortex of affected hemisphere on motor behavior
| Technique | Reference | Area of stimulation | No. of patients, mean age, lesion location, and time since stroke | Study design | Control condition | Stimulation parameters | Main results |
|---|---|---|---|---|---|---|---|
| rTMS, high frequency | Khedr et al., 2005 | Hand area of M1, affected hemisphere | 52 adults, 54 years, 26 cortical, 26 subcortical, 6–29 days | Single-blind, randomized, longitudinal, sham- controlled | 52 patients, 26 real TMS, 26 sham TMS (perpendicular to scalp) | Ten 10-s trains of 3-Hz stimulation with 50 s between each train, 120% rMT, 10 consecutive daily sessions | Scandinavian Stroke Scale, NIH Stroke Scale, and Barthel Index scale measured before rTMS, at end of the rTMS session, and 10 days later; real rTMS improved patients’ scores more than sham |
| rTMS, high frequency | Kim et al., 2006 | Hand area of M1, affected hemisphere | 15 adults, 54 years, 5 cortical, 10 subcortical, 4–41 months | Single-blind, crossover, sham- controlled | Real TMS, sham TMS (perpendicular to scalp) | 10 Hz, 80% rMT, 8 trains of 2 s duration (160 pulses), each train followed by sequential finger movement task | rTMS resulted in significantly larger increase in MEP amplitude than sham rTMS; plastic change positively associated with enhanced motor performance accuracy in sequential finger motor task |
| rTMS, high frequency | Malcolm et al., 2007 | Hand area of M1, affected hemisphere | 19 adults, 67 years, 11 cortical, 8 subcortical, average 4±3 years | Double-blind, longitudinal, randomized, sham- controlled | 19 patients, 9 real TMS, 10 sham TMS (sham coil) | 20 Hz (1200 pulses), 90% rMT, 10 consecutive, daily for 5 consecutive days followed by constraint- induced therapy | Regardless of group assignment, participants demonstrated significant gains on primary outcome measures: Wolf Motor Function Test and Motor Activity Log–Amount of Use. Participants receiving real rTMS failed to show differential improvement on either primary outcome measure |
| rTMS, high frequency | Yozbatiran et al., 2009 | Hand area of M1, affected hemisphere | 12 adults, 67 years, 4.7±4.5 years | No blinding, 2-center study design | Real TMS, no sham control | 20 Hz (1600 pulses), 90% rMT | 20-Hz rTMS was safe. Modest improvements seen, for example in grip strength, range of motion, and pegboard performance, up to 1 week after rTMS |
| rTMS, high frequency | Pomeroy et al., 2007 | Hand area of M1, affected hemisphere | 27 adults, 75 years, average 27 days | Single-blind, randomized, longitudinal | Patients divided in 4 groups: (1) real rTMS +real VMC, (2) real rTMS + placebo VMC, (3) placebo rTMS +real VMC, (4) placebo rTMS + placebo VMC (placebo= sham coil) | 1 Hz, 5 trains of 40 pulses, 3 min intertrain interval (total 200 pulses), 120% rMT, treatment: 8 working days | In real rTMS+real VMC group, MEP frequency increased 14% for biceps and 20% for triceps, whereas in placebo rTMS +placebo VMC group, it decreased 12% for biceps and 6% for triceps. For other groups there were changes of intermediate values |
| rTMS, high frequency | Ameli et al., 2009 | Hand area of M1, affected hemisphere | 29 adults, 56 years, 16 subcortical, 13 subcortical and cortical, 1–88 weeks | Single-blind, randomized, controlled | Real TMS, vertex TMS | 5 Hz, 1000 pulses, 80 rMT | 10-Hz rTMS improved movement kinematics in 14 of 16 patients with subcortical stroke, but not in patients with additional cortical stroke. rTMS over ipsilesional M1 reduced neural activity of contralesional M1 in 11 patients with subcortical stroke, but caused a widespread bilateral recruitment of primary and secondary motor areas in 7 patients with cortical stroke. Activity in ipsilesional M1 at baseline correlated with improvement of index finger tapping frequency induced by rTMS |
| rTMS, high frequency | Chang et al., 2010 | Hand area of M1, affected hemisphere | 28 adults, 56.6 years, average 13.4 days (subacute) | Single-blind, randomized, longitudinal, sham- controlled | 28 patients, 18 real TMS, 10 sham TMS | 10 Hz 1000 pulses, 90% rMT, 10 sessions over 2-week period+motor training | Motor function (Motoricity Index improved and Box and Block test) in both groups after treatment; real rTMS additionally improved motor function (Fugl–Meyer Assessment) of affected upper limb. Over 3 months after the stroke, the time and type of intervention for Motoricity Index of affected upper extremity showed significant interaction |
| rTMS, high frequency | Khedr et al., 2010 | Hand area of M1, affected hemisphere | 48 adults, 59.53 years, acute ischemic stroke of middle cerebral artery territory, 6.50± 3.63 days | Single-blind, randomized, longitudinal, sham- controlled | 48 patients, 16 real 3-Hz TMS, 16 real 10-Hz TMS, 16 sham TMS | 3 Hz (5 s, 50 trains), with total 750 pulses at 130% rMT, 5 Hz (2 s, 37 trains), with total 750 pulses at 100% rMT, sham TMS perpendicular to scalp, daily for 5 days in a row | Real rTMS produced greater improvement in motor disability scales than sham, which was evident even at 1 year follow-up. These improvements were associated with changes in cortical excitability over treatment interval |
| rTMS, high frequency | Koganemaru et al., 2010 | Hand area of M1, affected hemisphere | 9 adults, 51.6 years, 9 subcortical, average 24 months | Crossover (exp. 1, 2), longitudinal (exp. 3) | 9 patients (exp. 1), 9 healthy controls (exp. 2), PT+PNS, 5-Hz rTMS, PT+PNS+ 5-Hz rTMS | 5 Hz rTMS for 8 s (100% MT); exp. 3 12 times of rTMS+PNS+PT on the same 9 patients, with 1 session each day, on 2 separate days per week, for 6 weeks in total | Exp. 1: combined intervention (PT+PNS+TMS), but neither single intervention resulted in improvement of extensor movement and grip power along with reduction of flexor hypertonia in paretic upper limbs. Exp. 2: only combined intervention resulted in selective plastic changes of corticospinal excitability, motor threshold, and silent period for extensors. Exp. 3: combination of interventions over 6 weeks induced a beneficial effect that remained present to some extent 2 weeks later |
| tDCS, anodal | Hummel and Cohen, 2005 | Hand area of M1, affected hemisphere | 1 adult, 84-year- old man, subcortical, 107 months | Double-blind, crossover, sham- controlled | Anodal tDCS, sham tDCS | 1 mA, 20 min | tDCS led to improvements in pinch force, Jebsen–Taylor Hand Function Test, and simple RTs in paretic hand that outlasted stimulation period for at least 40 min. These changes were accompanied by increased corticomotor excitability and reduced intracortical inhibition to TMS |
