Abstract
Event-related desynchronization (ERD) of sensori-motor rhythms (SMR) can be used for online brain–machine interface (BMI) control, but yields challenges related to the stability of ERD and feedback strategy to optimize BMI learning. Here, we compared two approaches to this challenge in 20 right-handed healthy subjects (HS, five sessions each, S1–S5) and four stroke patients (SP, 15 sessions each, S1–S15). ERD was recorded from a 275-sensor MEG system. During daily training, motor imagery-induced ERD led to visual and proprioceptive feedback delivered through an orthotic device attached to the subjects’ hand and fingers. Group A trained with a heterogeneous reference value (RV) for ERD detection with binary feedback and Group B with a homogenous RV and graded feedback (10 HS and 2 SP in each group). HS in Group B showed better BMI performance than Group A (p < 0.001) and improved BMI control from S1 to S5 (p = 0.012) while Group A did not. In spite of the small n, SP in Group B showed a trend for a higher BMI performance (p = 0.06) and learning was significantly better (p < 0.05). Using a homogeneous RV and graded feedback led to improved modulation of ipsilesional activity resulting in superior BMI learning relative to use of a heterogeneous RV and binary feedback.
Index Terms: Brain–machine interface, event-related desynchronization, neurorehabilitation, stroke
I. Introduction
Based on the finding that power amplitudes of the sensori-motor rhythm (SMR) can be voluntarily modulated [1], various brain-computer and brain-machine interface (BMI) applications were developed that translate event-related desynchronization (ERD) or synchronization (ERS) into signals that control external devices [2], [3]. ERD/ERS offers quantification of stimulus-locked brain activity e.g., during motor imagery, compared to reference conditions (RC). Easiness of use made SMR-ERD, which reflects processing within the sensorimotor cortex [4], an ideal candidate to drive online BMI systems in the context of neurorehabilitation [5].
In contrast to BMI approaches that aim to replace lost function e.g., by allowing for continuous high-dimensional control of robotic devices that move completely paralyzed hands or legs, restorative (biofeedback) BMI systems aim at inducing (BMI-) use-dependent neuroplasticity that might facilitate motor recovery [6]–[8]. As in both approaches contingent feedback or reward plays a key role for acquiring control of neural activity, novel techniques that were recently successfully incorporated in BMI systems delivering feedback through direct stimulation of peripheral nerves [9], [10], dorsal root ganglia (DRG) [11] or cortical and sub-cortical brain regions [12]–[16] offer promising new perspectives for patient populations with compromised afferent pathways.
The development of restorative (biofeedback) BMI is closely related to the success of neurofeedback [17] and to data indicating that stroke patients with best motor recovery are the ones in whom ipsilesional cortical function is closer to that found in healthy controls [18], [19]. A negative correlation between impairment and activation in ipsilesional M1 during hand motions has been documented [20]–[23]. Thus, in a restorative BMI, adaptive plasticity of ipsilesional brain activity could be acquired through contingent reward of better SMR-ERD control associated with motor movement or imagery of the affected limb.
For implementation of such an approach, however, an optimal trade-off between BMI adaptability to the dynamic states of the brain and rewarding feedback for consistent reproduction of desired neurophysiologic activity that might facilitate motor recovery needs to be met. The nonstationary nature of ERD provides an objective rationale to implement adaptive methods [24]–[26], but it is not conclusively clear how to adapt and at what rate [27]. Various methods were described in the literature to address nonstationarity [28], [29] including application of adaptation techniques carried out at different BMI modules, e.g., spatial filtering of the signal [30], feature extraction [31] or at the classifier. Operationally, the purpose of a restorative BMI (induction of use-dependent neuroplasticity) would be to augment a desired neurophysiologic feature through systematic reward [6], [7], [32]. Our study addresses this issue by using different adaptation settings carried out at the level of the BMI normalizer coupled with afferent proprioceptive feedback.
One method for online classification of SMR-based ERD is based on the expression of power estimates in sample blocks recorded during a task condition (TC) relative to a reference value (RV). The RV is continuously updated by the power estimates of the RC and can be based on either 1) signal power estimates of a rest condition and TC preceding the actual sample block (heterogeneous RV) or on 2) the signal power estimates of the rest condition only (homogeneous RV). Both approaches comprise a certain degree of adaptability to account for potential changes of power values during the rest condition, e.g., due to fatigue [33], [34]. This is achieved by continuously updating the RV during the training based on the ITI’s (rest conditions) that immediately preceded the actual trial (Fig. 1). Moreover, the heterogeneous RV is additionally updated by the power values recorded during the previous task trials. This approach offers the advantage that the ERD detection threshold is reduced if the subject fails to persistently produce ERD of certain strength so that reliable BMI control is sustained and early frustration to control the BMI avoided. Furthermore, improvements in ERD production will make the task more challenging in the following trials giving the subject an incentive to improve further.
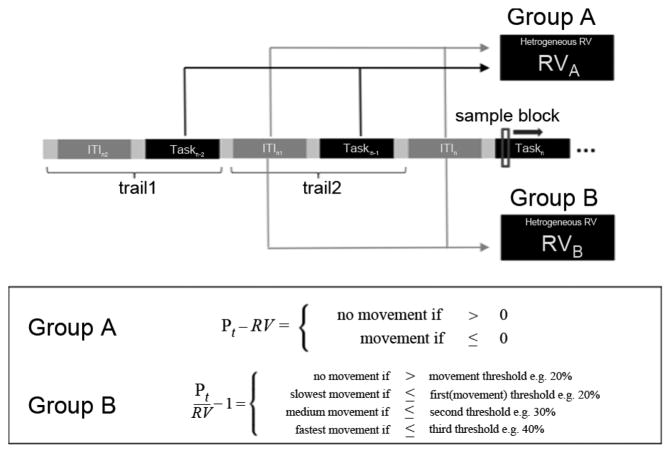
Fig. 1.
