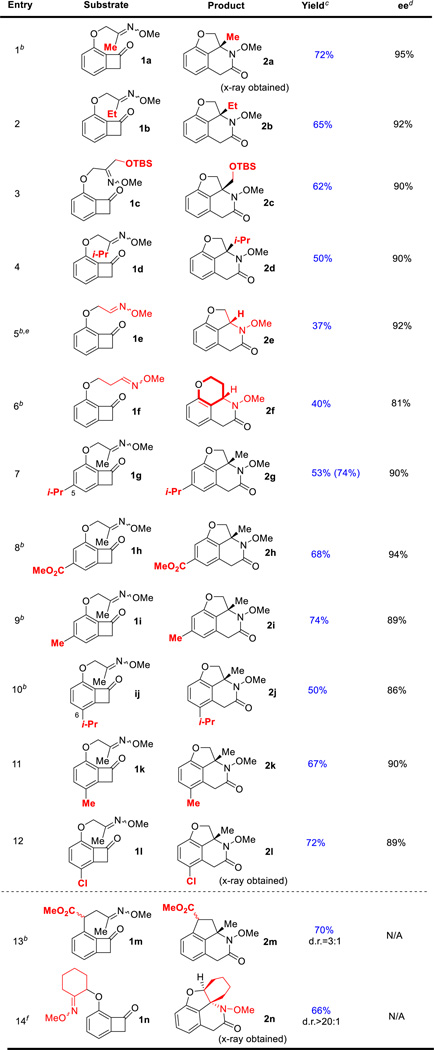
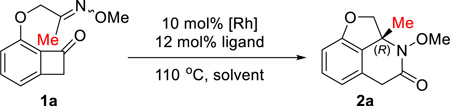
Abstract
Herein we describe the first enantioselective Rh-catalyzed carboacylation of oximes (imines) via C–C activation. In this transformation, the benzocyclobutenone C1–C2 bond is selectively activated by a low valent rhodium catalyst and subsequently the resulting two Rh–C bonds add across a C=N bond, which provides a unique approach to access chiral lactams. A range of polycyclic nitrogen-containing scaffolds were obtained in good yields with excellent enantioselectivity. Further derivatization of the lactam products led to a rapid entry to various novel fused heterocycles.
Graphical abstract
1. INTRODUCTION
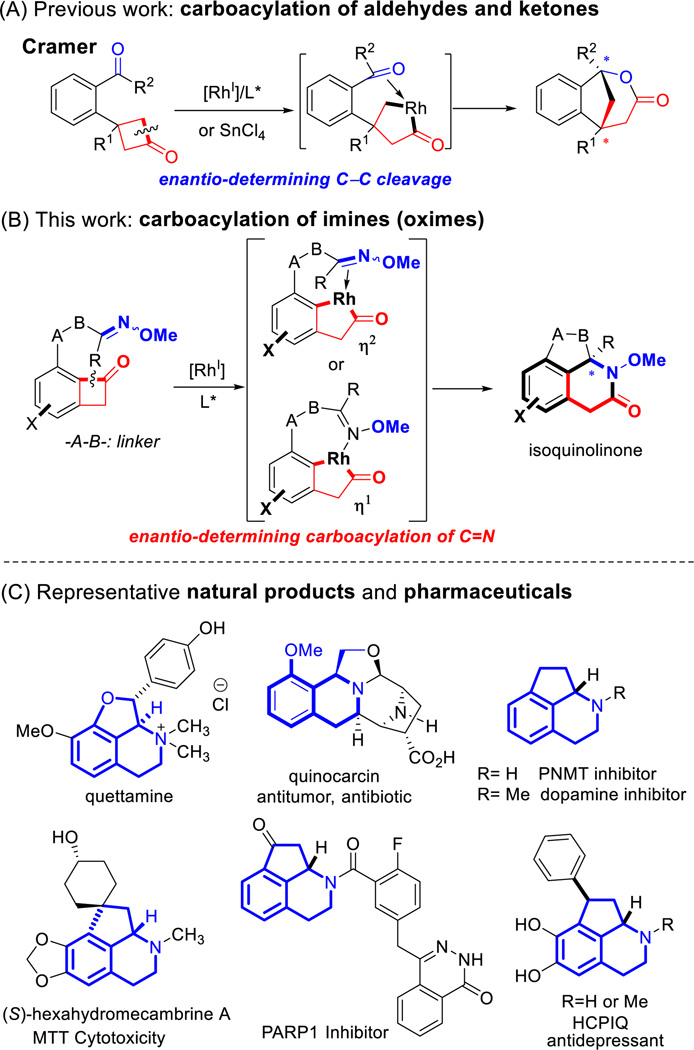
Transition metal-catalyzed C–C σ-bond cleavage of cyclic compounds followed by 2π-insertion has recently emerged as an attractive approach for preparing various ring systems.1 These methods generally operate at near pH and redox neutral conditions, and usually in a highly atom-economical fashion. However, the scope of the unsaturated 2π-units that can undergo such a “Cut & Sew” sequence2 have been primarily restricted to non-polar carbon-carbon multiple bonds, such as alkenes, alkynes and 1,3-dienes.1o Pioneering work by Cramer and coworkers demonstrated the first and asymmetric example of Rh-catalyzed carboacylation of aldehydes and ketones via enantiotopic C–C activation of cyclobutanones to access bridged lactones (Scheme 1A).3 Recently, the same group showed the same transformation can also be catalyzed by Lewis acids.4
 |
(1) |
Scheme 1.