| tDCS, anodal | Hummel et al., 2005 | Hand area of M1, affected hemisphere | 6 adults, 57 years, 1 cortical, 5 subcortical, 23–107 months | Double-blind, crossover, sham- controlled | Anodal tDCS, sham tDCS | 1 mA, 20 min | Jebsen–Taylor Hand Function Test measured in paretic hand improved significantly with anodal but not with sham tDCS. This effect outlasted the stimulation period and was present in every single patient tested. It correlated with an increment in motor cortical excitability within affected hemisphere and reduced short- interval intracortical inhibition |
| tDCS, anodal | Hummel et al., 2006 | Hand area of M1, affected hemisphere | 11 adults, 57 years, 18–107 months | Double-blind, crossover, sham- controlled | Anodal tDCS, sham tDCS | 1 mA, 20 min | Anodal tDCS shortened RTs and improved pinch force in paretic hand relative to sham, an effect present in patients with higher impairment |
| tDCS, anodal | Hesse et al., 2007 | Hand area of M1, affected hemisphere | 10 adults, 63 years, 8 cortical, 2 subcortical, 4–8 weeks | No blinding, longitudinal | Anodal tDCS, no sham or control condition | 1.5 mA, 7 min followed by robot- assisted arm training for 20 min every working day over 6 weeks | Arm function of 3 patients (2 with subcortical lesion) improved significantly. In remaining 7 patients, all with cortical lesions, arm function changed little |
| tDCS anodal | Celnik et al., 2009 | Hand area of M1, affected hemisphere | 9 adults, 55.3 years, 3 cortical and subcortical, 1 subcortical, 5 cortical, average 55.7± 5.57 months | Double-blind, crossover | PNS+tDCS, PNS+tDCS sham, tDCS+PNS sham, PNS sham+tDCS sham | Anodal, 1 mA, 20 min, 2 h of PNS or sham. In last 20 min PNS combined with 20 min of tDCS or sham. Motor training immediately after stimulation | PNS+tDCS resulted in 41.3% improvement in number of correct key presses relative to PNS sham+tDCS sham, 15.4% relative to PNS+tDCS sham, and 22.7% relative to tDCS+PNS sham. These performance differences were maintained 1 and 6 days after end of training |
NIH, National Institutes of Health; M1, primary motor cortex; MEP, motor evoked potential; PNS, peripheral nerve stimulation; PT, physical training; rMT, resting motor threshold; RT, reaction time; rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation; VMC, voluntary muscle contraction.
Table 40.3.
Summary of previous studies investigating the effectiveness of inhibitory and facilitatory rTMS or tDCS over primary motor cortex of unaffected and affected hemisphere on motor behavior
| Technique | Reference | Area of stimulation | No. of patients, mean age, lesion location, and time since stroke | Study design | Control condition | Stimulation parameters | Main results |
|---|---|---|---|---|---|---|---|
| tDCS, anodal, cathodal | Fregni et al., 2005 | Hand area of M1, unaffected hemisphere, affected hemisphere | 6 adults, 53.67 years, 3 cortical, 3 subcortical, 27.08 months | Single-blind, crossover, randomized, sham- controlled | Sham tDCS, anodal tDCS over affected hemisphere, cathodal tDCS over unaffected hemisphere | 1 mA, 20 min | Both anodal and cathodal tDCS improved motor performance in the Jebsen–Taylor Hand Function Test |
| tDCS, anodal, cathodal | Boggio et al., 2007 | Hand area of M1, unaffected hemisphere, affected hemisphere | 9 adults, 57 years, 9 subcortical, 13–85 months | Exp. 1: double- blind. Exp. 2: crossover, longitudinal; randomized, sham- controlled | Sham tDCS, anodal tDCS over affected hemisphere, cathodal tDCS over unaffected hemisphere | Intensity 1 mA, 20 min, administered once (exp. 1), only cathodal daily over 5 consecutive days (exp. 2) | Improvement in Jebsen–Taylor Hand Function Test immediately after tDCS. Greater cumulative improvement after 5 days of tDCS (lasting for 14 days) |
| tDCS, anodal, cathodal | Stagg et al., 2012 | Hand area of M1, unaffected hemisphere, affected hemisphere | 12 adults, 63.47 years, 7 subcortical, 6 cortical, average 33.8 months | Single-blind, randomized, crossover, sham- controlled | Sham tDCS, anodal tDCS over affected hemisphere, cathodal tDCS over unaffected hemisphere | 1 mA, 20 min | Anodal tDCS led to significant improvements in RTs with affected hand, associated with an increase in movement-related cortical activity in stimulated M1 and functionally interconnected regions. Cathodal tDCS led to a functional improvement only when compared with sham stimulation |
| rTMS, cTBS, iTBS | Talelli et al., 2007 | Hand area of M1, unaffected hemisphere, affected hemisphere | 6 adults, 57.7 years, 3 cortical, 3 subcortical, 31 months | Single-blind, crossover, sham- controlled | Real TMS, sham TMS (sham coil) | cTBS (300 pulses, 80% aMT), iTBS (600 pulses, 80% aMT) | cTBS suppressed MEPs evoked in healthy hands but did not change motor behavior or electrophysiology of paretic hands. iTBS transiently improved motor behavior and corticospinal output in paretic hands |
| rTMS, cTBS, iTBS | Di Lazzaro et al., 2008 | Hand area of M1, unaffected hemisphere, affected hemisphere | 12 adults, 69.4 years, 4 cortical, 8 subcortical, 12 healthy, average 5.1 days. Control subjects, mean age 63.2 years | Single-blind, No sham- controlled, but healthy control group, crossover | cTBS over unaffected hemisphere, iTBS over affected hemisphere | cTBS (600 pulses, 80% aMT), iTBS (800 pulses, 80 % aMT) | In patients, both iTBS and cTBS produced a significant increase in amplitude of MEPs evoked by stimulation of affected hemisphere. Effects in patients comparable to those in controls |
| rTMS, low frequency, high frequency | Khedr et al., 2009 | Hand area of M1, unaffected hemisphere, affected hemisphere | 36 adults, 57.9 years, 19 cortical, 17 subcortical, average 17.1 days | Single-blind, randomized, longitudinal, sham- controlled | 36 patients, 12 real 1-Hz TMS over unaffected hemisphere, 12 real 3-Hz TMS over affected hemisphere, 12 sham TMS | 1-Hz TMS, 100% rMT, 900 pulses, 3-Hz TMS, 130% rMT, 900 pulses, daily over 5 days | At 3-month time point, both real rTMS groups had improved significantly more in different rating scales (NIH Stroke Scale and Barthel Index Scale) than sham group; in addition, 1-Hz group performed better than 3-Hz group in NIH Stroke Scale |
| rTMS, cTBS, iTBS | Ackerley et al., 2010 | Hand area of M1, unaffected hemisphere, affected hemisphere | 10 adults, 60 years, 10 subcortical, average 28 months | Double-blind, crossover, sham- controlled | cTBS, over unaffected hemisphere, iTBS over affected hemisphere, sham (sham coil) | cTBS (600 pulses), iTBS (600 pulses), 90% aMT | TBS and training led to task- specific improvements in grip- lift. Specifically, cTBS of contralesional M1 led to an overall decrement in upper-limb function |
| rTMS, low frequency, high frequency | Emara et al., 2010 | Hand area of M1, unaffected hemisphere, affected hemisphere | 60 adults, 53.9 years, average 4.1 months | Single-blind, randomized, longitudinal, sham- controlled | 60 patients, 20 real 1-Hz TMS over unaffected hemisphere, 20 real 5-Hz TMS over affected hemisphere, 20 sham (perpendicular to scalp) | 1 Hz, 110120% rMT, 150 pulses, 5 Hz, 80–90% rMT, 750 pulses, 10 daily sessions+PT | Real rTMS improved finger tapping test, Activity Index score, and modified Rankin Scale in both groups. Effect sustained over 12-week observation period |