Upper: Design of the BMI training and computation of RV in Group A (N = 12) and B (N = 12). In Group A, RV for every incoming sample block of Taskn is computed as a mean based on power estimates of the preceding two rest condition intervals (ITIn−1 and ITIn−2) and task intervals (Taskn−1 and Taskn−2) (heterogeneous RV). In Group B, only power estimates of ITIn−1 and ITIn−2 are used to compute the RV for the sample blocks of Taskn (homogeneous RV). Lower: BMI settings for ERD detection in Group A and B are given in the box. In Group A, ERD is detected if the power value of the incoming sample block recorded during BMI training is smaller than RV (binary). In Group B, an ERD is detected if the incoming sample block value is smaller than a fixed value (e.g., 20% of homogeneous RV). Stronger ERD are translated to faster velocities of orthosis movements (graded feedback).
In contrast, a homogeneous RV requires setting a fixed ERD detection threshold relative to the RV. Accordingly, the subject has to reliably reach a specific ERD strength relative to the RV that is influenced by the signal power estimates of rest only to gain BMI control.
Another aspect in designing a BMI is the translation of the BMI signal into adequate feedback. Based on the assumption that rapid and accurate feedback is beneficial for learning [35], graded feedback to reward stronger ERD might increase the probability for reliable ERD detection in the course of the training. Thus, here we hypothesized that training on a homogeneous RV and graded feedback that reflects ERD strength will lead to better BMI learning compared to a BMI that is based on a heterogeneous RV and binary feedback. Presently, it is unknown which approach leads to better BMI learning and performance and, thus, induction of adaptive plasticity of ipsilesional brain activity in patients with extensive brain lesions.
II. Methods
A. Subjects
Twenty right-handed SMR-BMI-naïve healthy volunteers (10 male, 10 female, mean age: 28.3 years ±5.6) and four stroke patients (see Table I) were invited for five BMI training sessions (S1–S5, healthy subjects) on consecutive days. We also included four stroke patients (see Table I) who received 15 sessions (S1–S15) of daily BMI training. The handedness of each subject was evaluated by the Edinburgh Handedness Inventory [36]. All stroke patients suffered from subcortical lesions (for demographics, lesion location and extent see Table I) and were unable to extend their severely affected fingers. Participants were randomly assigned to one of two groups. Before entering the study, written informed consent to the study was given. The study protocol was approved by the National Institute of Neurologic Disorders and Stroke Institutional Review Board.
TABLE I.
Demographic Data of Participating Stroke Patients
| Patient | Age | Months after stroke | Lesion location and extent | Affected hand |
|---|---|---|---|---|
| 1 | 45 | 53 | Subcortical stroke involving the basal ganglia (putamen) and centrum semiovale subjacent to the right central sulcus. Some involvement of the anterior insula. | left |
| 2 | 34 | 19 | Palidum, striatum, thalamus, encephalomalacia affecting the descending fibers of the frontal and right temporal lobe. Slight ex vacuo dilatation of the right lateral ventricle. | left |
| 3 | 31 | 151 | Encephalomalacia of the left centrum semiovale affecting the corona radiata, posterior limb of the capsula interna, globus pallidus, putamen and white matter of the medial temporal lobe. | right |
| 4 | 64 | 63 | Subcortical stroke affecting | left |
B. Design
In Group A, desynchronization was identified during BMI training based on a heterogeneous RV, while in Group B a homogenous RV was used (Fig. 1). Subjects were instructed to use visuo-kinesthetic motor imagery (MI) of moving their left hand to generate contralateral ERD.
C. Electrophysiologic Recordings
Neuromagnetic brain activity was recorded at a sampling rate of 600 Hz using a CTF 275 MEG system (CTF Systems, Inc., Port Coquitlam, BC, Canada) composed of a whole-head array of 275 radial first-order gradiometer/SQUID channels housed in a magnetically shielded room (Vacuumschmelze, Hanau, Germany). Synthetic third gradient balancing was used to remove background noise online. A video system was used to constantly monitor the subjects. Online electromyography (EMG) recordings were obtained from the m. brachioradialis and m. flexor carpiulnaris muscles of both arms using radiotranslucent surface electrodes (Biopac, inter-electrode distance: 2 cm).
D. Online Classification of ERD
ERD were computed based on the power method described by Pfurtscheller (1979) [4] using the following equations:
| (1) |
| (2) |
Pt is the power estimate in a given frequency band of the t sample block. RV is reference value.
For online classification various possibilities for choosing the composition and length of the RC exist. Preferring a reliable online detection of ERD that is rather insensitive to inter- and intra-session changes of maximum power values during rest and task suggests to choose a RV that is continuously updated by both, Pt and PREF values during BMI control (heterogeneous RV). With such an approach, an ERD is usually detected if the incoming sample block values are smaller than RV (as in Group A).
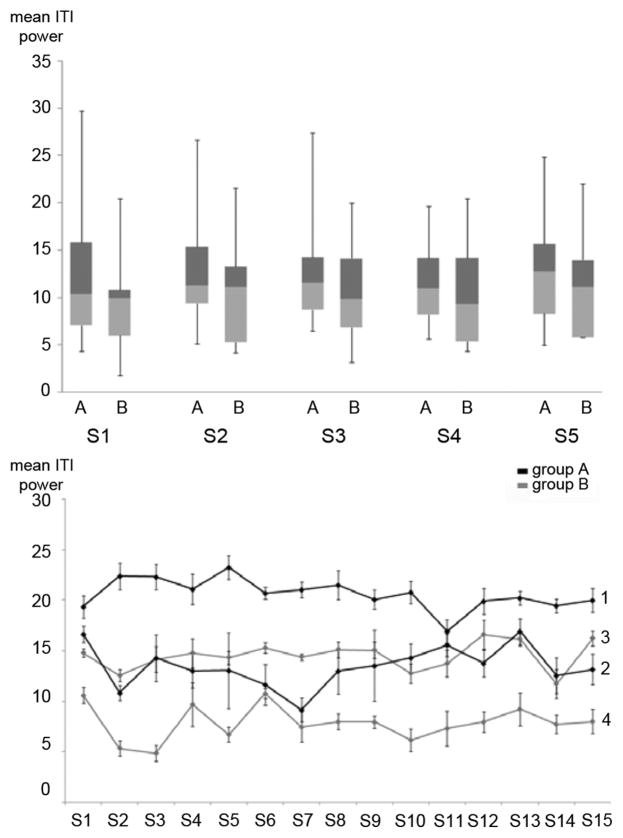
Given that the power estimates during rest condition (e.g., inter-trial-interval, ITI) are rather stable (as indicated in Fig. 3, lower graph), individual ERD thresholds for graded proprioceptive feedback can be set. In Group B each sample block value became interpreted as ERD if it was equal or stronger than 20% relative to RC. In stroke patients, a threshold of 15% was chosen.