Carboacylation of C=X Bonds via Transition- Metal-Catalyzed C–C Activation.
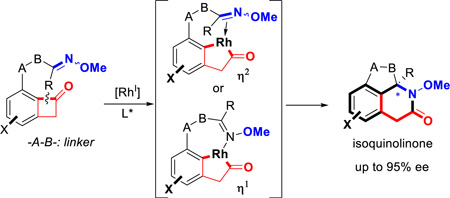
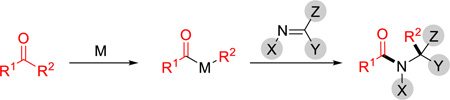
Stimulated by the pivotal role of amide-bond formation in organic synthesis and the pharmaceutical importance of nitrogen-containing heterocycles, we have been fascinated by the transition metal-catalyzed carboacylation of C=N bonds. Initiated by oxidative addition into ketone α C–C bonds, the resulting two M–C bonds, including one M–acyl bond, can add across an imine C=N bond to form an amide (eq 1), which, to the best of our knowledge, has not been reported previously. Elegant work by Chi and coworkers, involving an organocatalyst-promoted ring-opening of cyclobutenones followed by a formal enantioselective hetero-Diels-Alder reaction with sulfonyl and isatin imines, represents the closest example.5 Given the importance of optically enriched lactam moieties in bioactive compounds,6 herein a Rh-catalyzed enantioselective intramolecular carboacylation of oximes (imines) is described via C–C activation of benzocylcobutenones7,8,9 to access chiral fused lactams (Scheme 1B). Considering the prevalence of hydroisoquinolines and isoquinolinones in natural products and pharmaceuticals,10 this method also provides a unique entry to these scaffolds (Scheme 1C).
Previously, we have demonstrated the C1–C2 bond of benzo-cyclobutenones can be selectively cleaved to allow subsequent intramolecular insertion of alkenes and alkynes.8 The challenges of developing enantioselective carboacylation of C=N double bonds are three-fold: a) unlike ketones/aldehydes, imines tend to undergo E/Z isomerization and exist as a mixture of geometric isomers, which may complicate the enantio-determining process; b) due to the Lewis basicity of the nitrogen, imines can coordinate with metals in either an η1 or η2 mode, which is a distinct feature from the olefin/alkyne insertion;11 (c) imine hydrolysis can be a competitive side reaction.
2. RESULTS AND DISCUSSION
2.1 Condition optimization
To explore the aforementioned challenges, we sought the use of oximes as an imine equivalent due to their bench stability and the ease of cleavage of N–O bond for introducing various substituents on the nitrogen (vide infra, eq 2 and Scheme 2). Consequently, benzocyclobutenone 1a containing a ketoxime group with variable E/Z ratios (1:1 to 4:1) was employed as the initial substrate, and various catalytic conditions were explored (Table 1). Due to the different nature between C=C and C=N bonds, the conditions with [Rh(cod)Cl]2 and dppb that previously worked best8a for olefin insertion only yielded a small amount of product (entry 1, Table 1). In contrast, through examining of various rhodium pre-catalysts, the corresponding cationic complex showed much higher reactivity (entry 2, Table 1). Regarding the solvent effect, THF was found to be optimal, and 1,4-dioxane also provided a reasonable yield (entries 2–5, Table 1). It is likely that both solvents can stabilize the active rhodium intermediates during the reaction via weak coordination. In addition, the pre-made and in situ generated rhodium complexes (with dppb ligand) exhibited similar reactivity (entry 6, Table 1). Meanwhile, a survey of the counter-ion effect indicated tetrafluoroborate is the best counter-ion for this reaction (Table S1). Hence, [Rh(cod)(CH3CN)2]BF4 was selected as the initial pre-catalyst for further investigation of the enantioselective transformation.
Scheme 2.
Synthetic Applications I
Table 1.
