Abstract
IMPORTANCE
Salvage surgery for recurrent head and neck squamous cell carcinoma (HNSCC) carries substantial risks of morbidity and mortality. Risk factors for death within 1 year should be better defined.
OBJECTIVES
To report preoperative oncologic prognostic factors predictive of short-term (<1 year) survival after salvage surgery in patients with HNSCC, to assess whether preoperative age and comorbidity predicts 1-year mortality, and to report hospital courses after salvage surgery within 1 year.
DESIGN, SETTING, AND PARTICIPANTS
A retrospective medical record review of 191 patients with recurrent HNSCC treated with salvage surgery from January 1, 2003, through December 31, 2013, at a tertiary academic center.
INTERVENTIONS
Surgical salvage of HNSCC (larynx, oral cavity, oropharynx, or hypopharynx) with curative intent.
MAIN OUTCOMES AND MEASURES
Primary outcome was survival 1 year after salvage surgery. Secondary outcomes were length of inpatient hospital stay, days of admissions, and skilled nursing facility disposition within 1 year stratified by survival status. Presalvage Charlson–Age Comorbidity Index (CACI) was calculated. Associations among CACI, oncologic risk factors, and risk of death within 1 year after salvage surgery are investigated using multivariable analysis.
RESULTS
Of 191 patients studied, 53 (27.7%) died within 1 year after salvage surgery. Patients who died within 1 year had more total inpatient admissions (P < .001), longer total length of stay (P < .001), and higher risk of discharge to a skilled nursing facility (P < .001) and spent 17.3% (interquartile range, 5.2-36.3) of their remaining days in the hospital. Independent risk factors for death within 1 year are CACI (relative risk [RR], 1.43; 95% CI, 1.16-1.76), primary T3 or T4 stage (RR, 2.34; 95% CI, 1.27-4.31), and disease-free interval of less than 6 months (RR, 5.61; 95% CI, 1.78-16.7).
CONCLUSIONS AND RELEVANCE
Medical comorbidity and age as measured by the CACI, primary T3 or T4 stage, and short disease-free interval must be considered in selecting patients ideal for surgical salvage surgery for recurrent HNSCC. Patients with these risk factors should be more strongly considered for palliative measures.
Surgery is frequently the only available treatment modality for recurrent head and neck squamous cell carcinoma (HNSCC) despite increased morbidity and lower success rates compared with surgery in the setting of primary disease.1,2 In many cases, critical clinical decisions for each patient must be made to determine whether the potential benefits of salvage surgery outweigh its risks, morbidity, and economic costs.
Careful selection of patients eligible for salvage surgery should be based on disease and patient characteristics. Previous studies3-7 have successfully identified oncologic risk factors of poor outcome after salvage surgery, including greater primary tumor and nodal stage, short disease-free interval (DFI), positive margins, a non-laryngeal cancer site, and history of radiation therapy.
Many candidates for surgical salvage unfortunately possess other medical comorbidities that will adversely affect their survival. A previous study8 found that nearly 40% of 65-year-old Medicare patients underwent surgery in the year of their death.9 With increasing numbers of HNSCC in elderly patients, factors that can negatively affect the postoperative outcomes, such as age or comorbidities, become essential in making treatment decisions. Although age and comorbidities negatively affect survival in patients with HNSCC, literature that evaluates age and comorbidities as predictors of mortality specifically after salvage surgery in patients with recurrent HNSCC is lacking.10-13
The primary goal of this study is to identify preoperative risk factors for 1-year mortality after salvage surgery for recurrent HNSCC. The secondary goal is to evaluate measures of disease burden that affect quality of life, including length of stay (LOS) in the hospital, number of admissions, and patient disposition to a skilled nursing facility (SNF) within 1 year after salvage surgery. By defining the disease and comorbidity characteristics and the treatment course of those with short-term mortality or poor quality of life, we hope our study can help accurately guide the treating physicians and contribute to the informed decision-making process for patients with recurrent HNSCC.
Methods
Approval for this study was obtained from the institutional review board of the University of Pittsburgh Medical Center. This retrospective medical record review included patients who underwent salvage surgery for recurrent squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, or larynx for primary site and regional recurrences from January 1, 2003, through December 31, 2013, as stored in our institutional head and neck clinical database. Patients were excluded if no complete record of their primary treatment was available, for a lack of curative intent for the salvage surgery, for surgery for a second primary tumor, or if there was less than 1 year of follow-up after surgery for recurrence unless death occurred.