| rTMS, low frequency, high frequency | Sasaki et al., 2011 | Hand area of M1, unaffected hemisphere, affected hemisphere | 29 adults, 65.7 years, 6–29 days | Single-blind, randomized, longitudinal, sham- controlled | 29 patients, 11 real 1-Hz TMS over unaffected hemisphere, 9 real 10-Hz TMS over affected hemisphere, 9 sham (perpendicular to scalp) | 1 Hz, 1800 pulses, 10 Hz, 1000 pulses, 5 days, consecutive sessions | Both real rTMS groups had significant increases in both grip strength and tapping frequency. More increase in 10-Hz group vs sham |
| rTMS, low frequency, high frequency, bihemispheric | Takeuchi et al., 2009 | Hand area of M1, unaffected hemisphere, affected hemisphere | 30 adults, 59.3 years, 30 subcortical, average 28.8 months | Double-blind, randomized | 30 patients, 10 real bihemispheric TMS (alternating 1 Hz vs 10 Hz, 1000 pulses for each hemisphere, 90% rMT), 10 real 1-Hz TMS over unaffected hemisphere and sham 10 Hz over unaffected hemisphere, 10 real 10-Hz TMS over affected hemisphere and sham 1-Hz TMS over unaffected hemisphere | 1 Hz vs 10 Hz, 1000 pulses for each hemisphere, 90% rMT, after TMS 15 min motor training | Bilateral and 1-Hz rTMS improved acceleration in paretic hand. Compared with 1 Hz, bihemispheric rTMS decreased inhibitory function of affected motor cortex and enhanced effect of motor training on pinch force. 10-Hz rTMS had no effect on motor function |
aMT, active motor threshold; cTBS, continuous theta-burst stimulation; exp., experiment; iTBS, intermittent theta-burst stimulation; M1. primary motor cortex; MEP, motor evoked potential; NIH, National Institutes of Health; PT, physical training; RT, reaction time; rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation.
Bihemispheric regulation
Recent stimulation techniques proposed to target simultaneously the ipsilesional and contralesional regions (Takeuchi et al., 2009; Lindenberg et al., 2010b, 2012b; Bolognini et al., 2011). Lindenberg and colleagues (2010b) combined bihemispheric tDCS and simultaneous physical/occupational therapy. The authors found an improvement of motor function (WMFT scores) in comparison with the sham group, and increased activation of ipsilesional motor regions during paced movements of the affected limb, as assessed by fMRI. These effects outlasted the NBS application by at least 1 week. Furthermore, the same authors (Lindenberg et al., 2012b) reported that the effects of multiple sessions did not lead to a linear stimulus/response function, a finding of relevance for the design of upcoming trials.
Bolognini et al. (2011) investigated the neurophysiological and behavioral effects of bihemispheric tDCS combined with constraint-induced movement therapy (CIMT). These authors reported behavioral gains in the Jebsen–Taylor Hand Function Test (JHFT), handgrip strength, and FMA score after real but not sham tDCS. TMS neurophysiological measurements showed a reduction in transcallosal inhibition from the intact to the affected hemisphere accompanied by increased ipsilesional corticospinal excitability only in the real tDCS/CIMT group. Neurophysiological changes in this study correlated with the magnitude of the behavioral gains: MEP amplitudes correlated with ipsilesional M1 excitability and with FMA scores. Conversely, contralesional-to-ipsilesional M1 transcallosal inhibition showed an inverse correlation with FMA and JHFT scores. Both real and sham TMS groups showed a reduction in corticospinal excitability of the unaffected hemisphere. See Table 40.4 for details of these studies.
Table 40.4.
Summary of previous studies investigating the effectiveness of bilateral tDCS over primary motor cortex of unaffected and affected hemisphere on motor behavior
| Technique | Reference | Area of stimulation | No. of patients, mean age, lesion location, and time since stroke | Study design | Control condition | Stimulation parameters | Main results |
|---|---|---|---|---|---|---|---|
| tDCS, bihemispheric | Lindenberg et al., 2010b | Hand area of M1s | 20 adults, 58 years, 55.7±5.57 months | Single-blind, randomized, sham- controlled | 20 patients, 10 real bihemispheric (anodal over affected hemisphere and cathodal over unaffected hemisphere), 10 sham bihemispheric | 1.5 mA, 30 min with simultaneous physical OT for 60 min, 5 consecutive sessions | Improvement of motor function significantly greater in real rTMS group (20.7% in Fugl–Meyer and 19.1% in Wolf Motor Function Test scores) compared with sham group (3.2% in Fugl–Meyer and 6.0% in Wolf Motor Function Test scores). Effects lasted 1 week. In real TMS group, stronger activation of intact ipsilesional motor regions during paced movements of affected limb found postintervention vs no changes in control group |
| tDCS, bihemispheric | Lindenberg et al., 2012b | Hand area of M1s | 10 adults, 50.3 years, 20.3±20.6 months | Exp. 1: no controls. Exp 2: double- blind, randomized, sham controlled | First treatment: 10 patients bihemispheric tDCS (anodal over affected hemisphere and cathodal over unaffected hemisphere). Second treatment: 4 of 10 patients and 6 new patients |
1.5 mA, 30 min with simultaneous physical OT for 60 min, 5-day intervention (1st treatment)+a second 5-day intervention separated from first by 2–29 days (mean 9.9±9.4 days) (2nd treatment) | First 5-day period yielded an increase in Upper-Extremity Fugl–Meyer (UE-FM) scores by 5.9±2.4 points (16.6±10.6%). Second 5-day period resulted in further meaningful, although significantly lower, gains with additional improvement of 2.3±1.4 points in UE-FM compared with end of first 5-day period (5.5±4.2%). Overall mean change after the two periods was 8.2±2.2 points (22.9±11.4%) |
| tDCS, bihemispheric | Bolognini et al., 2011 | Hand area of M1s | 14 adults, 46.7 years, average 37 months | Double-blind, randomized | 14 patients, 7 real bihemispheric tDCS (anodal over affected hemisphere and cathodal over unaffected hemisphere) and 7 sham bihemispheric | 2 mA, 40 min, combined with CIMT | Patients in both groups demonstrated gains on Jebsen–Taylor Hand Function Test, Handgrip Strength, Motor Activity Log Scale, and Fugl–Meyer Motor Score. Gains larger in real tDCS group. Neurophysiological measurements showed a reduction in transcallosal inhibition from intact to affected hemisphere, and increased corticospinal excitability in affected hemisphere only in real tDCS/CIMT group. Such neurophysiological changes correlated with the magnitude of behavioral gains. Both groups showed a reduction in corticospinal excitability of unaffected hemisphere |
CIMT, constraint-induced movement therapy; M1, primary motor cortex; OT, occupational therapy; rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation.