Selected Pre-Evaluation of Reaction Conditionsa
 | ||||
|---|---|---|---|---|
| Entry | Catalyst/Ligand | Solvent | Yieldb | er (R:S)c |
| 1 | [Rh(cod)CI]2/dppb | THF | <20% | N/A |
| 2d | [Rh(cod)dppb]BF4 | THF | 58% (66%) | N/A |
| 3d | [Rh(cod)dppb]BF4 | 1,4-dioxane | 40% (60%) | N/A |
| 4d | [Rh(cod)dppb]BF4 | PhCI | 22% (47%) | N/A |
| 5d | [Rh(cod)dppb]BF4 | Toluene | 7% (8%)f | N/A |
| 6e | [Rh(cod)(CH3CN)2]BF4/dppb | THF | 57% | N/A |
| 7 | [Rh(cod)(CH3CN)2]BF4/(R)-DTBM-SEGPHOS | THF | 23% | 38.5:61.5 |
| 8 | [Rh(cod)(CH3CN)2]BF4/(R)-xyl-SEGPHOS | THF | 54% | 7:93 |
| 9 | [Rh(cod)(CH3CN)2]BF4/(S,S)-DIOP | THF | 54% | 51.6:48.4 |
| 10 | [Rh(cod)(CH3CN)2]BF4/(R)-SYNPHOS | THF | 39% | 25:75 |
| 11 | [Rh(cod)(CH3CN)2]BF4/(S)-BINAP | THF | 20% | 78:22 |
| 12 | [Rh(cod)(CH3CN)2]BF4/(R)-tol-BINAP | THF | 50% | 16:84 |
| 13 | [Rh(cod)(CH3CN)2]BF4/(R)-xyl-BINAP | THF | 72% | 4:96 |
| 14 | [Rh(cod)(CH3CN)2]BF4/(R)-H8-BINAP | THF | 71% | 10:90 |
| 15 | [Rh(cod)(CH3CN)2]BF4/(R)-xyl-H8-BINAP | THF | 52% | 6:94 |
| 16 | [Rh(cod)(CH3CN)2]BF4/(R)-xyl-MeO-BIPHEP | THF | 60% | 8:92 |
| 17 | [Rh(cod)(CH3CN)2]BF4/(R)-SDP | THF | 32% | 97:3 |
| 18 | [Rh(cod)(CH3CN)2]BF4/(R)-xyl-SDP | THF | 46% (63%) | 99.5:0.5 |
 | ||||
Unless otherwise mentioned, the reaction was run with 10 mol % rhodium complex (based on the metal) and 12 mol % ligand on a 0.1 mmol scale at 110 °C for 48 h; numbers in parenthesis are yields based on recovered starting material (brsm).
Isolated yield.
Determined by chiral HPLC.
110 °C for 12 h and then 130 °C for 24 h
130 °C.
NMR yield using mesitylene as the internal standard.
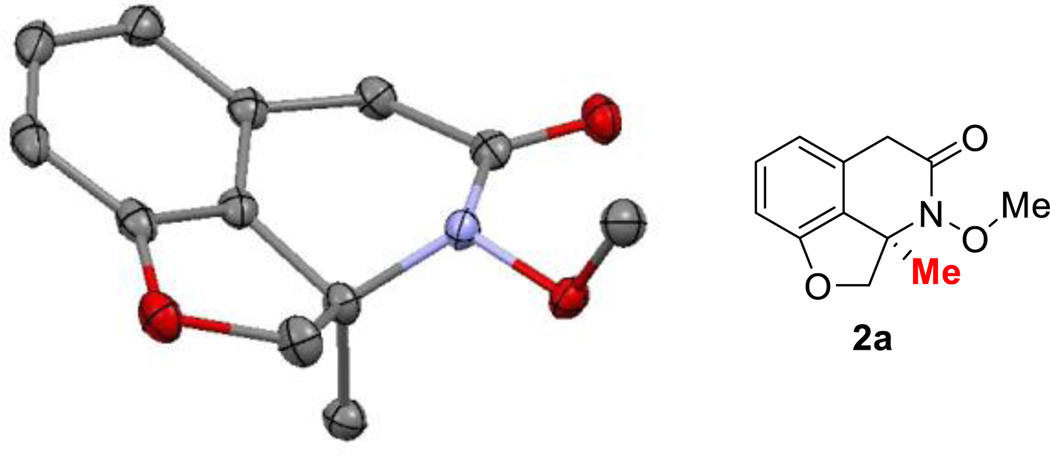
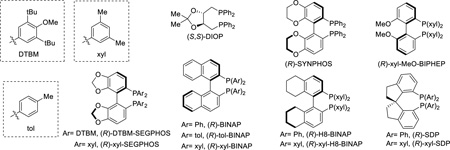
A range of chiral bi-dentate phosphine ligands were examined. DTBM-SEGPHOS and DIOP, which previously gave excellent enantioselectivity8b for the olefin insertion, only resulted in low yields and poor enantioselectivity (entries 7 and 9, Table 1). Surprisingly, xylyl-substituted SEGPHOS provided 86% ee (entry 8, Table 1). Subsequently, ligands with different backbones, such as SYNPHOS, BINAP, H8-BINAP and MeO-BIPHEP, were evaluated (entries 10–16, Table 1). Again, the xylyl-based ligands showed significantly higher enantioselectivity than their phenyl and tolyl analogues. In particular, good yield (72%) and excellent ee (92%) can be obtained with xyl-BINAP (entry 13, Table 1). While efforts to further improve the enantioselectivity using xyl-BINAP remained unfruitful, the xyl-SDP ligand, first developed by Zhou and coworkers,12 was found to give almost perfect enantioselectivity (99% ee) (entry 18, Table 1). Such a high enantioselectivity of this transformation is remarkable, because it suggested that, although involving C–N formation, both E and Z isomers of the oxime substrate can be converted to the same enantiomer of the product. The absolute configuration (the R isomer) was confirmed by the micro-focused X-ray crystallography (Figure 1).