Data extracted from the medical record included demographic data (sex, age, race, alcohol use, and smoking status), oncologic data from the primary tumor (tumor markers, pT, pN, tumor grade, extracapsular spread, surgical margin status, histologic type, tumor recurrence, and adjuvant therapies), and administrative data (International Classification of Diseases, Ninth Revision [ICD-9], codes for medical comorbidities, total LOS, number of admissions within 1 year of salvage surgery, and disposition from hospital to an SNF). Follow-up information was obtained for each patient, including current state of health, time to death, and death related to disease or from other factors. Five patients were unavailable for follow-up.
The Charlson–Age Comorbidity Index(CACI) was calculated using ICD-9 codes abstracted from hospital administrative data (Table 1). The CACI score was then calculated as described originally by Charlson etal14 by adding weighed scores of corresponding comorbid diseases. These ICD-9 codes were chosen by a previously validated CACI algorithm as described by Quan et al.15 Diagnoses made after the date of salvage surgery were not included in the calculation. The resultant CACI scores were then determined by first calculating the weighed score for each comorbid disease separately and subsequently adding the age factor to result in a single score.9 Independent medical record review was performed for a subset of patients to ensure agreement between administrative data and manual record review.
Table 1.
Demographic Characteristics, Clinicopathologic Information, and Univariate Association of Oncologic Risk Factors and 1-Year Mortality
| No. (%) of Patientsa |
||||
|---|---|---|---|---|
| Survival |
||||
| Variable | All (N = 191) | ≥1 Year (n = 138) | <1 Year (n = 53) | P Value |
| Cancer subsite | ||||
| Larynx | 72 (37.7) | 56 (40.6) | 16 (30.2) | <.001b |
| Oral cavity | 72 (37.7) | 50 (36.2) | 22 (41.5) | |
| Oropharynx | 38 (19.9) | 27 (19.6) | 11 (20.8) | |
| Hypopharyx | 9 (4.7) | 5 (3.6) | 4(7.5) | |
| Sex | ||||
| Female | 55 (28.8) | 40 (29.0) | 15 (28.3) | .93 |
| Male | 136 (71.2) | 98 (71.0) | 38 (71.7) | |
| Smoking history | ||||
| Never | 27 (14.1) | 15 (10.9) | 12 (22.6) | .04b |
| Former or current | 158 (82.7) | 118 (85.5) | 40 (75.5) | |
| Alcohol history | ||||
| None | 50 (26.2) | 35 (25.4) | 15 (28.3) | .95 |
| Former or current alcohol history | 122 (63.9) | 86 (62.3) | 36 (67.9) | |
| T stagec | ||||
| 1 | 72 (37.7) | 63 (45.7) | 9 (17.0) | <.001b |
| 2 | 61 (31.9) | 42 (30.4) | 19 (35.8) | |
| 3 | 35 (18.3) | 19 (13.8) | 16 (30.2) | |
| 4 | 19 (9.9) | 10 (7.2) | 9 (17.0) | |
| N stagec | ||||
| N0/1 | 135 (70.7) | 103 (74.6) | 32 (60.4) | .02b |
| N2 or less | 46 (24.1) | 27 (19.6) | 19 (35.8) | |
| Closure | ||||
| Free flap | 30 (15.7) | 17 (12.3) | 13 (24.5) | .05b |
| Primary closure or pedicledflap | 97 (50.8) | 73 (52.9) | 24 (45.3) | |
| Primary radiotherapy | ||||
| Yes | 93 (48.7) | 69 (50.0) | 24 (45.3) | .56 |
| No | 98 (51.3) | 69 (50.0) | 29 (55.7) | |
| Adjuvant therapy | ||||
| None | 103 (53.9) | 80 (58.0) | 23 (43.4) | .009b |
| Radiation | 31 (16.2) | 20 (14.5) | 11 (20.8) | |
| Radiation and chemotherapy | 20 (10.5) | 9 (6.5) | 11 (20.8) | |
| Disease-free interval,mo | ||||
| <6 | 91 (47.6) | 56 (40.6) | 35 (66.0) | .002b |
| ≥6 | 100 (52.4) | 82 (59.4) | 18 (34.0) | |
| Primary extracapsular spread | ||||
| Yes | 17 (8.9) | 5 (3.6) | 12 (22.6) | .89 |
| No | 8 (4.2) | 2 (1.4) | 6 (11.3) | |
| HPV status | ||||
| Positive | 9 (4.7) | 7 (5.1) | 2 (3.8) | .43 |
| Negative | 25 (13.1) | 14 (10.1) | 11 (20.8) | |
| Primary perineural invasion | ||||
| Yes | 26 (13.6) | 14 (10.1) | 12 (22.6) | .20 |
| No | 49 (25.7) | 35 (25.4) | 14 (26.4) | |
| Primary mandible procedure | ||||
| Yes | 20 (10.5) | 12 (8.7) | 8 (15.1) | .29 |
| No | 144 (75.4) | 105 (76.1) | 39 (73.6) | |
| Primary positive margin | ||||
| Yes | 9 (4.7) | 6 (4.3) | 3 (5.7) | >.99 |
| No | 74 (38.7) | 47 (34.1) | 27 (50.9) | |
Total No. (%) discrepancies are a result of data points answered as unknown or not applicable.