CONCLUSION
In summary, there is an increasing body of literature on possible beneficial effects of modulation of cortical excitability in the ipsilesional or contralesional M1s, or a combination of both on motor function. These effects have so far been stronger when NIBS is applied in close relationship with motor training (Takeuchi et al., 2008, 2009; Chang et al., 2010; Koganemaru et al., 2010; Lindenberg et al., 2010b, 2012b; Bolognini et al., 2011; Avenanti et al., 2012). The benefit of coupling NIBS with physical practice may rely on Hebbian principles of synaptic plasticity (Buonomano and Merzenich, 1998). Moreover, data suggest that the effects of facilitation or inhibition of ipsilesional or contralesional M1, respectively, may relate not only to modulation of local changes in cortical excitability, but also to the induction of reorganization in distributed neural networks.
To date, no significant adverse effects have been reported in stroke patients, such as induction of an epileptic seizure. Thus, rTMS and tDCS appear to be safe techniques when used according to current safety guidelines regarding intensity, frequency, and duration of stimulation (Poreisz et al., 2007; Rossi et al., 2009).
Well designed studies (i.e., multicenter, longitudinal, sham-controlled) are needed to determine how effective these techniques are in motor rehabilitation after stroke. It is likely that the outcome of NIBS interventional approaches may be influenced by fluctuations in resting brain activity before or after stimulation, by homeostatic plasticity, as conceptualized by the Binenstock–Cooper–Monroe theory (Bienenstock et al., 1982; Ziemann et al., 2004), and by the specific timing between rehabilitative therapy and cortical stimulation (Avenanti et al., 2012). Additionally, state dependency (i.e., a targeted region’s varying response to stimulation based on its previous state of activity) should be studied in the context of combination of rehabilitation with stimulation (Silvanto et al., 2008).
In the future, it is possible that specific markers will be developed to help identify patients who can benefit the most from NIBS interventions based on individual patterns of cortical activation (Nowak et al., 2008; Ameli et al., 2009), white-matter microstructural integrity (Stinear et al., 2007; Lindenberg et al., 2012a), or presence of certain genetic polymorphisms (Kleim et al., 2006; Cheeran et al., 2009b; Fritsch et al., 2010).
References
- Abraham W, Bear M. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Ackerley SJ, Stinear CM, Barber PA, et al. Combining theta burst stimulation with training after subcortical stroke. Stroke. 2010;41:1568–1572. doi: 10.1161/STROKEAHA.110.583278. [DOI] [PubMed] [Google Scholar]
- Ameli M, Grefkes C, Kemper F, et al. Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol. 2009;66:298–309. doi: 10.1002/ana.21725. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Coccia M, Ladavas E, et al. Low-frequency rTMS promotes use-dependent motor plasticity in chronic stroke: A randomized trial. Neurology. 2012;78:256–264. doi: 10.1212/WNL.0b013e3182436558. [DOI] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N, Cohen LG. Brain–computer interfaces: communication and restoration of movement in paralysis. J Physiol. 2007;579:621–636. doi: 10.1113/jphysiol.2006.125633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Alonso-Alonso M, Mansur CG, et al. Hand function improvement with low-frequency repetitive transcranial magnetic stimulation of the unaffected hemisphere in a severe case of stroke. Am J Phys Med Rehabil. 2006;85:927–930. doi: 10.1097/01.phm.0000242635.88129.38. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Nunes A, Rigonatti SP, et al. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25:123–129. [PubMed] [Google Scholar]
- Bolognini N, Vallar G, Casati C, et al. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in post-stroke patients. Neurorehabil Neural Repair. 2011;25:819–829. doi: 10.1177/1545968311411056. [DOI] [PubMed] [Google Scholar]
- Bolton DA, Cauraugh JH, Hausenblas HA. Electromyogram-triggered neuromuscular stimulation and stroke motor recovery of arm/hand functions: a meta-analysis. J Neurol Sci. 2004;223:121–127. doi: 10.1016/j.jns.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci. 1996;144:160–170. doi: 10.1016/s0022-510x(96)00222-5. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Valls-Solé J, Pascual-Leone A, et al. Rapid modulation of human cortical motor outputs following ischaemic nerve block. Brain. 1993;116:511–525. doi: 10.1093/brain/116.3.511. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Nitsche MA, Bolognini N, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5:175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch E, Weber C, Cohen LG, et al. Think to move: a neuromagnetic brain–computer interface (BCI) system for chronic stroke. Stroke. 2008;39:910–917. doi: 10.1161/STROKEAHA.107.505313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch ER, Modir Shanechi A, Fourkas AD, et al. Parietofrontal integrity determines neural modulation associated with grasping imagery after stroke. Brain. 2012;135:596–614. doi: 10.1093/brain/awr331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Netz J, Wessling M, et al. Remote changes in cortical excitability after stroke. Brain. 2003;126:470–481. doi: 10.1093/brain/awg044. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Wessling M, Netz J, et al. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair. 2008;22:4–21. doi: 10.1177/1545968307301769. [DOI] [PubMed] [Google Scholar]
- Byrnes ML, Thickbroom GW, Phillips BA, et al. Long-term changes in motor cortical organization after recovery from subcortical stroke. Brain Res. 2001;889:278–287. doi: 10.1016/s0006-8993(00)03089-4. [DOI] [PubMed] [Google Scholar]
- Carey JR, Evans CD, Anderson DC, et al. Safety of 6-Hz primed low-frequency rTMS in stroke. Neurorehabil Neural Repair. 2008;22:185–192. doi: 10.1177/1545968307305458. [DOI] [PubMed] [Google Scholar]
- Carey JR, Anderson DC, Gillick BT, et al. 6-Hz primed low-frequency rTMS to contralesional M1 in two cases with middle cerebral artery stroke. Neurosci Lett. 2010;469:338–342. doi: 10.1016/j.neulet.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauraugh JH, Kim SB. Stroke motor recovery: active neuromuscular stimulation and repetitive practice schedules. J Neurol Neurosurg Psychiatry. 2003;74:1562–1566. doi: 10.1136/jnnp.74.11.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celnik P, Webster B, Glasser DM, et al. Effects of action observation on physical training after stroke. Stroke. 2008;39:1814–1820. doi: 10.1161/STROKEAHA.107.508184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celnik P, Paik NJ, Vandermeeren Y, et al. Effects of combined peripheral nerve stimulation and brain polarization on performance of a motor sequence task after chronic stroke. Stroke. 2009;40:1764–1771. doi: 10.1161/STROKEAHA.108.540500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WH, Kim YH, Bang OY, et al. Long-term effects of rTMS on motor recovery in patients after subacute stroke. J Rehabil Med. 2010;42:758–764. doi: 10.2340/16501977-0590. [DOI] [PubMed] [Google Scholar]