Figure 1.
Crystal structure of compound 2a at 50% probability level with absolute stereochemistry, Hydrogen atoms are omitted for clarity
The enantioselective version of the reaction was further optimized with xyl-SDP as the ligand (Table 2). Changing the solvent from THF to 1,4-dioxane slightly increased the yield (entry 2, Table 2) and employing [Rh(cod)2]BF4 as the pre-catalyst gave a cleaner reaction (entry 3, Table 2). In addition, adding the catalyst in two portions also improved the yield (entry 4, Table 2). How-ever, the reaction yield remained moderate despite intensive conditions screened using xyl-SDP ligand alone (Table S2). The impressively high enantioselectivity with the rigid SDP ligands suggested a well-controlled transition state, but the moderate yields indicated the stability of the catalyst could be an issue. Efforts of adding a mono-dentate ligand, such as PPh3 or P(C6F5)3,13 to stabilize the catalyst intermediate remained unsuccessful due to side reactions triggered by these rhodium-mono-dentate-phosphine complexes (Table S2).
Table 2.
Studies of the Mixed-ligand Conditions and Control Experimentsa
 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst/Ligand | Additives | Solvent | Yieldb | er(R:S)c |
| 1 | [Rh(cod)(CH3CN)2]BF4/(R)-xyl-SDP | none | THF | 46% (63%) | 99.5:0.5 |
| 2 | [Rh(cod)(CH3CN)2]BF4/(R)-xyl-SDP | none | 1,4-dioxane | 51% (85%) | 99:1 |
| 3 | [Rh(cod)2]BF4/(R)-xyl-SDP | none | 1,4-dioxane | 53% (90%) | 99:1 |
| 4d | [Rh(cod)2]BF4/(R)-xyl-SDP | none | 1,4-dioxane | 55% (85%) | 99:1 |
| 5 | [Rh(cod)2]BF4/(S)-xyl-BINAP | none | 1,4-dioxane | 79% | 96:4 |
| 6 | [Rh(cod)2]BF4/(R)-xyl-SDP : (S)-xyl-BINAP = 1:1 | none | 1,4-dioxane | 63% | 97:3 |
| 7 | [Rh(cod)2]BF4/(R)-xyl-SDP : (R)-xyl-BINAP = 1:1 | none | 1,4-dioxane | 75% | 45:55 |
| 8 | [Rh(cod)2]BF4/(R)-xyl-SDP : (rac)-xyl-BINAP = 1:1 | none | 1,4-dioxane | 74% | 66:34 |
| 9d | [Rh(cod)2]BF4/(R)-xyl-SDP : (S)-xyl-BINAP = 1:1 | none | 1,4-dioxane | 72% | 97.2:2.5 |
| 10d | [Rh(cod)(CH3CN)2]BF4/(R)-xyl-SDP: (S)-xyl-BINAP = 1:1 | none | 1,4-dioxane | 61% | 97:3 |
| 11d | [Rh(cod)2]BF4/(R)-xyl-SDP : (S)-xyl-BINAP = 2:1 | none | 1,4-dioxane | 60% | 98.5:1.5 |
| 12d | [Rh(cod)2]BF4/(R)-xyl-SDP : (S)-xyl-BINAP = 5:1 | none | 1,4-dioxane | 54% | 99:1 |
| variations from entry 9 | |||||
| 13 | w/o Rh catalysts | none | 1,4-dioxane | 0% | N/A |
| 14 | w/o ligand | none | 1,4-dioxane | 0% | N/A |
| 15 | w/o catalyst & ligand | none | 1,4-dioxane | 0% | N/A |
| 16 | w/o catalyst & ligand | 20 mol% ZnCI2 | 1,4-dioxane | 0% | N/A |
| 17 | w/o catalyst & ligand | 20 mol% AICI3 | 1,4-dioxane | 0% | N/A |
| 18 | w/o catalyst & ligand | 20 mol% B(C6F5)3 | 1,4-dioxane | 0% | N/A |
Unless otherwise mentioned, the reaction was run with 10 mol % rhodium complex (based on the metal) and 12 mol % ligand on a 0.1 mmol scale at 110 °C for 48 h; numbers in parenthesis are yields based on recovered starting material (brsm).
Isolated yield.
Determined by chiral HPLC.
Rhodium complex (5 mol%) and mixed ligands (6 mol%) were added initially; the reaction mixture was stirred at 110 °C for 24 h before another portion of the same catalyst was added.