Statistically significant (P < .05).
Staging of primary disease at the time of initial diagnosis.
Patients were stratified by 1-year survival status. Overall survival was calculated from the date of the salvage surgery. Patient and tumor characteristics were tested for univariate association with survival less than 1 year or 1 year or greater using χ2 tests, Fisher exact tests, and logistic regression as appropriate. Factors that were associated with survival (P < .05) were then included in a multivariable logistic regression model. Odds ratios were computed to measure the factors associated with survival of less than 1 year while controlling for the effects of the other covariates. The comorbidities that make up the CACI were then individually assessed for association with survival less than 1 year after salvage surgery, and relative risks (RRs) were reported. Association between the total comorbidity score and overall survival was evaluated by log-rank tests and Kaplan-Meier curves, stratified by comorbidity scores of 6 and 8 separately.
The total number of hospital admissions and the LOS during the year after salvage surgery, for general and otolaryngology-specific visits, are described along with the percentage of days in the year after the salvage surgery that were spent in the hospital. They are compared across survival groups and comorbidity strata using t tests. A χ2 test of proportions is used to compare the proportion of individuals with a history of SNF discharge across survival groups and comorbidity scores. Statistical tests were 2-sided, and analyses were conducted using SAS and STAT statistical software, version 9.4 (SAS Institute Inc), and R, version 3.1.1 (R Foundation for Statistical Computing).
Results
A total of 191 patients (72 with cancer of the larynx, 72 with oral cavity cancer, 38 with oropharynx cancer, and 9 with hypopharynx cancer) met the inclusion criteria. Mean follow-up was 20 months (interquartile range [IQR], 10-34 months). Univariate association of oncologic risk factors and 1-year mortality rates with demographic and clinicopathologic information is presented in Table 1. Fifty-three of 191 patients died within 1 year after surgery, yielding a 1-year overall survival rate after salvage surgery of 72%. Of patients who survived less than 1 year, the median survival after salvage surgery was 5 months compared with 26 months for 159 patients who survived 1 year or more. This number represented 27.7% of the entire cohort.
On univariate analysis, primary cancer subsite had a significant effect on 1-year survival, with the laryngeal site having the best prognosis and the hypopharyngeal site having the worst (P < .001). Smoking (P = .04), advanced primary T stage (P < .001), advanced primary nodal stage (N2 or higher) (P = .02), adjuvant therapy with radiation or chemoradiation after primary surgery (P = .006), and DFI shorter than 6 months before recurrence (P = .001) were associated with less than 1-year survival after salvage surgery. Sex, age, alcohol history, human papillomavirus status, margin status, extracapsular spread, perineural invasion, and mandible procedure of primary surgery were not associated with mortality within 1 year after surgery for recurrent disease within this cohort (Table 1).
The CACI outcomes and univariate association of age, comorbidities, and 1-year mortality are given in Table 2. The mean (SD) CACI score was 6.5 (2.7). The most common comorbidities were pulmonary disease (50 [26.2%]), diabetes mellitus (35 [18.3%]), myocardial infarction (19 [9.9%]), and peripheral vascular disease (19 [9.9%]). No single common medical comorbidity was predictive of 1-year mortality by itself, except for rheumatic disease (RR, 2.93; 95% CI, 1.78-4.84) and severe liver disease (RR, 3.52; 95% CI, 2.81-4.45). Both these comorbidities were rare in this patient population. Neck metastasis was present in 45 of 191 patients (23.6%) and was a significant predictor of mortality (RR, 2.78; 95% CI, 1.82-4.24). Age alone was also not a significant predictor of 1-year mortality (RR, 1.22; 95% CI, 0.98-1.51). When the total CACI was computed, however, for every unit increase in total CACI score, the odds of death within 1 year after salvage surgery increased by 30% (RR, 1.30; 95% CI, 1.15-1.47).
Table 2.