Fig. 3.
Stability of inter- and intraday mean power values during inter-trial intervals (ITI, rest condition). Upper: Healthy subjects, error bars indicate minimum and maximum of all data. Power values are shown for each group (A,B) across sessions (S1–S5). Lower: Stroke patients, black = group A; gray = group B; each line represents the mean power values of intra-session ITI’s on S1–S15 (STD indicated by error bar, numbers at the end of each line indicates which patients data is represented). While absolute mean power values during rest show a large inter-individual variability, average variances of intraday power estimates range between 8%–12%. This stability of power values allow fixed thresholds for ERD detection (e.g., if decrease of mean power estimates during task performance reach 20%).
The BCI2000 Software platform1 was used to implement online BMI control of an orthotic device [37]. BCI2000 is based on a system model that consists of four modules (source, signal processing, user application, and operator interface) [38] and incorporates customizable signal filtering as well as extraction of signal features for translation into device control signals. Computation of ERD involved the power spectrum estimation (an autoregressive model of order 16 using the Yule-Walker algorithm) of the ongoing MEG signal associated with the specified SMR rhythm frequency range (11–14 Hz) and comparison of the resulting values with a RV. A custom made module implemented in the BCI2000 chain allowed to set fixed thresholds for various speed levels of the orthosis. The update rate for the orthotic device was at 153 ms.
E. MEG Sensor Selection
Before the first training session, a screening session was performed to identify the frequency that showed strongest ERD within the range of 11–14 Hz over right (healthy subjects) or ipsilesional (stroke patients) central sensor space. In this area, three MEG sensors were chosen that showed strongest desynchronization during motor imagery. To estimate the ERD, power estimates were calculated for every sensor separately and then averaged. On all training days, head localization relative to MEG sensors was kept constant and the same sensors were used as in S1. ERD detection thresholds and thresholds for different speed levels of the orthosis were computed and set-up in Group B before each training session. As stated above, the purpose of the BMI was to facilitate activity in the ipsilesional motor network in stroke patients or in the “active” motor network of healthy subjects.
F. BMI Training
In both groups, BMI training consisted of 575 trials (1725 trials in stroke patients) distributed over five (15) sessions. In Group B, each training session was preceded by a screening run with 23 trials to determine mean power values during RC and task.
In Group A, desynchronization was identified if three subsequent incoming sample blocks had equal or lower values than RV and translated into visual and proprioceptive feedback delivered through steady extension of the subject’s fingers by an orthotic device (see Fig. 2). Movement of the orthotic device would stop if the incoming sample block was greater than RV. After each trial, the subjects’ fingers were moved back passively to a neutral position.
Fig. 2.
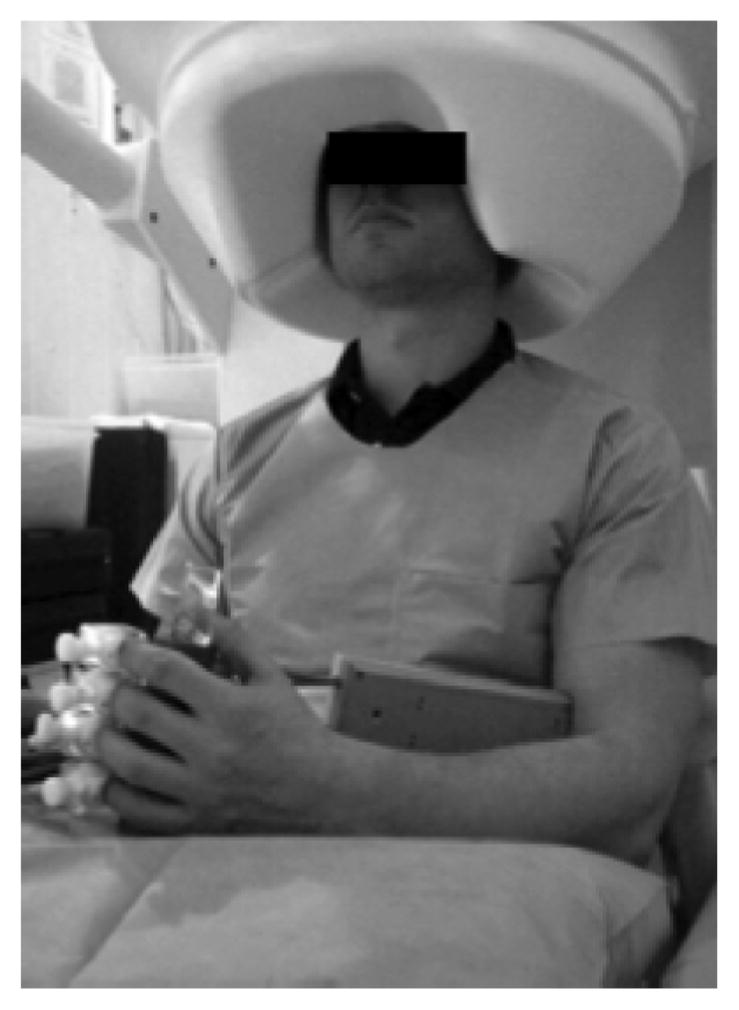
A 275-sensor MEG was used for recording of oscillatory brain activity. An orthotic device affixed to the subject’s hand and fingers delivered proprioceptive feedback. Onset and end of task was indicated by an auditory stimulus.
In Group B, a threshold (Tr) was identified that allowed good differentiation between rest and task power values, so that false positive classification of desynchronization during rest would range below 10%. In healthy subjects that threshold was 20% and 15% in stroke patients (a value to be found reliably beyond one standard deviation (SD) of the mean ITI power). Depending on the maximum strength of desynchronization (relation of the incoming sample block’s value to the RV in %) three speed levels for orthosis movement were set for the training. Optimal desynchronization in both groups would result in identical position of the orthotic device at the end of the task. BMI training was interrupted when EMG activity during imagery exceeded baseline activity recorded at rest.