However, instead of using xyl-SDP (12 mol%) alone, when 6 mol% xyl-BINAP and 6 mol% xyl-SDP were used together, a significantly higher yield (72%) and excellent ee (95%) were obtained (entry 9, Table 2). It is worth noting that the R enantiomers of xyl-BINAP and xyl-SDP gave opposite enantioselectivity (entry 7, Table 2), thus the (S)-xyl-BINAP was coupled with (R)-xyl-SDP. We hypothesized that, while giving lower enantioselectivity than xyl-SDP, xyl-BINAP has a higher catalytic activity and lifetime. Indeed, when an equimolar (R)-xyl- SDP and (R)-xyl-BINAP were used together, the major enantiomer of the product was dictated by the xyl-BINAP ligand (entry 7, Table 2). It was also reasonable to observe that only 32% ee (instead of ~50% ee if two ligands were equally efficient) was provided when (R)-xyl-SDP was used in combination with rac-xyl-BINAP (entry 8, Table 2). In addition, [Rh(cod)2]BF4 was confirmed to be a better pre-catalyst for the mixed ligand system (entry 10, Table 2). Although increasing the xyl-SDP/BINAP ratio further enhanced the ee (entries 11 and 12, Table 2), the yields were nevertheless compromised.
Further control experiments suggested that both rhodium and ligands were crucial for the success of the reaction (entries 13–15, Table 2). Finally, to examine whether this transformation could be catalyzed by Lewis acids alone, a number of Lewis acids were surveyed (entries 16–18, Table 2 and Table S2); however, none provided the desired product.
2.2 Substrate Scope
With the optimized conditions in hand (entry 9, Table 2), we next investigated the substrate scope. First, substitutes on the ketoxime with various steric properties all underwent the desired carboacylation giving excellent enantioselectivity (≥ 90% ee, Table 3). It is not surprising that when the steric bulk of the oxime substituent increased from methyl to ethyl and to isopropyl, the reactivity diminished and a higher temperature (130 °C) was required (entries 2–4, Table 3). Although the aldoxime substrate (1e) suffered from a competing β-H elimination issue and the lactam product (2e) was unstable at high temperatures, a moderate yield (37%) with an excellent ee (92%) can nevertheless be obtained by using xyl-SDP alone as the ligand. It is encouraging to observe that 6-membered rings can also be formed generating an interesting 6-6-6 fused lactam (entry 6, Table 3). Moreover, both electron-donating and withdrawing groups on the arene can be well tolerated (entries 7–12, Table 3). In particular, both C5 and C6-substituted benzocyclobutenones are competent substrates.
Table 3.
Substrate Scopea
Reaction conditions: [Rh(cod)2]BF4 (5 mol %), (R)-xyl-SDP (3 mol %), (S)-xyl-BINAP (3 mol%), 1,4-dioxane, 130 °C; another portion of the same catalyst was added after 24h.
Reaction temperature was 110 °C.
Isolated yield; numbers in the parenthesis are brsm yields.
Determined by chiral HPLC.
(R)-xyl-SDP (12 mol%) alone was used.
[Rh(CH3CN)2(cod)]BF4 (20 mol%) and (R)-xyl-BINAP (25 mol%) were used.
Next, we aimed at replacing the ether linker with a carbon-based one. Substrate 1m with a pre-existing stereocenter underwent smooth transformation to give the fused lactam in 70% yield with 3:1 d.r. (entry 13, Table 3). Finally, the substrate containing a cyclic oxime was examined for this transformation (entry 14, Table 3). We were concerned that the relatively rigid conformation of the six-membered ring with a fixed orientation would hinder the carboacylation process. To our delight, with a higher catalyst loading the desired tetracyclic scaffold containing two adjacent stereocenters can nonetheless be provided in a good yield and excellent diastereoselectivity (>20:1 d.r.). The structure of product 2n was confirmed by both 2D-NMR and X-ray diffraction analysis.