- Cheeran B, Cohen L, Dobkin B, et al. The future of restorative neurosciences in stroke: driving the translational research pipeline from basic science to rehabilitation of people after stroke. Neurorehabil Neural Repair. 2009a;23:97–107. doi: 10.1177/1545968308326636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran BJ, Ritter C, Rothwell JC, et al. Mapping genetic influences on the corticospinal motor system in humans. Neuroscience. 2009b;164:156–163. doi: 10.1016/j.neuroscience.2009.01.054. [DOI] [PubMed] [Google Scholar]
- Chollet F, DiPiero V, Wise RJ, et al. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol. 1991;29:63–71. doi: 10.1002/ana.410290112. [DOI] [PubMed] [Google Scholar]
- Christie BR, Abraham WC. Priming of associative long-term depression in the dentate gyrus by theta frequency synaptic activity. Neuron. 1992;9:79–84. doi: 10.1016/0896-6273(92)90222-y. [DOI] [PubMed] [Google Scholar]
- Chrysikou EG, Hamilton RH. Noninvasive brain stimulation in the treatment of aphasia: exploring interhemispheric relationships and their implications for neurorehabilitation. Restor Neurol Neurosci. 2011;29:375–394. doi: 10.3233/RNN-2011-0610. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Traversa R, Rossini PM. Post-stroke reorganization of brain motor output to the hand: a 2–4 month follow-up with focal magnetic transcranial stimulation. Electroencephalogr Clin Neurophysiol. 1997;105:438–450. doi: 10.1016/s0924-980x(97)00052-0. [DOI] [PubMed] [Google Scholar]
- Conforto AB, Cohen LG, dos Santos RL, et al. Effects of somatosensory stimulation on motor function in chronic cortico-subcortical strokes. J Neurol. 2007;254:333–339. doi: 10.1007/s00415-006-0364-z. [DOI] [PubMed] [Google Scholar]
- Conforto AB, Ferreiro KN, Tomasi C, et al. Effects of somatosensory stimulation on motor function after subacute stroke. Neurorehabil Neural Repair. 2010;24:263–272. doi: 10.1177/1545968309349946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforto AB, Anjos SM, Saposnik G, et al. Transcranial magnetic stimulation in mild to severe hemi-paresis early after stroke: a proof of principle and novel approach to improve motor function. J Neurol. 2012;259:1399–1405. doi: 10.1007/s00415-011-6364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008a;63:272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- Cramer SC. Repairing the human brain after stroke. II. Restorative therapies. Ann Neurol. 2008b;63:549–560. doi: 10.1002/ana.21412. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Moore CI, Finklestein SP, et al. A pilot study of somatotopic mapping after cortical infarct. Stroke. 2000;31:668–671. doi: 10.1161/01.str.31.3.668. [DOI] [PubMed] [Google Scholar]
- Dafotakis M, Grefkes C, Wang L, et al. The effects of 1 Hz rTMS over the hand area of M1 on movement kinematics of the ipsilateral hand. J Neural Transm. 2008;115:1269–1274. doi: 10.1007/s00702-008-0064-1. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, et al. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch JE, Merians AS, Adamovich S, et al. Development and application of virtual reality technology to improve hand use and gait of individuals post-stroke. Restor Neurol Neurosci. 2004;22:371–386. [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, et al. Modulating cortical excitability in acute stroke: a repetitive TMS study. Clin Neurophysiol. 2008;119:715–723. doi: 10.1016/j.clinph.2007.11.049. [DOI] [PubMed] [Google Scholar]
- Dimyan MA, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of functional recovery mechanisms after stroke. Neurorehabil Neural Repair. 2010;24:125–135. doi: 10.1177/1545968309345270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol. 2011;7:76–85. doi: 10.1038/nrneurol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP, Suner S, Sanes JN. Dynamic organization of primary motor cortex output to target muscles in adult rats. II. Rapid reorganization following motor nerve lesions. Exp Brain Res. 1990;79:492–503. doi: 10.1007/BF00229319. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Hess G, Sanes JN. Substrates and mechanisms for learning in motor cortex. In: Bloedel J, Ebner T, Wise SP, editors. Acquisition of Motor Behavior in Vertebrates. MIT Press; Cambridge, MA: 1996. pp. 363–386. [Google Scholar]
- Duque J, Hummel F, Celnik P, et al. Transcallosal inhibition in chronic subcortical stroke. Neuroimage. 2005;28:940–946. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Emara TH, Moustafa RR, Elnahas NM, et al. Repetitive transcranial magnetic stimulation at 1Hz and 5Hz produces sustained improvement in motor function and disability after ischaemic stroke. Eur J Neurol. 2010;17:1203–1209. doi: 10.1111/j.1468-1331.2010.03000.x. [DOI] [PubMed] [Google Scholar]
- Ertelt D, Small S, Solodkin A, et al. Action observation has a positive impact on rehabilitation of motor deficits after stroke. Neuroimage. 2007;36:T164–T173. doi: 10.1016/j.neuroimage.2007.03.043. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, et al. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feydy A, Carlier R, Roby-Brami A, et al. Longitudinal study of motor recovery after stroke: recruitment and focusing of brain activation. Stroke. 2002;33:1610–1617. doi: 10.1161/01.str.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis Z. A comprehensive review of the effects of rTMS on motor cortex excitability and inhibition. Clin Neurophysiol. 2006;117:2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio P, Mansur C, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16:1551–1555. doi: 10.1097/01.wnr.0000177010.44602.5e. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Valle AC, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37:2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- Frey SH, Fogassi L, Grafton S, et al. Neurological principles and rehabilitation of action disorders: computation, anatomy, and physiology (CAP) model. Neurorehabil Neural Repair. 2011;25:6S–20S. doi: 10.1177/1545968311410940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, et al. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- Fries W, Danek A, Witt TN. Motor responses after transcranial electrical stimulation of cerebral hemispheres with a degenerated pyramidal tract. Ann Neurol. 1991;29:646–650. doi: 10.1002/ana.410290612. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp. 1994;2:56–78. [Google Scholar]