G. Data Analysis
In order to have a measure that allows comparison across groups, BMI performance was defined as % of orthosis movement time relative to total training time. This measure reflects the number of incoming sample blocks classified as ERD in each group. BMI learning was defined as improvement of BMI performance between the first and last training sessions (S1–S5, healthy subjects; S1–S15, stroke patients). To evaluate the rate of false positive ERD classification in each group as an indirect measure of volitional (intended) movement, the sensitivity index SI, (3) for each subject and session was calculated and averaged across group. To assess BMI learning in both groups, an ANOVA for repeated measures (ANOVARM) with “session” as the within-subject factor and “group” as between subject factor was performed. Statistical significance was assumed when p < 0.05
| (3) |
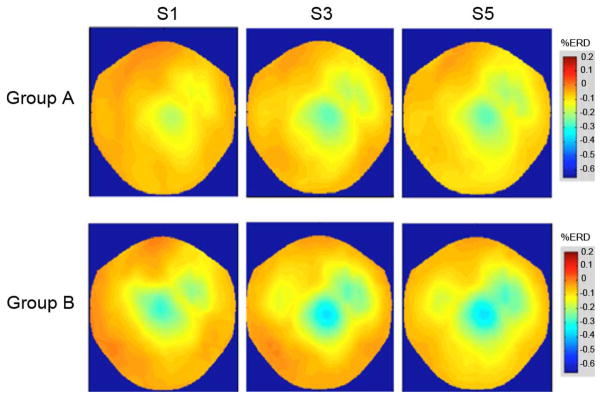
Hit rate was defined as the relative number of sample blocks during the trials in which ERD were detected according to (1) and (2). “False_alarm_rate” was defined as the relative number of sample blocks in which ERD were detected during the ITI. To illustrate changes in amplitude and extent of desynchronization at 11 Hz from session to session, ERD maps of the MEG sensor space in S1, S3, and S5 were calculated for each healthy subject separately and then averaged over group.
III. Results
A. Healthy Subjects
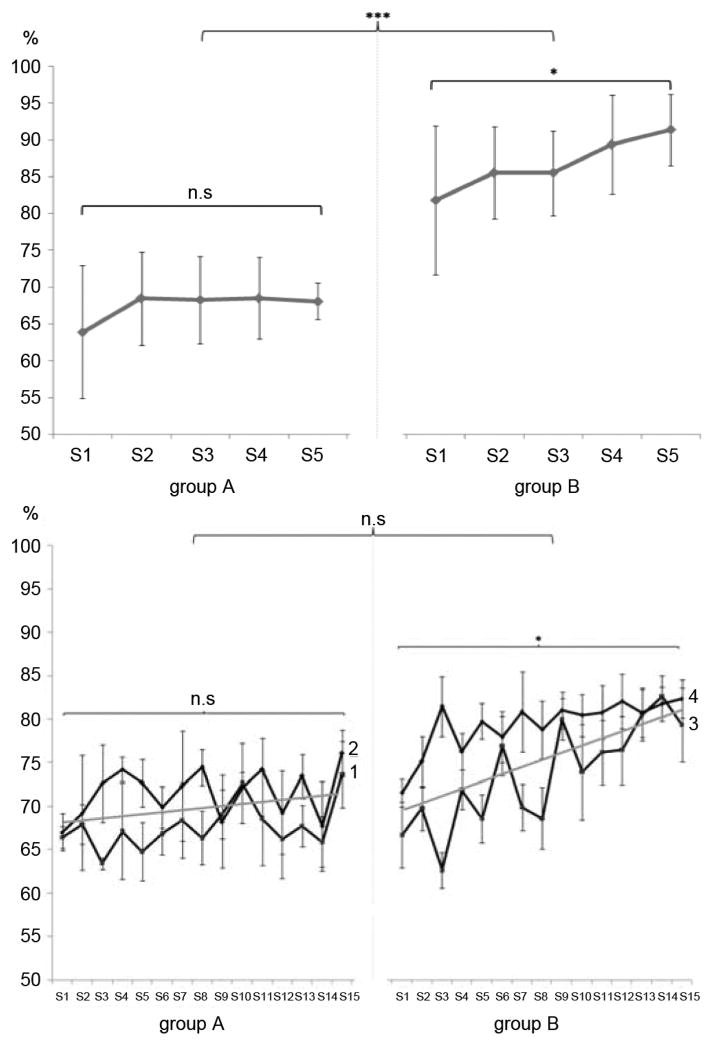
BMI performance: While Group A showed a mean performance of 67.5 ± 6.74% over all sessions, Group B reached 86.7 ± 5.85%. ANOVARM with factors “group” and “session,” showed a main effect for “group” (F(1, 18) = 39.26, p < 0.001) and “session” (F(4, 15) = 3.508, p < 0.05).
BMI learning: Repeated measures ANOVA with factors “group” and “session” showed a main effect for “group” (F(1, 18) = 16.325, p < 0.05) and “session” (F(4, 72) = 5.670, p < 0.01) (Fig. 4, upper graph). Bonferroni corrected post-hoc analysis of BMI learning from S1–S5 showed significant BMI learning only in Group B (M: 9.5, 95% CI [0.7; 1.13], p < 0.05), but not A (M:4.0, 95% CI [0.3; 0.8], p = 0.19, n.s., Fig. 4, upper graph). An independent samples t-test was used to evaluate the difference of BMI learning between Group A and B, showing that Group B learned significantly better than A (M:5.531, 95% CI [1.75; 9.308], p < 0.01). SI: SI values differed significantly between both groups (F(1, 18) = 16.325), p < 0.001). While SI improved from S1 to S2 in both groups, only Group B continued to show a trend for further increase (Fig. 5, upper graph). Topographical ERD maps: While both groups improved mean ERD strength and spatial distribution across sessions, Group B had mean ERD values of up to 46% (compared to 34% in Group A) (Fig. 6).
Fig. 4.
Mean BMI performance over sessions in Group A and B. Upper: Healthy subjects (n = 10 in each group). Lower: Stroke patients (n = 4, each subject is shown individually, numbers at the end of each line indicate of which patient’s data is shown).