CACI Outcomes and Univariate Association of Age Comorbidities and 1-Year Mortality
| 1 Year After Salvage Surgery |
||||
|---|---|---|---|---|
| Comorbidity | Weight for CACI Calculation | No. (%) of Patients (N = 191) | 1-Year Mortality Rate, No. (%) | RR of Mortality Within 1 Year of Salvage Surgery (95% CI) |
| Myocardial infarction | 1 | 19 (9.9) | 6 (31.6) | 1.12 (0.55-2.26) |
| Congestive heart failure | 1 | 12 (6.3) | 6 (50) | 1.84 (0.99-3.41) |
| Peripheral vascular disease | 1 | 19 (9.9) | 4 (21) | 0.71 (0.29-1.76) |
| Stroke | 1 | 7 (3.7) | 2 (29) | 0.99 (0.30-3.29) |
| Dementia | 1 | 2 (1.0) | 1 (50) | 1.76 (0.43-7.17) |
| Pulmonary | 1 | 50 (26.2) | 16 (32) | 1.17 (0.72-1.90) |
| Rheumatic disease | 1 | 5 (2.6) | 4 (80) | 2.93 (1.78-4.84)a |
| Peptic ulcer disease | 1 | 2 (1.0) | ... | ... |
| Liver disease, mild | 1 | 6 (3.1) | 3 (50) | 1.79 (0.77-4.12) |
| Diabetes mellitus | 1 | 30 (15.7) | 11 (37) | 1.35 (0.79-2.31) |
| Diabetes with end-organ damage | 2 | 5 (2.6) | 2 (40) | 1.41 (0.47-4.23) |
| Paralysis | 2 | 1 (0.5) | ... | ... |
| Renal disease | 2 | 6 (3.1) | 3 (50) | 1.79 (0.78-4.12) |
| Any tumor | 2 | 191 (100) | ... | ... |
| Liver disease, severe | 3 | 1 (0.5) | 1 (100) | 3.52 (2.81-4.45)a |
| Metastasisb | 4 | 45 (23.6) | 25 (56) | 2.78 (1.82-4.24)a |
| Human Immunodeficiency virus infection | 6 | 0 | ... | ... |
| Age, y | ||||
| 0-49 | ... | 21 (11.0) | 4 (8) | 1.22 (0.98-1.51)a |
| 50-59 | ... | 19 (9.9) | 0 (0) | |
| 60-69 | ... | 49 (25.7) | 13 (24) | |
| 70-79 | ... | 67 (35.1) | 16 (30) | |
| 80-89 | ... | 45 (23.6) | 18 (44) | |
| 90-99 | ... | 9 (4.7) | 2 (4) | |
| Total comorbidity score, OR (95% CI) | ... | ... | ... | 1.30 (1.15-1.47)a |
Abbreviations: CACI, Charlson-Age Comorbidity Index; OR, odds ratio; RR, relative risk; ellipses, data not applicable.
Statistically significant (P < .05).
Metastasis indicates neck metastasis of N2 or higher or distant metastasis.
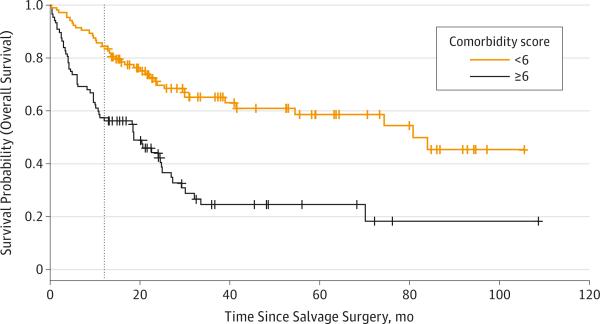
The mean CACI score was significantly higher for those with less than 1 year survival compared with those with survival of 1 year or more (8 vs 6, P = .001). To put this into perspective, a 59-year-old patient (1 point) with a history of myocardial infarction (1 point), congestive heart failure (1 point), and primary HNSCC (2 points) would have a CACI score of 5. A 65-year-old patient (2 points) with primary HNSCC (2 points) and neck metastasis (4 points) would have a CACI score of 8. To evaluate the CACI as an indicator of mortality, Kaplan-Meier survival curves were created after patients were divided into 2 categories: CACI score of 6 or higher (112 patients) vs less than 6 (79 patients), which reveals a significant difference in overall survival (P < .001) (Figure).
Figure. Kaplan-Meier Plot for Overall Survival of Patients After Salvage Surgery, Stratified by Comorbidity Score.
Patients with Charlson–Age Comorbidity Index (CACI) scores less than 6 had a survival advantage. Estimated median survival was 19 months (95% CI, 10-25 months) for the group with CACI scores of 6 or higher and 81 months (95% CI, 41 months to not reached) for the group with CACI scores less than 6. Number at risk was 104 for CACI score less than 6 and 87 for CACI score of 6 or higher.