2.3 Synthetic Applications
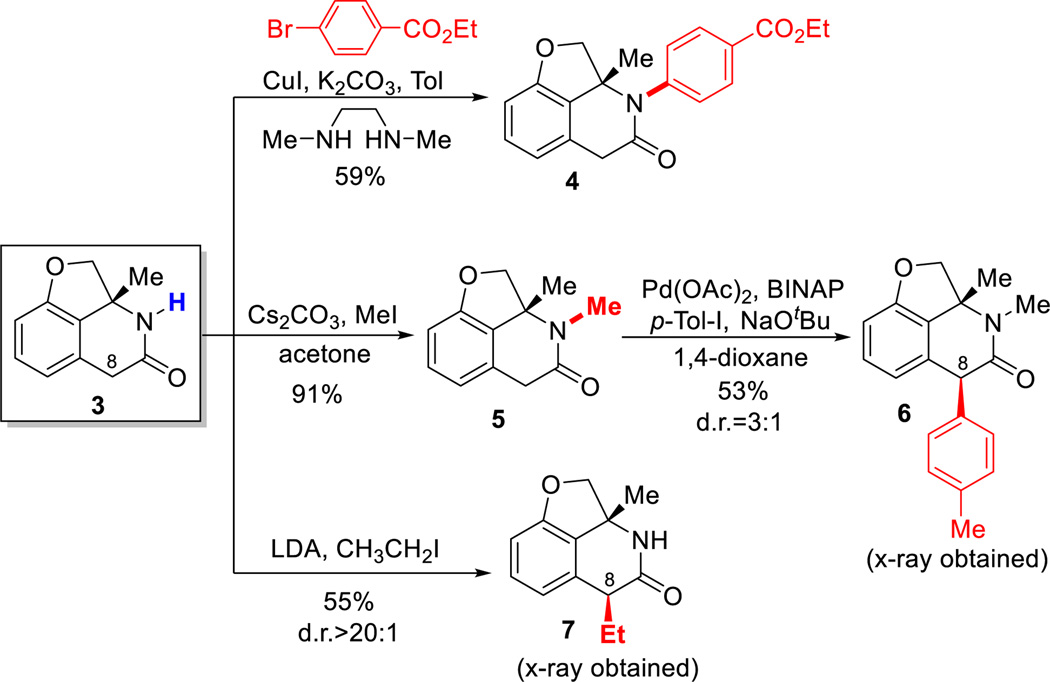
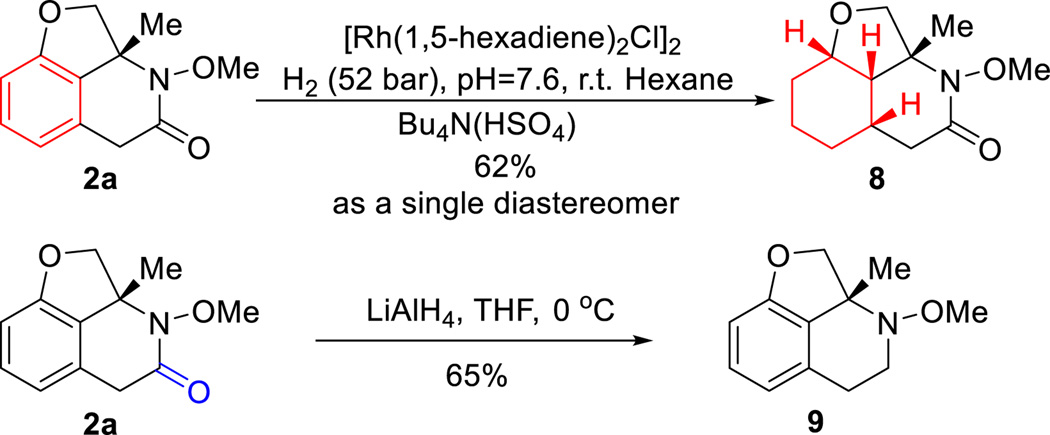
One advantage of using oximes as the C=N coupling partner is that the O–N bond can be easily cleaved using various reductants.14 For example, treatment of lactam 2a with Mo(CO)6 provided the free amide in 86% yield (eq 2). N-arylated and alkylated lactams can be conveniently obtained under cross-coupling15 and SN2 conditions (Scheme 2). While the benzocyclobutenone substrates with a C8-substituent were not reactive under carboacylation conditions, arylation and alkylation at the C8-position can be efficiently achieved through post functionalization. In particular, complete diastereoselectivity (7) was obtained for the alkylation reaction, suggesting an excellent convex-face-controlled situation. The relative stereochemistry was unambiguously confirmed by X-ray diffraction analysis.
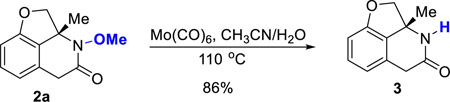 |
(2) |
Furthermore, a new saturated scaffold can be efficiently constructed using a mild Rh-catalyzed hydrogenation protocol.16 Two interesting features should be noted: 1) the reaction gives a perfect diastereoselectivity; 2) the N-OMe bond and the amide moiety remained intact after the reaction. In addition, a complementary LiAlH4 reduction smoothly provided the corresponding N-OMe piperidine.17
3. CONCLUSIONS
In summary, we have developed a highly enantioselective Rh-catalyzed carboacylation of oxime C=N bonds via C–C activation. Using this method, unique polycyclic lactam scaffolds can be efficiently accessed from benzocyclobutenone-coupled oximes. The reaction conditions do not use a strong acid or base, and are overall redox neutral. High enantioselectivity can be achieved despite using a mixture of the E/Z isomers of the oximes. Considering the novelty of these structures, the potential pharmaceutical applications of the fused heterocyclic products are being investigated. Moreover, given the importance of amide-bond formation, this catalytic asymmetric C–C activation method should also have broad implications beyond this work. Detailed mechanistic studies and expansion of the reaction scope to other 2π-insertion reactions are ongoing in our laboratory.