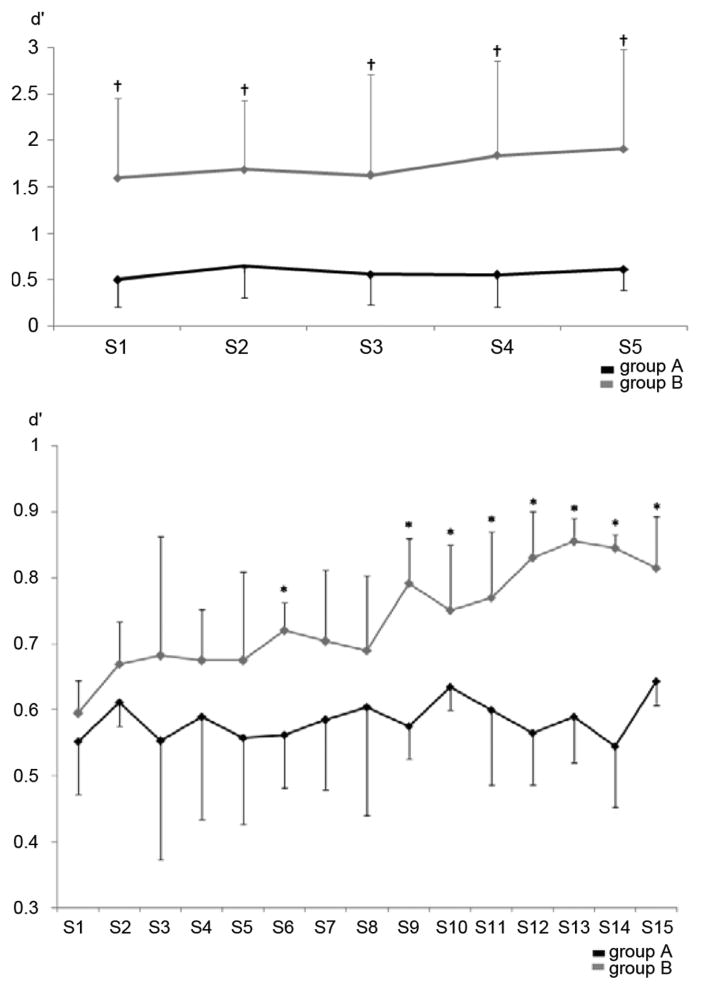
Fig. 5.
Mean sensitivity index (SI) in healthy subjects (upper graph, p < 0.001 by ANOVARM, main effect for‚ group indicated by †) and stroke patients (lower graph, p < 0.05 by ANOVARM, significant post-hoc comparison between groups indicated by *). The SI reflects the separation between mean power values during rest (ITI) and the task condition according to the ERD detection threshold used in Group A, respectively Group B. A high index indicates good separation and a low false positive ERD detection rate during the training. While there is a clear difference in healthy subjects between absolute SI values of each session in both groups, stroke patients showed very similar values at the beginning of the training, but only Group B showed improvements of SI values after S8.
Fig. 6.
Mean topographical ERD maps of in S1, S3, and S5 for Group A (upper row) and B (lower row) at 11 Hz (healthy subjects). Averaging was performed based on the assumption of a similar signal source in the sensori-motor cortex. While both groups show some increase in mean ERD-strength, this increase is more pronounced in Group B with a peak over the vertex. Both groups showed an enlargement of ERD in the course of the training.
B. Stroke Patients
BMI performance: Group A: Patient 1 reached a mean performance of 67.7 ± 2.66% while patient 2 showed a mean performance of 71.6 ± 2.79%. Group B: Patient 3 reached a mean performance of 73.6 ± 5.83% while patient 4 showed a mean performance of 79.4 ± 3.01% (Fig. 4, lower graph). ANOVARM with factors “group” and “session,” showed a main effect for “session” (F(14 140) = 9.79, p < 0.001), but not for “group” ((F(1, 10) = 4.382, p = 0.063), n.s.). BMI learning: Mean performance increase in Group B was higher over sessions resulting in a overall gain of 12.58 ± 1.56% (Group A: 6.28 ± 3.17%). Repeated measures ANOVA with factors “group” and “session” showed a main effect for “session” (F(4, 72) = 5.670, p < 0.001), but not for “group.” Bonferroni corrected post-hoc tests showed significant BMI learning from session 1–15 only in Group B (M:11.7, 95% CI [10.2; 12.9]; p < 0.05), but not in A (M:6.3, 95% CI [22.2; 12.9]; p = 0.194, n.s.) (Fig. 4, lower graph).
A two-tailed t-test for independent samples was used to evaluate the difference of BMI learning between Group A and B. There was significantly more learning in Group B than A (M:3.57, 95% CI [6.65; 0.48], p < 0.05). SI: ANOVARM with factors “group” and “session” indicated a main effect for “group” (F(1, 18) = 139.00), p < 0.001)) and “session” (F(14, 87.82) = 13.428, p < 0.05). While SI was similar at the beginning of the training in all patients, only Group B improved after S8 (see Fig. 5). Post-hoc t-test for independent samples indicated significant differences of SI in S9–15 (p < 0.05).
IV. Discussion
These results, though preliminary in stroke patients due to the small n, indicate that the combination of a homogeneous RV for ERD detection with graded feedback based on ERD strength leads to better BMI performance and learning than a heterogeneous RV with binary feedback. Thus, this training strategy may offer a better way to improve modulation of ipsilesional activity in the context of restorative BMI use in neurorehabilitation.
By design, a BMI based on a heterogeneous RV and binary feedback reduces the probability of early frustration during BMI learning and offers good adaptability to day-to-day and intraday variability of mean power estimates during rest and task condition intervals, but is also more susceptible to inconsistencies of voluntary SMR modulation during the BMI training: in a subject who fails to optimally desynchronize during a trial, e.g., due to distraction or fatigue that leads to a delay in desynchronization or weaker ERD, the heterogeneous RV will be adapted accordingly resulting in a lower threshold for ERD detection in the following trial and, thus, reduce the necessity to consistently generate strong ERD.