Multivariable association of age and comorbidities, oncologic risk factors, and 1-year mortality are given in Table 3.
Table 3.
Multivariate Association of Age Comorbidities, Oncologic Risk Factors, and 1-Year Mortality
| Effect | OR (95% CI) | P Value |
|---|---|---|
| Total comorbidity scorea | 1.43 (1.16-1.76) | <.001b |
| N stage (N0/1 vs N2) | 0.70 (0.15-3.42) | .66 |
| Closure (invasive vs noninvasive) | 2.45 (0.74-8.05) | .14 |
| Adjuvant radiation (vs no adjuvant) therapy | 1.74 (0.46-6.68) | .30 |
| Adjuvant CRT (vs no adjuvant therapy) | 0.70 (0.09-5.25) | .52 |
| Tstage(T3 or 4 vs T1 or 2) | 2.34 (1.27-4.31) | .006b |
| Former or current smoker (vs never smoker) | 0.50 (0.14-1.81) | .29 |
| Disease free for <6 mo | 5.61 (1.78-16.7) | .003b |
Abbreviations: CRT, chemotherapy and radiation therapy; OR, odds ratio.
Total comorbidities were treated as a continuous variable (ie, for every unit increase in total Charlson-Age Comorbidity Index score, the odds of death within 1 year after salvage increased by 43%).
Statistically significant (P < .05).
Variables that were significant on univariate analysis were included in the initial multivariable analysis: total CACI score, primary T stage, primary N stage, primary free-flap or regional reconstruction, use of radiation as part of initial treatment modality, and DFI. For every unit increase in total CACI score, the odds of death within 1 year after salvage surgery increased by 43% (RR, 1.43; 95% CI, 1.16-1.76) while controlling for other factors. In addition to the total CACI score, primary T3 or T4 stage (RR, 2.34; 95% CI, 1.27-4.31) and DFI of less than 6 months (RR, 5.61; 95% CI, 1.78-16.7) remained as significant independent predictors of 1-year mortality.
Hospitalizations and discharge disposition within 1 year after salvage surgery are given in Table 4. Overall, our cohort had a mean (SD) of 1.7 (1.5) admissions, among which 1.2 (0.9) admissions were specific for otolaryngology, including index salvage surgery visit. Median total inpatient LOS within a 1-year period was 11 days (IQR, 3-22 days), among which a median of 9 days (IQR, 2-13 days) were specific to otolaryngology. Of the year after salvage surgery or time to death, a median of 3.3% of patients’ time was spent in the hospital (IQR, 1.1-8.8) as a whole. Fifty of 191 patients (26.2%) were discharged at least once to an SNF in a year.
Table 4.
The 1-Year Expectant Course of Hospitalization After Salvage Surgery
| Variable | No. (%) of Patients (N = 191) | No. of Inpatient Admissions Within 1 Year, Mean (SD) | Total Inpatient LOS, Median (IQR), d | Discharged to SNF Within 1 Year of Salvage Surgery, No. (%) | Life in the Year After Salvage Surgery Spent in the Hospital, Median (IQR), % |
|---|---|---|---|---|---|
| Survival, y | |||||
| ≥1 | 138 (72.3) | 1.4 (1.2) | 9 (2-16) | 25 (18.1) | 2.5 (0.5-4.4) |
| <1 | 53 (27.7) | 2.5 (1.7) | 19 (12-29) | 25 (47.2) | 17.3 (5.2-36.3) |
| P value | <.001a | <.001a | <.001a | <.001a | |
| Comorbidity score 6 cutoff | |||||
| <6 | 104 (54.5) | 1.5 (1.4) | 9 (2-16) | 18 (17.3) | 2.5 (0.5-4.4) |
| ≥6 | 87 (45.5) | 2.0 (1.5) | 15 (6-25.5) | 32 (36.8) | 5.8 (2.2-15.6) |
| P value | .01a | .003a | .002a | .001a |
Abbreviations: IQR, interquartile range; LOS, length of stay; SNF, skilled nursing facility.
Statistically significant (P < .05).
Patients were then stratified by their 1-year survival status. Those with less than 1-year survival had more total admissions within 1-year follow-up (mean, 2.5 vs 1.4; P < .001). Median LOS for total inpatient stay was longer for those with less than 1-year survival (19 vs 9 days, P < .001). A higher percentage of patients with less than 1 year survival had a history of an SNF discharge than those with survival of 1 year or more (47.2% vs 18.1%, P < .001). Notably, those with less than 1-year survival spent a higher percentage of their life in the year after surgery as inpatients than those who survived for 1 year or more after surgery (median, 17.3% vs 2.5%; P < .001).