4. EXPERIMENTAL SECTION
General Conditions for the Rh-catalyzed Carboacylation of C=N Bonds
In a nitrogen filled glove box, an 8 mL vial was charged with the benzocyclobutenone substrate (1a to 1n, 0.1 mmol), [Rh(cod)2]BF4 (2.1 mg, 0.005 mmol, 5 mol%), (R)-xyl-SDP (2.1 mg, 0.003 mmol, 3 mol%) and (S)-xyl-BINAP (2.2 mg, 0.003 mmol, 3 mol%) {or [Rh(cod)2]BF4 (2.1 mg, 0.005 mmol, 5 mol%), (R)-xyl-SDP (4.2 mg, 0.006 mmol, 6 mol%) for 1e; [Rh(CH3CN)2(cod)]BF4 (3.7 mg, 0.01 mmol, 10 mol %) and (R)-xyl-BINAP (9.2 mg, 0.0125 mmol, 12.5 mol%) for 1n}. After adding 2 mL 1,4-dioxane, the vial was capped and stirred at room temperature for 5 minutes. The solution was then maintained at 110 °C (1a, 1e, 1f, 1h, 1i, 1j, 1m) or 130 °C (1b, 1c, 1d, 1g, 1k, 1l, 1n) for 24h before another portion of the same catalyst were added. After the reaction was maintained at the same temperature for another 24h, it was cooled to room temperature and purified by silica gel flash chromatography (CAM stain was used to visualize the location of the sample on TLC plate).
Supplementary Material
Scheme 3.
Synthetic Applications II
Acknowledgments
We thank CPRIT, NIGMS (R01GM109054) and the Welch Foundation (F 1781) for research grants. G.D. is a Searle Scholar and Sloan fellow. We thank Dr. M. C. Young for proofreading the manuscript. We also thank Dr. V. Lynch and Dr. M. C. Young for X-ray structures. Johnson Matthey is acknowledged for a generous donation of Rh salts. We are grateful to Ms. V. Garza for her kind assistance on HPLC. Chiral Technologies is thanked for their generous donation of chiral HPLC columns.
Footnotes
ASSOCIATED CONTENT
Supporting Information Experimental procedures; spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interests.
REFERENCES
- 1.For selected reviews on C–C activation see: Jones WD. Nature. 1993;364:676. Murakami M, Ito Y. Top. Organomet. Chem. 1999;3:97. Rybtchinski B, Milstein D. Angew. Chem. Int. Ed. 1999;38:870. doi: 10.1002/(SICI)1521-3773(19990401)38:7<870::AID-ANIE870>3.0.CO;2-3. Jun C-H. Chem. Soc. Rev. 2004;33:610. doi: 10.1039/b308864m. Satoh T, Miura M. Top. Organomet. Chem. 2005;14:1. Necas D, Kotora M. Curr. Org. Chem. 2007;11:1566. Crabtree RH. Chem. Rev. 1985;85:245. Ruhland K. Eur. J. Org. Chem. 2012:2683. Korotvicka A, Necas D, Kotora M. Curr. Org. Chem. 2012;16:1170. Seiser T, Saget T, Tran DN, Cramer N. Angew. Chem. Int. Ed. 2011;50:7740. doi: 10.1002/anie.201101053. Murakami M, Matsuda T. Chem. Commun. 2011;47:1100. doi: 10.1039/c0cc02566f. Dermenci A, Coe JW, Dong G. Org. Chem. Front. 2014;1:567. doi: 10.1039/c4qo00053f. (m) C–C bond activation. Dong G, editor. Topics in Current Chemistry. Vol. 346. Berlin: Springer-Verlag; 2014. Chen F, Wang T, Jiao N. Chem. Rev. 2014;114:8613. doi: 10.1021/cr400628s. Souillart L, Cramer N. Chem. Rev. 2015;115:9410. doi: 10.1021/acs.chemrev.5b00138.
- 2.For two seminal works: South MS, Liebeskind LS. J. Am. Chem. Soc. 1984;106:4181. Murakami M, Itahashi T, Ito Y. J. Am. Chem. Soc. 2002;124:13976. doi: 10.1021/ja021062n.