Homogeneous RV coupled with graded feedback facilitates BMI learning and excludes false positive detection of ERD, an issue relevant if subjects have difficulties to focus on the task as it often occurs in stroke patients. Inattention e.g., will not lead to a change of the feedback-threshold in the following runs. Furthermore, graded feedback allows better offline differentiation of BMI learning and performance depending on e.g., the amount of time the subject reached different speed levels during the BMI training, leading to improvements in motor control of the orthosis. This is an important issue for day-to-day comparability of ERD throughout the BMI training in stroke patients, assuming that stronger ERD correlate with increased activity of the underlying neuronal populations. Using a homogeneous RV with graded feedback, though, has the disadvantage that it requires daily determination of thresholds for different speed levels related to ERD strength.
Subjects in Group A showed some improvement of mean performance from S1 to S2 that was, however, not significant due to high variance. In the following sessions there was no further improvement, except for a decrease of variance (Fig. 4). It is likely that subjects in Group A did not improve their mean performance because stronger desynchronization would result in an adaptation of the RV making it more difficult (and metabolically costly) in the following trials to sustain the previously reached level of performance. Topographical ERD maps (Fig. 6) of both groups substantiate this assumption. While subjects in Group B showed successive increase in ERD strength, this steady increase could not be seen in Group A. This finding is further supported by the evaluation of the SI (Fig. 5) that indicates that in Group A the distribution of mean power values during task and ITI is in higher proximity. Our finding suggests that a heterogeneous RV with binary feedback results in an intermediate desynchronization level while stabilizing performance by improving consistency of ERD production so that a stable level of feedback is sustained. An intermediate desynchronization level, however, may lead to a higher amount of false positive ERD detections and, thus, lower performance levels compared to levels reached in Group B. The decrease of variance in the course of the training at the same performance level supports the assumption that the subjects in Group A tend to sustain an intermediate desynchronization strength with increasing consistency. This might reflect the attempt of the brain to optimize metabolic cost of BMI control minimizing a cost function according to optimal control theory [39], [40].
While in the patient population, both groups showed some increase of mean BMI performance over the course of 15 sessions (Fig. 4, lower graph), only patients of Group B showed significant BMI learning from S1 to S15.
A potential disadvantage of graded feedback with fixed ERD thresholds in patients with stroke could be that inattention or fatigue might lead to early frustration, as any drop in ERD strength will be reflected immediately by the movement speed of the orthosis. While it could be expected that higher absolute power values reached during ITI imply a higher capacity for desynchronization [41], we could not find such a correlation in stroke patients (Fig. 3, lower graph). On the contrary: high amplitudes with little variance during rest, respectively ITI, in stroke patients might indicate extensive idling of neuronal networks in the ipsilesional hemisphere as a sign of impairment. While recent studies showed that stroke patients can successfully learn to control a SMR-based BMI [3], [6], [8] this is, to our knowledge, the first study that investigated online-translation of ERD into graded proprioceptive feedback in a homogeneous RV environment. It suggests that fast adaptation of BMI parameters e.g., by using a heterogeneous RV, coupled with binary feedback might not be optimal for BMI learning associated with ERD production compared to less adaptive approaches and graded feedback. These findings might be of relevance to identify optimal parameters and frameworks for restorative BMI applications in stroke patients. Future work should dissociate further the relative contribution of RV and feedback type on BMI learning.
Acknowledgments
The work of S. Soekadar was supported by the Intramural Research Program (IRP) of the National Institute of Neurological Disorders and Stroke (NINDS), National Institues of Health (NIH), the German Federal Ministry of Education and Research (BMBF # 01GQ0831) and the Deutsche Forschungsgemeinschaft (DFG). The work of M. Witkowski was supported by the IRP of the NINDS/NIH, the DFG and the BMBF (01GQ0831). The work of N. Birbaumer was supported by the BMBF (01GQ0831) and the DFG. The work of L. G. Cohen is supported by the IRP of the NINDS/NIH and the Center for Neuroscience and Regenerative Medicine, Uniformed Services University of Health Sciences, Bethesda, MD.
S. R. Soekadar and M. Witkowski would like to thank K. Kunzelmann, F. Brasil, and M. Curado for their assistance in collecting the data, and M. Stephan for her helpful comments and advice on data analysis.
Biographies
Surjo R. Soekadar studied medicine in Mainz, Heidelberg, and Baltimore.
He is currently a Research Fellow at the Human Cortical Physiology and Stroke Neurorehabilitation Section (HCPS) at the National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH). His scientific interests include cortical plasticity in the context of brain-machine interface (BMI) applications, brain stimulation and neural mechanisms of learning and memory.
Dr. Soekadar received various prices such as the NIH-DFG Research Career Transition Award (2008) and the NIH Fellows Award for Research Excellence (2011).
Matthias Witkowski received the M.S. degree in computational life science from the University of Lübeck, Germany. He is currently a Ph.D. candidate at the Faculty of Mathematics and Natural Sciences, University of Tübingen, Germany.
His research interests include effects of noninvasive brain stimulation on cortical and subcortical neurophysiology and behavior, magnetoencephalography and advancement of beamformer algorithms in the context of online BMI-control.
Jürgen Mellinger is a Research Associate at the University of Tübingen, Germany, and Chief Software Engineer in the BCI2000 project. He has extensively worked on the development and application of BCI systems in patient populations.
Ander Ramos received the M.S. degree in biomedical engineering from the Johns Hopkins University, Baltimore, MD, and industrial engineering from the University of Munich, Germany. He is currently a Ph.D. candidate at the Graduate School of Neural and Behavioral Science, University of Tübingen, Germany.
His research interests include neuroprosthetics and application of neuro-robotics in stroke rehabilitation.
Niels Birbaumer received the Ph.D. degree in psychology from the University of Vienna, in 1969.
Since 1975, he has been full professor at the University of Tübingen (Institute of Medical Psychology and Behavioral Neurobiology) and invented the thought-translation device (TDD) enabling completely paralyzed ALS patients to communicate based on voluntary control of brain activity.
Dr. Birbaumer is Fellow of the Academy of Behavioral Medicine Research and fellow of the Society of Behavioral Medicine. He received the Leibniz Price, Albert-Einstein World Award and Helmholtz Medaille by the Berlin Branden-burgische Akademie der Wissenschaften.