Patients were also stratified for their CACI score. Those with a CACI score of 6 or greater had more total admissions within 1 year of follow-up (mean, 2.0 vs 1.5; P = .01), although admissions specific for otolaryngology were not statistically different (mean, 1.2 vs 1.2; P > .99). Median LOS for total inpatient stay was longer for those with a CACI score of 6 or greater (15 vs 9 days, P = .003), whereas otolaryngology-specific LOS was not different among patients with a comorbidity score of 6 or greater compared with those with a comorbidity score less than 6 (9.1 vs 8.5, P = .08). A higher percentage of patients had a history of SNF discharge within 1 year after the salvage surgery in those with a CACI score of 6 or greater than in those with CACI scores less than 6 (36.7% vs 17.3%, P = .002). Of note, those with comorbidity scores less than 6 spent less of their time in the year after the salvage surgery as inpatients (median, 2.5% vs 5.8%; P = .001).
Patients were also stratified for DFI and primary T stage. Those with a DFI of less than 6 months had more total admissions within 1-year follow-up (mean, 2.0 vs 1.5; P = .03) and spent more of their time in the year after the salvage surgery as inpatients (median, 4.4% vs 2.7%; P = .04). No difference was found in median LOS for total inpatient stay (12 vs 10 days, P = .07) and in the SNF discharge rate (25.3% vs 25.6%, P = .33). When stratified for primary T stage, those with primary T3 or T4 cancer had a longer LOS for total in patient stay (mean, 14 vs 10 days; P = .02) and spent more of their time in the year after the salvage surgery as inpatients (median, 5.5% vs 2.7%; P = .02), whereas the number of total admissions (1.9 vs 1.6, P = .18) and SNF discharge rate did not differ (20.3% vs 29.4%, P = .08).
Discussion
Although the 1-year survival rate of 72% after salvage surgery in our institution is acceptable, a subset of patients remain who do not benefit from salvage surgery and die within 1 year or spend a substantial amount of their remaining days in the hospital or a nursing home. Salvage surgery offers the best chance of achieving locoregional control and prolonged survival for patients with resectable recurrences and favorable performance status, yet it adds significant morbidity to all with potentially increased mortality in some patients.16 Patient- and tumor-specific factors affect survival outcome after salvage surgery in patients with recurrent HNSCC. The selection of patients who will most benefit from salvage surgery remains a challenging question. Our study considers disease and patient characteristics as factors that affect survival of patients and therefore may bridge this gap in knowledge.
Oncologic outcomes in our study align well with previous findings. Location of recurrence is also a known factor, with a previous study1 reporting laryngeal site as having the best post-salvage prognosis and hypopharyngeal site as having the worst outcome after surgery. Our cohort had similar patterns with regard to site-specific predictors of survival; patients with laryngeal HNSCC had the greatest 1-year postsalvage survival rate at 78%, and hypopharyngeal cancer was significantly worse at 56%. Matoscevic et al3 found that primary T stage was an important factor for survival across tumor subsites, with a higher stage shortening survival by a mean of 48 months. Schwartz et al4 found that patients with primary stage I or II had improved overall survival time compared with patients with later stages. Previous studies6,7 have found that the DFI affects survival after salvage surgery. One such study6 identified that a DFI of 9 months or less made a 20% difference in 5-year overall survival in their cohort. Another study7 found a DFI of 6 months or less significantly affected survival after salvage surgery. We similarly found that patients with a DFI of 6 months or less had worse survival outcomes after salvage surgery.
The Charlson Comorbidity Index has been used extensively to predict survival in HNSCC and other cancers.8,10,17 In a comparison of various comorbidity indexes in patients with HNSCC, there was no advantage in using a disease-specific index when compared with the Charlson Comorbidity Index to predict survival.18 The CACI used in this study incorporates age as a factor that contributes to comorbidity of patients, and we found that this modified index is a significant predictor of death within 1 year of salvage surgery. This finding has particular importance because the landscape of HNSCC is becoming more heterogeneous, with increasing numbers of elderly patients and young patients diagnosed as having HNSCC (2 groups with clearly different preoperative age and comorbidities).8,19 Therefore, the CACI represents a valid system to be used in our patient population to predict outcomes after salvage surgery. Those patients with a CACI score of at least 6 should be strongly counseled on their increased risk of mortality within the next year even with salvage surgery.