- Fritsch B, Reis J, Martinowich K, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan M, Marchal G, Viader F, et al. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol. 1996;40:216–226. doi: 10.1002/ana.410400213. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Bushara K, Sailer A, et al. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129:791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches. Brain. 2011;134:1264–1276. doi: 10.1093/brain/awr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Eickhoff SB, Nowak DA, et al. Dynamic intra- and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. Neuroimage. 2008a;41:1382–1394. doi: 10.1016/j.neuroimage.2008.03.048. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, Eickhoff SB, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008b;63:236–246. doi: 10.1002/ana.21228. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, Wang LE, et al. Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling. Neuroimage. 2010;50:233–242. doi: 10.1016/j.neuroimage.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147–150. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- Hesse S, Werner C, Schonhardt EM, et al. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: a pilot study. Restor Neurol Neurosci. 2007;25:9–15. [PubMed] [Google Scholar]
- Hortobagyi T, Taylor JL, Petersen NT, et al. Changes in segmental and motor cortical output with contralateral muscle contractions and altered sensory inputs in humans. J Neurophysiol. 2003;90:2451–2459. doi: 10.1152/jn.01001.2002. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, et al. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Hummel F, Cohen L. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabil Neural Repair. 2005;19:14–19. doi: 10.1177/1545968304272698. [DOI] [PubMed] [Google Scholar]
- Hummel F, Cohen L. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5:708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Voller B, Celnik P, et al. Effects of brain polarization on reaction times and pinch force in chronic stroke. BMC Neurosci. 2006;7:73. doi: 10.1186/1471-2202-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel FC, Steven B, Hoppe J, et al. Deficient intra-cortical inhibition (SICI) during movement preparation after chronic stroke. Neurology. 2009;72:1766–1772. doi: 10.1212/WNL.0b013e3181a609c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillard A, Martin CD, Garambois K, et al. Vicarious function within the human primary motor cortex? A longitudinal fMRI stroke study. Brain. 2005;128:1122–1138. doi: 10.1093/brain/awh456. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, et al. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin-Lang A, Luft AR, Sawaki L, et al. Modulation of human corticomotor excitability by somatosensory input. J Physiol. 2002;540:623–633. doi: 10.1113/jphysiol.2001.012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuda W, Abo M, Kaito N, et al. Six-day course of repetitive transcranial magnetic stimulation plus occupational therapy for post-stroke patients with upper limb hemi-paresis: a case series study. Disabil Rehabil. 2010a;32:801–807. doi: 10.3109/09638280903295474. [DOI] [PubMed] [Google Scholar]
- Kakuda W, Abo M, Kobayashi K, et al. Low-frequency repetitive transcranial magnetic stimulation and intensive occupational therapy for poststroke patients with upper limb hemiparesis: preliminary study of a 15-day protocol. Int J Rehabil Res. 2010b;33:339–345. doi: 10.1097/MRR.0b013e32833cdf10. [DOI] [PubMed] [Google Scholar]
- Kakuda W, Abo M, Kobayashi K, et al. Application of combined 6-Hz primed low-frequency rTMS and intensive occupational therapy for upper limb hemiparesis after stroke. Neurorehabilitation. 2011;29:365–371. doi: 10.3233/NRE-2011-0714. [DOI] [PubMed] [Google Scholar]
- Kakuda W, Abo M, Shimizu M, et al. A multi-center study on low-frequency rTMS combined with intensive occupational therapy for upper limb hemiparesis in post-stroke patients. The NEURO Investigators. J Neuroeng Rehabil. 2012;9:4. doi: 10.1186/1743-0003-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Fathy N, et al. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65:466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Abdel-Fadeil MR, Farghali A, et al. Role of 1 and 3 Hz repetitive transcranial magnetic stimulation on motor function recovery after acute ischaemic stroke. Eur J Neurol. 2009;16:1323–1330. doi: 10.1111/j.1468-1331.2009.02746.x. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Etraby AE, Hemeda M, et al. Long-term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurol Scand. 2010;121:30–37. doi: 10.1111/j.1600-0404.2009.01195.x. [DOI] [PubMed] [Google Scholar]
- Kim YH, Park JW, Ko MH, et al. Facilitative effect of high frequency subthreshold repetitive transcranial magnetic stimulation on complex sequential motor learning in humans. Neurosci Lett. 2004;367:181–185. doi: 10.1016/j.neulet.2004.05.113. [DOI] [PubMed] [Google Scholar]
- Kim YH, You SH, Ko MH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37:1471–1476. doi: 10.1161/01.STR.0000221233.55497.51. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. Mechanisms of hemispheric interaction in man. In: Kinsbourne M, Smith WL, editors. Hemispheric Disconnection and Cerebral Function. Charles C. Thomas; Springfield: 1974. pp. 260–285. [Google Scholar]
- Kinsbourne M. Dichotic imbalance due to isolated hemisphere occlusion or directional rivalry? Brain Lang. 1980;11:221–224. doi: 10.1016/0093-934x(80)90123-6. [DOI] [PubMed] [Google Scholar]
- Kirton A, Chen R, Friefeld S, et al. Contralesional repetitive transcranial magnetic stimulation for chronic hemiparesis in subcortical paediatric stroke: a randomised trial. Lancet Neurol. 2008;7:507–513. doi: 10.1016/S1474-4422(08)70096-6. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9:735–737. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hutchinson S, Theoret H, et al. Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology. 2004;62:91–98. doi: 10.1212/wnl.62.1.91. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Theoret H, Pascual-Leone A. Suppression of ipsilateral motor cortex facilitates motor skill learning. Eur J Neurosci. 2009;29:833–836. doi: 10.1111/j.1460-9568.2009.06628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koganemaru S, Mima T, Thabit MN, et al. Recovery of upper-limb function due to enhanced use-dependent plasticity in chronic stroke patients. Brain. 2010;133:3373–3384. doi: 10.1093/brain/awq193. [DOI] [PubMed] [Google Scholar]
- Kolominsky-Rabas P, Weber M, Gefeller O, et al. Epidemiology of ischaemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32:2735–2740. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S, Studenski S, Duncan P, et al. Persisting consequences of stroke measures by the Stroke Impact Scale. Stroke. 2002;33:1840–1844. doi: 10.1161/01.str.0000019289.15440.f2. [DOI] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Differential impairment of individuated finger movements in humans after damage to the motor cortex or the corticospinal tract. J Neurophysiol. 2003;90:1160–1170. doi: 10.1152/jn.00130.2003. [DOI] [PubMed] [Google Scholar]
- Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil Neural Repair. 2009;23:313–319. doi: 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]
- Liepert J, Hamzei F, Weiller C. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve. 2000a;23:1761–1763. doi: 10.1002/1097-4598(200011)23:11<1761::aid-mus14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Liepert J, Storch P, Fritsch A, et al. Motor cortex disinhibition in acute stroke. Clin Neurophysiol. 2000b;111:671–676. doi: 10.1016/s1388-2457(99)00312-0. [DOI] [PubMed] [Google Scholar]