Leonardo G. Cohen received the M.D. degree from the University of Buenos Aires. He completed his neurology residency at Georgetown University.
After his postdoctoral training in clinical neurophysiology at the Department of Neurology, University of California (Irvine) and in motor control and movement disorders at the Human Motor Control Section, National Institute of Neurological Disorders and Stroke (NINDS), he became Chief of the Human Cortical Physiology and Stroke Neurorehabilitation Section (HCPS), NINDS, in 1998. His research interests include mechanisms underlying plastic changes in the human central nervous system and the development of novel therapeutic approaches for recovery of function after brain lesions.
Footnotes
Contributor Information
Surjo R. Soekadar, Email: surjo@soekadar.com, Human Cortical Physiology and Stroke Neurorehabilitation Section (HCPS), National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Bethesda, MD 20892 USA, and also with the Institute for Medical Psychology and Behavioral Neurobiology (IMP), University of Tübingen, 72076 Tübingen, Germany
Matthias Witkowski, Email: matthias.witkowski@gmx.de, Cortical Physiology and Stroke Neurorehabilitation Section (HCPS), National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Bethesda, MD 20892 USA, and also with the Institute for Medical Psychology and Behavioral Neurobiology (IMP), University of Tübingen, 72076 Tübingen, Germany.
Jürgen Mellinger, Email: juergen.mellinger@uni-tuebingen.de, Institute for Medical Psychology and Behavioral Neurobiology (IMP), University of Tübingen, 72076 Tübingen, Germany.
Ander Ramos, Email: ander.ramos@gmail.com, Institute for Medical Psychology and Behavioral Neurobiology (IMP), University of Tübingen, 72076 Tübingen, Germany, and also with Fatronik-Tecnalia Germany, 72070 Tübingen, Germany.
Niels Birbaumer, Email: niels.birbaumer@uni-tuebingen.de, Institute for Medical Psychology and Behavioral Neurobiology (IMP), University of Tübingen, 72076 Tübingen, Germany, and also with the Ospedale san Camillo, IRCCS, Alberoni, 70-30126 Venezia, Italy.
Leonardo G. Cohen, Email: cohenl@ninds.nih.gov, Human Cortical Physiology and Stroke Neurorehabilitation Section (HCPS), National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Bethesda, MD 20892 USA
References
- 1.Sterman MB. EEG biofeedback: Physiological behavior modification. Neurosci Biobehav Rev. 1981;1(5):405–412. doi: 10.1016/0149-7634(81)90036-1. [DOI] [PubMed] [Google Scholar]
- 2.Pfurtscheller G, Graimann B, Huggins JE, Levine SP. Brain-computer communication based on the dynamics of brain oscillations. Suppl Clin Neurophysiol. 2004;57:583–591. doi: 10.1016/s1567-424x(09)70398-8. [DOI] [PubMed] [Google Scholar]
- 3.Buch E, et al. Think to move: A neuromagnetic brain-computer interface (BCI) system for chronic stroke. Stroke. 2008;39:910–917. doi: 10.1161/STROKEAHA.107.505313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfurtscheller G, Aranibar A. Evaluation of event-related desynchronization (ERD) preceding and following self-paced movement. Electroencephgr Clin Neurophysiol. 1979;46:138–146. doi: 10.1016/0013-4694(79)90063-4. [DOI] [PubMed] [Google Scholar]
- 5.Vaughan TM, Wolpaw JR, Donchin E. EEG-based communication: Prospects and problems. IEEE Trans Rehabil Eng. 1996 Dec;4(4):425–430. doi: 10.1109/86.547945. [DOI] [PubMed] [Google Scholar]
- 6.Birbaumer N, Cohen LG. Brain-computer interfaces: Communication and restoration of movement in paralysis. J Physiol. 2007;579:621–636. doi: 10.1113/jphysiol.2006.125633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly J, et al. Feasibility of a new application of noninvasive brain computer interface (BCI): A case study of training for recovery of volitional motor control after stroke. J Neurol Phys Ther. 2009;33:203–211. doi: 10.1097/NPT.0b013e3181c1fc0b. [DOI] [PubMed] [Google Scholar]
- 8.Broetz D, et al. Combination of brain-computer interface training and goal-directed physical therapy in chronic stroke: A case report. Neurorehabil Neural Repair. 2010;24:674–679. doi: 10.1177/1545968310368683. [DOI] [PubMed] [Google Scholar]
- 9.Kim SS, Mihalas S, Russell A, Dong Y, Bensmaia S. Does afferent heterogeneity matter in conveying tactile feedback through peripheral nerve stimulation? IEEE Trans Neural Syst Rehabil Eng. 2011 Oct;19(5) doi: 10.1109/TNSRE.2011.2160560. [DOI] [PubMed] [Google Scholar]
- 10.Horch K, Meek S, Taylor T, Hutchinson D. Object discrimination with an artificial hand using electrical stimulation of peripheral tactile and proprioceptive pathways with intrafascicular electrodes. IEEE Trans Neural Syst Rehabil Eng. 2011 Oct;19(5) doi: 10.1109/TNSRE.2011.2162635. [DOI] [PubMed] [Google Scholar]
- 11.Weber DJ, London BM, Hokanson JA, Ayers CA, Torres RR, Zaaimi B, Miller LE. Limb-state information encoded by peripheral and central somatosensory neurons: Implications for an afferent interface. IEEE Trans Neural Syst Rehabil Eng. 2011 Oct;19(5) doi: 10.1109/TNSRE.2011.2163145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkatraman S, Carmena J. Active sensing of target location encoded by cortical microstimulation. IEEE Trans Neural Syst Rehabil Eng. 2011 Jun;19(3):317–324. doi: 10.1109/TNSRE.2011.2117441. [DOI] [PubMed] [Google Scholar]