Comorbidity is unfortunately common within our patient population. We found that 59% of our patients undergoing salvage surgery had a CACI of 6 or higher. This finding is similar to the reported literature, with medical comorbidity in patients with head and neck cancer having been previously found to be common. Piccirillo and Vlahiotis11 concluded that the degree of comorbid disease burden was high among patients with HNSCC, second only to that of patients with lung and colorectal cancer. Another study20 found comorbidity to be a major risk factor for complications and mortality in patients with HNSCC, citing the severity of comorbid disease as directly related to worse outcomes. Our results reveal a similar link between comorbidity and worse outcomes after salvage surgery in patients with recurrent HNSCC after adjusting for other oncologic risk factors. This finding reveals that comorbidity as measured by the CACI is an independent risk factor for 1-year mortality after salvage surgery in this specific patient population. Also, not unexpectedly, those with higher CACIs had a significantly worse hospital course after salvage surgery: more total inpatient admissions, longer total LOS, higher chance of discharge to an SNF, and higher percentage of time spent in the hospital within 1 year after surgery. In a separate analysis, similar patterns were partly observed for patients with a DFI of less than 6 months (more total inpatient admissions and higher percentage of time spent in the hospital) and a history of primary T3 or T4 stage cancer (higher percentage of time spent in the hospital). This finding implies that patients with significant comorbidities with a larger primary disease burden have longer recovery time after salvage surgery, as would be expected.
This study is not free of limitations. Clinical stages for recurrent cancer were not included in the analysis. The follow-up information of a single academic institution was used to make a conclusion about the hospital course of the cohort, which could be underestimated for certain patients who frequent hospitals outside our system. Deduction of precise disease-specific benefit of salvage surgery may be difficult because disease-specific survival was not used as an outcome variable. However, we believe that any death, no matter the cause, within 1 year (overall survival) is an important measure to define the benefit of salvage surgery when comorbidities are included as a major predictive variable in the analysis.
There are significant differences in hospital course between patients who survived less than 1 year after salvage surgery compared with patients who survived 1 year or more. These results reveal potentially costly consequences of surgical salvage for those who did not benefit from salvage surgery. Patients with less than 1-year survival had longer total LOS, higher number of total admissions, and a higher chance of being discharged to an SNF leading up to their death. Of note, they spent nearly 20% of their time in the hospital between salvage surgery and death. These findings suggest a poor quality of life among those patients who die within 1 year after salvage surgery. On the basis of our results and our findings with regard to oncologic and comorbidity risk factors, a surgeon should consider palliative care options for those patients with high comorbidity scores, high primary T stage, and short DFI (<6 months). This information could prove valuable in helping to counsel patients and their families facing recurrent HNSCC and the subsequent management decisions.
Conclusions
Most patients will survive at least 1 year after surgery for recurrent HNSCC, but many will not. Careful selection of patients for salvage surgery for recurrent HNSCC is essential for optimizing outcomes after salvage surgery and decreasing short-term patient mortality. Along with certain oncologic factors, such as primary T3 or T4 stage and short DFI, comorbidity and age as measured by the CACI score should be taken into consideration when selecting patients for salvage surgery treatment. The CACI score can be a useful adjunct to preoperative evaluation and patient counseling. The treatment decision for aggressive therapy must ultimately justify the risks, morbidity, and high economic costs that are associated with it.
Acknowledgments
Funding/Support: This work was partially funded by grant UL1 TL1TR000005 from the University of Pittsburgh Clinical Scientist Training Program and Clinical and Translational Science Institute (Mr Kim).
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
Author Contributions: Mr Kim had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: J. Kim, S. Kim, Albergotti, Johnson, Gildener-Leapman.
Acquisition, analysis, or interpretation of data: J. Kim, S. Kim, Albergotti, Choi, Kaplan, Abberbock, Gildener-Leapman.
Drafting of the manuscript: J. Kim, Choi, Abberbock.
Critical revision of the manuscript for important intellectual content: J. Kim, S. Kim, Albergotti, Kaplan, Johnson, Gildener-Leapman.
Statistical analysis: J. Kim, Choi, Kaplan, Abberbock.
Obtained funding: J. Kim, S. Kim.
Administrative, technical, or material support: Albergotti, Kaplan.
Study supervision: S. Kim, Albergotti, Johnson, Gildener-Leapman.
Conflict of Interest Disclosures: None reported.
Previous Presentation: This study was presented at the Annual Meeting of the American Head and Neck Society; April 22, 2015; Boston, Massachusetts.