- Liepert J, Zittel S, Weiller C. Improvement of dexterity by single session low-frequency repetitive transcranial magnetic stimulation over contralesional motor cortex in acute stroke: a double-blind placebo-controlled crossover trial. Restor Neurol Neurosci. 2007;25:461–465. [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, et al. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010a;74:280–287. doi: 10.1212/WNL.0b013e3181ccc6d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu L, et al. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010b;75:2176–2184. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Zhu LL, Rüber T, et al. Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum Brain Mapp. 2012a;33:1040–1051. doi: 10.1002/hbm.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Zhu LL, Schlaug G. Combined central and peripheral stimulation to facilitate motor recovery after stroke: the effect of number of sessions on outcome. Neurorehabil Neural Repair. 2012b;26:479–483. doi: 10.1177/1545968311427568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AC, Guarino P, Krebs HI, et al. Multicenter randomized trial of robot-assisted rehabilitation for chronic stroke: methods and entry characteristics for VA ROBOTICS. Neurorehabil Neural Repair. 2009;23:775–783. doi: 10.1177/1545968309338195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Markert J, Sauseng P, et al. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci. 2006;26:6096–6102. doi: 10.1523/JNEUROSCI.4564-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loubinoux I, Carel C, Pariente J, et al. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. Neuroimage. 2003;20:2166–2180. doi: 10.1016/j.neuroimage.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Luft AR, McCombe-Waller S, Whitall J, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA. 2004;292:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm MP, Triggs WJ, Light KE, et al. Repetitive transcranial magnetic stimulation as an adjunct to constraint-induced therapy: an exploratory randomized controlled trial. Am J Phys Med Rehabil. 2007;86:707–715. doi: 10.1097/PHM.0b013e31813e0de0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur CG, Fregni F, Boggio PS, et al. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology. 2005;64:1802–1804. doi: 10.1212/01.WNL.0000161839.38079.92. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Nelson RJ, Stryker MP, et al. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224:591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Facchini S, Boroojerdi B, et al. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol. 2000;111:344–349. doi: 10.1016/s1388-2457(99)00243-6. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, et al. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nair DG, Hutchinson S, Fregni F, et al. Imaging correlates of motor recovery from cerebral infarction and their physiological significance in well-recovered patients. Neuroimage. 2007;34:253–263. doi: 10.1016/j.neuroimage.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair DG, Renga V, Lindenberg R, et al. Optimizing recovery potential through simultaneous occupational therapy and non-invasive brain-stimulation using tDCS. Restor Neurol Neurosci. 2011;29:411–420. doi: 10.3233/RNN-2011-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netz J, Lammers T, Hömberg V. Reorganization of motor output in the non-affected hemisphere after stroke. Brain. 1997;120:1579–1786. doi: 10.1093/brain/120.9.1579. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann, et al. Transcranial direct current stimulation: state of the art. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Dafotakis M, et al. Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Arch Neurol. 2008;65:741–747. doi: 10.1001/archneur.65.6.741. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Bösl K, Podubeckà J, et al. Noninvasive brain stimulation and motor recovery after stroke. Restor Neurol Neurosci. 2010;28:531–544. doi: 10.3233/RNN-2010-0552. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes F, et al. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- Oliveri M. Brain stimulation procedures for treatment of contralesional spatial neglect. Restor Neurol Neurosci. 2011;29:421–425. doi: 10.3233/RNN-2011-0613. [DOI] [PubMed] [Google Scholar]
- Page SJ, Levine P, Leonard A. Mental practice in chronic stroke: results of a randomized, placebo-controlled trial. Stroke. 2007;38:1293–1297. doi: 10.1161/01.STR.0000260205.67348.2b. [DOI] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci. 2008;28:5631–5640. doi: 10.1523/JNEUROSCI.0093-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy VM, Cloud G, Tallis RC, et al. Transcranial magnetic stimulation and muscle contraction to enhance stroke recovery: a randomized proof-of-principle and feasibility investigation. Neurorehabil Neural Repair. 2007;21:509–517. doi: 10.1177/1545968307300418. [DOI] [PubMed] [Google Scholar]
- Pomeroy V, Aglioti SM, Mark VW, et al. Neurological principles and rehabilitation of action disorders: rehabilitation interventions. Neurorehabil Neural Repair. 2011;25:33S–43S. doi: 10.1177/1545968311410942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poreisz C, Boros K, Antal A, et al. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007;72:208–214. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Rehme AK, Fink GR, von Cramon DY, et al. The role of the contralesional motor cortex for motor recovery in the early days after stroke assessed with longitudinal fMRI. Cereb Cortex. 2011;21:756–768. doi: 10.1093/cercor/bhq140. [DOI] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, et al. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 2008;586:325–351. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner CIE, Woldag H, Atanasova R, et al. Change of facilitation during voluntary bilateral hand activation after stroke. J Neurol Sci. 2005;239:25–30. doi: 10.1016/j.jns.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics – 2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, et al. Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Calautti C, Pauri F, et al. Post-stroke plastic reorganisation in the adult brain. Lancet Neurol. 2003;2:493–502. doi: 10.1016/s1474-4422(03)00485-x. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Altamura C, Ferreri F, et al. Neuroimaging experimental studies on brain plasticity in recovery from stroke. Eura Medicophys. 2007;43:241–254. [PubMed] [Google Scholar]
- Sandrini M, Umiltà C, Rusconi E. The use of transcranial magnetic stimulation in cognitive neuroscience: a new synthesis of methodological issues. Neurosci Biobehav Rev. 2011;35:516–536. doi: 10.1016/j.neubiorev.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Suner S, Donoghue JP. Dynamic organization of primary motor cortex output to target muscles in adult rats. I. Long-term patterns of reorganization following motor or mixed peripheral nerve lesions. Exp Brain Res. 1990;79:479–491. doi: 10.1007/BF00229318. [DOI] [PubMed] [Google Scholar]