- 13.O’Doherty JE, Lebedev MA, Li Z, Nicolelis MAL. Towards a brain-machine-brain interface: Virtual active touch using randomly patterned intracortical microstimulation. IEEE Trans Neural Syst Rehabil Eng. 2011 Oct;19(5) doi: 10.1109/TNSRE.2011.2166807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koivuniemi A, Andrew OK. Asymmetric Versus Symmetric electric pulses for intracortical microstimulation. IEEE Trans Neural Syst Rehabil Eng. 2011;19(5) doi: 10.1109/TNSRE.2011.2166563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heming E, Choo R, Davies J, Kiss Z. Designing a thalamic somatosensory neural prosthesis: Consistency and persistence of percepts evoked by electrical stimulation. IEEE Trans Neural Syst Rehabil Eng. 2011 Oct;19(5) doi: 10.1109/TNSRE.2011.2152858. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Khalil H, Oweiss K. Neural feedback for instantaneous spatiotemporal modulation of afferent pathways in bi-directional brain machine interfaces. IEEE Trans Neural Syst Rehabil Eng. 2011 Oct;19(5) doi: 10.1109/TNSRE.2011.2162003. [DOI] [PubMed] [Google Scholar]
- 17.Birbaumer N, et al. Neurofeedback and brain-computer interface clinical applications. Int Rev Neurobiol. 2009;86:107–117. doi: 10.1016/S0074-7742(09)86008-X. [DOI] [PubMed] [Google Scholar]
- 18.Ward NS, et al. Longitudinal changes in cerebral response to proprioceptive input in individual patients after stroke: An FMRI study. Neurorehabil Neural Repair. 2006;20:398–405. doi: 10.1177/1545968306286322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turton A, et al. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101:316–328. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- 20.Platz T, et al. Brain activation pattern as assessed with multi-modal EEG analysis predict motor recovery among stroke patients with mild arm paresis who receive the arm ability training. Restor Neurol Neurosci. 2002;20:21–35. [PubMed] [Google Scholar]
- 21.Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calautti C, et al. The relationship between motor deficit and hemisphere activation balance after stroke: A 3t fMRI study. Neuroimage. 2007;34:322e3. doi: 10.1016/j.neuroimage.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 23.Johansen-Berg H, et al. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Nat Acad Sci USA. 2002;99:14518e23. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McFarland DJ, Sarnacki WA, Wolpaw JR. Should the parameters of a BCI translation algorithm be continually adapted? J Neurosci Methods. 199:103–107. doi: 10.1016/j.jneumeth.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vidaurre C, Schlögl A, Cabeza R, Scherer R, Pfurtscheller G. A fully on-line adaptive BCI. IEEE Trans Biomed Eng. 2006 Jun;53(6):1214–1219. doi: 10.1109/TBME.2006.873542. [DOI] [PubMed] [Google Scholar]
- 26.Shenoy P, Krauledat M, Blankertz B, Rao RP, Müller KR. Towards adaptive classification for BCI. J Neural Eng. 2006;3:R13–23. doi: 10.1088/1741-2560/3/1/R02. [DOI] [PubMed] [Google Scholar]
- 27.Krausz G, Scherer R, Korisek G, Pfurtscheller G. Critical decision-speed and information transfer in the graz brain-computer interface. Appl Psychophysiol Biofeedback. 2003;28:233–240. doi: 10.1023/a:1024637331493. [DOI] [PubMed] [Google Scholar]
- 28.Von Bünau P, Meinecke FC, Király FC, Müller KR. Finding stationary subspaces in multivariate time series. Phys Rev Lett. 2009;103:214101. doi: 10.1103/PhysRevLett.103.214101. [DOI] [PubMed] [Google Scholar]
- 29.Kawanabe M, Vidaurre C, Scholler S, Müller KR. Robust common spatial filters with a maxmin approach. Proc. IEEE Eng. Med. Biol. Soc. Conf; 2009; 2009. pp. 2470–2473. [DOI] [PubMed] [Google Scholar]
- 30.Vidaurre C, Blankertz B. Towards a cure for BCI illiteracy. Brain Topogr. 2010;23:194–198. doi: 10.1007/s10548-009-0121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lotte F, Guan C, Ang KK. Comparison of designs towards a subject-independent brain-computer interface based on motor imagery. Proc. IEEE Eng. Med. Biol. Soc. Soc; 2009; 2009. pp. 4543–4536. [DOI] [PubMed] [Google Scholar]
- 32.Abe M, Schambra H, Wassermann EM, Luckenbaugh D, Schweighofer N, Cohen LG. Reward improves long-term retention of a motor memory through induction of offline memory gains. Curr Biol. 2011;21:557–562. doi: 10.1016/j.cub.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Dynamics of the human EEG during prolonged wakefulness: Evidence for frequency-specific circadian and homeostatic influences. Neurosci Lett. 1997;239:121–124. doi: 10.1016/s0304-3940(97)00904-x. [DOI] [PubMed] [Google Scholar]
- 34.Lemke M. Correlation between EEG and driver’s actions during prolonged driving under monotonous conditions. Accid Anal Prev. 1982;14:7–17. [Google Scholar]
- 35.Bilodeau EA. Information feedback. In: Bilodeau EA, editor. Acquisition of Skill. New York: Academic; 1966. [Google Scholar]
- 36.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 37.Schalk G, et al. BCI2000: A general-purpose brain-computer interface (BCI) system. IEEE Trans Biomed Eng. 2004 Jun;51(6):1034–1043. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- 38.Schalk G. Effective brain-computer interfacing using BCI2000. Proc IEEE Eng Med Biol Soc Conf; 2009; 2009. pp. 5498–5501. [DOI] [PubMed] [Google Scholar]
- 39.Scott SH. Optimal feedback control and the neural basis of volitional motor control. Nat Rev Neurosci. 2004;5:532–546. doi: 10.1038/nrn1427. [DOI] [PubMed] [Google Scholar]
- 40.Jackson A, Fetz E. Interfacing with the computational brain. IEEE Trans Neural Syst Rehabil Eng. 2011 Oct;19(5) doi: 10.1109/TNSRE.2011.2158586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blankertz B, Sannelli C, Halder S, Hammer EM, Kübler A, Müller KR, Curio G, Dickhaus T. Neurophysiological predictor of SMR-based BCI performance. Neuroimage. 2010;51:1303–1309. doi: 10.1016/j.neuroimage.2010.03.022. [DOI] [PubMed] [Google Scholar]