REFERENCES
- 1.Zafereo ME, Hanasono MM, Rosenthal DI, et al. The role of salvage surgery in patients with recurrent squamous cell carcinoma of the oropharynx. Cancer. 2009;115(24):5723–5733. doi: 10.1002/cncr.24595. [DOI] [PubMed] [Google Scholar]
- 2.Wong LY, Wei WI, Lam LK, Yuen AP. Salvage of recurrent head and neck squamous cell carcinoma after primary curative surgery. Head Neck. 2003;25(11):953–959. doi: 10.1002/hed.10310. [DOI] [PubMed] [Google Scholar]
- 3.Matoscevic K, Graf N, Pezier TF, Huber GF. Success of salvage treatment: a critical appraisal of salvage rates for different subsites of HNSCC. Otolaryngol Head Neck Surg. 2014;151(3):454–461. doi: 10.1177/0194599814535183. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz GJ, Mehta RH, Wenig BL, Shaligram C, Portugal LG. Salvage treatment for recurrent squamous cell carcinoma of the oral cavity. Head Neck. 2000;22(1):34–41. doi: 10.1002/(sici)1097-0347(200001)22:1<34::aid-hed6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Kowalski LP. Results of salvage treatment of the neck in patients with oral cancer. Arch Otolaryngol Head Neck Surg. 2002;128(1):58–62. doi: 10.1001/archotol.128.1.58. [DOI] [PubMed] [Google Scholar]
- 6.Stell PM. Time to recurrence of squamous cell carcinoma of the head and neck. Head Neck. 1991;13(4):277–281. doi: 10.1002/hed.2880130403. [DOI] [PubMed] [Google Scholar]
- 7.Koo BS, Lim YC, Lee JS, Choi EC. Recurrence and salvage treatment of squamous cell carcinoma of the oral cavity. Oral Oncol. 2006;42(8):789–794. doi: 10.1016/j.oraloncology.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Sikora AG, Toniolo P, DeLacure MD. The changing demographics of head and neck squamous cell carcinoma in the United States. Laryngoscope. 2004;114(11):1915–1923. doi: 10.1097/01.mlg.0000147920.66486.bc. [DOI] [PubMed] [Google Scholar]
- 9.Kwok AC, Semel ME, Lipsitz SR, et al. The intensity and variation of surgical care at the end of life: a retrospective cohort study. Lancet. 2011;378(9800):1408–1413. doi: 10.1016/S0140-6736(11)61268-3. [DOI] [PubMed] [Google Scholar]
- 10.Bøje CR, Dalton SO, Primdahl H, et al. Evaluation of comorbidity in 9388 head and neck cancer patients: a national cohort study from the DAHANCA database. Radiother Oncol. 2014;110(1):91–97. doi: 10.1016/j.radonc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Piccirillo JF, Vlahiotis A. Comorbidity in patients with cancer of the head and neck: prevalence and impact on treatment and prognosis. Curr Oncol Rep. 2006;8(2):123–129. doi: 10.1007/s11912-006-0047-z. [DOI] [PubMed] [Google Scholar]
- 12.Boruk M, Chernobilsky B, Rosenfeld RM, Har-El G. Age as a prognostic factor for complications of major head and neck surgery. Arch Otolaryngol Head Neck Surg. 2005;131(7):605–609. doi: 10.1001/archotol.131.7.605. [DOI] [PubMed] [Google Scholar]
- 13.Ouellette JR, Small DG, Termuhlen PM. Evaluation of Charlson-Age Comorbidity Index as predictor of morbidity and mortality in patients with colorectal carcinoma. J Gastrointest Surg. 2004;8(8):1061–1067. doi: 10.1016/j.gassur.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 14.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 15.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 16.Ho AS, Kraus DH, Ganly I, Lee NY, Shah JP, Morris LG. Decision making in the management of recurrent head and neck cancer. Head Neck. 2014;36(1):144–151. doi: 10.1002/hed.23227. [DOI] [PubMed] [Google Scholar]
- 17.Land LH, Dalton SO, Jørgensen TL, Ewertz M. Comorbidity and survival after early breast cancer: a review. Crit Rev Oncol Hematol. 2012;81(2):196–205. doi: 10.1016/j.critrevonc.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Piccirillo JF, Spitznagel EL, Jr, Vermani N, Costas I, Schnitzler M. Comparison of comorbidity indices for patients with head and neck cancer. Med Care. 2004;42(5):482–486. doi: 10.1097/01.mlr.0000124254.88292.a1. [DOI] [PubMed] [Google Scholar]
- 19.Toner M, O'Regan EM. Head and neck squamous cell carcinoma in the young: a spectrum or a distinct group? part 2. Head Neck Pathol. 2009;3(3):249–251. doi: 10.1007/s12105-009-0137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrier MB, Spuesens EB, Le Cessie S, Baatenburg de Jong RJ. Comorbidity as a major risk factor for mortality and complications in head and neck surgery. Arch Otolaryngol Head Neck Surg. 2005;131(1):27–32. doi: 10.1001/archotol.131.1.27. [DOI] [PubMed] [Google Scholar]