- Sasaki N, Mizutani S, Kakuda W, et al. Comparison of the effects of high- and low-frequency repetitive transcranial magnetic stimulation on upper limb hemiparesis in the early phase of stroke. J Stroke Cerebrovasc Dis. 2011 doi: 10.1016/j.jstrokecerebrovasdis.2011.10.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Sathian K, Buxbaum LJ, Cohen LG, et al. Neurological principles and rehabilitation of action disorders: common clinical deficits. Neurorehabil Neural Repair. 2011;25:21S–32S. doi: 10.1177/1545968311410941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaechter JD, Fricker ZP, Perdue KL, et al. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp. 2009;30:3461–3474. doi: 10.1002/hbm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambra HM, Sawaki L, Cohen LG. Modulation of excitability of human motor cortex (M1) by 1 Hz transcranial magnetic stimulation of the contralateral M1. Clin Neurophysiol. 2003;114:130–133. doi: 10.1016/s1388-2457(02)00342-5. [DOI] [PubMed] [Google Scholar]
- Sharma N, Pomeroy VM, Baron JC. Motor imagery: a backdoor to the motor system after stroke? Stroke. 2006;37:1941–1952. doi: 10.1161/01.STR.0000226902.43357.fc. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton N, Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn Sci. 2008;12:447–454. doi: 10.1016/j.tics.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Sporns O. Networks of the Brain. MIT Press; Cambridge, MA: 2010. [Google Scholar]
- Stagg CJ, Bachtiar V, O’Shea J, et al. Cortical activation changes underlying stimulation-induced behavioral gains in chronic stroke. Brain. 2012;135:276–284. doi: 10.1093/brain/awr313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, et al. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Swayne OB, Rothwell JC, Ward NS, et al. Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cereb Cortex. 2008;18:1909–1922. doi: 10.1093/cercor/bhm218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi N, Chuma T, Matsuo Y, et al. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36:2681–2686. doi: 10.1161/01.STR.0000189658.51972.34. [DOI] [PubMed] [Google Scholar]
- Takeuchi N, Tada T, Toshima M, et al. Inhibition of the unaffected motor cortex by 1 Hz repetitive transcranial magnetic stimulation enhances motor performance and training effect of the paretic hand in patients with chronic stroke. J Rehabil Med. 2008;40:298–303. doi: 10.2340/16501977-0181. [DOI] [PubMed] [Google Scholar]
- Takeuchi N, Tada T, Toshima M, et al. Repetitive transcranial magnetic stimulation over bilateral hemispheres enhances motor function and training effect of paretic hand in patients after stroke. J Rehabil Med. 2009;41:1049–1054. doi: 10.2340/16501977-0454. [DOI] [PubMed] [Google Scholar]
- Talelli P, Greenwood RJ, Rothwell JC. Arm function after stroke: neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin Neurophysiol. 2006;117:1641–1659. doi: 10.1016/j.clinph.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Talelli P, Greenwood RJ, Rothwell JC. Exploring theta burst stimulation as an intervention to improve motor recovery in chronic stroke. Clin Neurophysiol. 2007;118:333–342. doi: 10.1016/j.clinph.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Sandrini M, Cohen LG. Modulation of motor learning and memory formation by non-invasive cortical stimulation of the primary motor cortex. Neuropsychol Rehabil. 2011;21:650–675. doi: 10.1080/09602011.2011.605589. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res. 2007;180:583–593. doi: 10.1007/s00221-007-0991-3. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Zanette G. Modulation of ipsilateral motor cortex in man during unimanual finger movements of different complexities. Neurosci Lett. 1998;244:121–124. doi: 10.1016/s0304-3940(98)00150-5. [DOI] [PubMed] [Google Scholar]
- Tombari D, Loubinoux I, Pariente J, et al. A longitudinal fMRI study: in recovering and then in clinically stable sub-cortical stroke patients. Neuroimage. 2004;23:827–839. doi: 10.1016/j.neuroimage.2004.07.058. [DOI] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Bassi A, et al. Mapping of motor cortical reorganization after stroke: a brain stimulation study with focal magnetic pulses. Stroke. 1997;28:110–117. doi: 10.1161/01.str.28.1.110. [DOI] [PubMed] [Google Scholar]
- Turton A, Wroe S, Trepte N, et al. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101:316–328. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan A, Sandrini M. Combining transcranial direct current stimulation and neuroimaging: novel insights in understanding neuroplasticity. J Neurophysiol. 2012;107:1–4. doi: 10.1152/jn.00557.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- Wang L, Yu C, Chen H, et al. Dynamic functional reorganization of the motor execution network after stroke. Brain. 2010;133:1224–1238. doi: 10.1093/brain/awq043. [DOI] [PubMed] [Google Scholar]
- Ward N. Assessment of cortical reorganisation for hand function after stroke. J Physiol. 2011;589:5625–5632. doi: 10.1113/jphysiol.2011.220939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, et al. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, et al. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129:809–819. doi: 10.1093/brain/awl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann E, Epstein C, Ziemann U, et al. The Oxford Handbook of Transcranial Stimulation. Oxford University Press; Oxford: 2008. [Google Scholar]
- Webster BR, Celnik PA, Cohen LG. Noninvasive brain stimulation in stroke rehabilitation. NeuroRx. 2006;3:474–481. doi: 10.1016/j.nurx.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C, Chollet F, Friston KJ, et al. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol. 1992;31:463–472. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- Williams JA, Pascual-Leone A, Fregni F. Interhemispheric modulation induced by cortical stimulation and motor training. Phys Ther. 2010;90:398–410. doi: 10.2522/ptj.20090075. [DOI] [PubMed] [Google Scholar]
- Wittenberg GF, Chen R, Ishii K, et al. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003;17:48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- Woldag H, Lukhaup S, Renner C, et al. Enhanced motor cortex excitability during ipsilateral voluntary hand activation in healthy subjects and stroke patients. Stroke. 2004;35:2556–2559. doi: 10.1161/01.STR.0000144651.07122.da. [DOI] [PubMed] [Google Scholar]
- Yozbatiran N, Alonso-Alonso M, See J, et al. Safety and behavioral effects of high-frequency repetitive transcranial magnetic stimulation in stroke. Stroke. 2009;40:309–312. doi: 10.1161/STROKEAHA.108.522144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LL, Lindenberg R, Alexander MP, et al. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41:910–915. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Ilic TV, Pauli C, et al. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci. 2004;24:1666–1672. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Nitsche MA, et al. Consensus: Motor cortex plasticity protocols. Brain Stimul. 2008;1:164–182. doi: 10.1016/j.brs.2008.06.006. [DOI] [PubMed] [Google Scholar]