- 3.Souillart L, Cramer N. Angew. Chem. Int. Ed. 2014;53:9640. doi: 10.1002/anie.201405834. [DOI] [PubMed] [Google Scholar]
- 4.Souillart L, Cramer N. Chem. Eur. J. 2015;21:1863. doi: 10.1002/chem.201406135. [DOI] [PubMed] [Google Scholar]
- 5.Li B-S, Wang Y, Jin Z, Zheng P, Ganguly R, Chi YR. Nature Comm. 2015;6:6207. doi: 10.1038/ncomms7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For examples of drugs containing a chiral lactam moiety see: St Georgiev V, Van Inwegen RG, Carlson P. Eur. J. Med. Chem. 1990;25:375. Mekonnen B, Weiss E, Katz E, Ma J, Ziffer H, Kyle DE. Biorg. Med. Chem. 2000;8:1111. doi: 10.1016/s0968-0896(00)00049-3. Ding Y-S, Lin K-S, Logan J, Benveniste H, Carter P. J. Neurochem. 2005;94:337. doi: 10.1111/j.1471-4159.2005.03202.x. Lin K-S, Ding Y-S. Biorg. Med. Chem. 2005;13:4658. doi: 10.1016/j.bmc.2005.04.062. Altomare C, Carotti A, Casini G, Cellamare S, Ferappi M, Gavuzzo E, Mazza F, Pantaleoni G, Giorgi R. J. Med. Chem. 1988;31:2153. doi: 10.1021/jm00119a016. Altomare C, Cellamare S, Carotti A, Casini G, Ferappi M, Gavuzzo E, Mazza F, Carrupt P-A, Gaillard P, Testa B. J. Med. Chem. 1995;38:170. doi: 10.1021/jm00001a022.
- 7.For a seminal mechanistic study on metal-mediated cleavage of benzocyclobutenone C𠄼 bonds see: Huffman MA, Liebeskind LS, Pennington WT. Organometallics. 1990;9:2194. Huffman MA, Liebeskind LS, Pennington WT. Organometallics. 1992;11:255. For a detailed computational mechanistic study of Rh-catalyzed C–C activation of benzocyclobutenones see: Lu G, Fang C, Xu T, Dong G, Liu P. J. Am. Chem. Soc. 2015;137:8274. doi: 10.1021/jacs.5b04691.
- 8.(a) Xu T, Dong G. Angew. Chem. Int. Ed. 2012;51:7567. doi: 10.1002/anie.201202771. [DOI] [PubMed] [Google Scholar]; (b) Xu T, Ko HM, Sagave NA, Dong G. J. Am. Chem. Soc. 2012;134:20005. doi: 10.1021/ja309978c. [DOI] [PubMed] [Google Scholar]; (c) Xu T, Savage NA, Dong G. Angew. Chem. Int. Ed. 2014;53:1891. doi: 10.1002/anie.201310149. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Xu T, Dong G. Angew. Chem. Int. Ed. 2014;53:10733. doi: 10.1002/anie.201404802. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Chen P-h, Xu T, Dong G. Angew. Chem. Int. Ed. 2014;53:1674. doi: 10.1002/anie.201310100. [DOI] [PubMed] [Google Scholar]
- 9.For selected examples involving 2π-insertion to related rhodacyclopen-tanones: references 2b, 3 and Matsuda T, Fujimoto A, Ishibashi M, Murakami M. Chem. Lett. 2004;33:876. Shaw MH, Me-likhova EY, Kloer DP, Whittingham WG, Bower JF. J. Am. Chem. Soc. 2013;135:4992. doi: 10.1021/ja401936c. Ko HM, Dong G. Nature Chemistry. 2014;6:739. doi: 10.1038/nchem.1989. Parker E, Cramer N. Organometallics. 2014;33:780. Souillart L, Parker E, Cramer N. Angew. Chem. Int. Ed. 2014;53:3001. doi: 10.1002/anie.201311009. Shaw MH, McCreanor NG, Whittingham WG, Bower JF. J. Am. Chem. Soc. 2015;137:463. doi: 10.1021/ja511335v. Shaw MH, Croft RA, Whittingham WG, Bower JF. J. Am. Chem. Soc. 2015;137:8054. doi: 10.1021/jacs.5b05215.
- 10.For examples of drugs containing a tetrahydroisoquinoline moiety see: Omar H, Hashim NM, Zajmi A, Nordin N, Abdelwahab SI, Azizan AHS, Hadi AHA, Ali HM. Molecules. 2013;18:8994. doi: 10.3390/molecules18088994. Zarga MA, Miana GA, Shamma M. Tetrahedron Lett. 1981;22:541. Grunewald GL, Sall DJ, Monn JA. J. Med. Chem. 1988;31:433. doi: 10.1021/jm00397a029. Kim JH, Ryu YB, Lee WS, Kim YH. Bioorg. Med. Chem. 2014;22:6047. doi: 10.1016/j.bmc.2014.09.004. Ye N, Chen CH, Chen T, Song Z, He JX, Huan XJ, Song SS, Liu Q, Chen Y, Ding J, Xu Y, Miao ZH, Zhang A. J. Med. Chem. 2013;56:2885. doi: 10.1021/jm301825t. Parraga J, Galan A, Sanz MJ, Cabedo N, Cortes D. Eur. J. Med. Chem. 2015;90:101. doi: 10.1016/j.ejmech.2014.11.009. For a comprehensive review of the chemistry and biology of the tetrahydroisoquinoline alkaloids see: Scott JD, Williams RM. Chem. Rev. 2002;102:1669. doi: 10.1021/cr010212u. For isoquinolinones with novel vasorelaxation activity see: Lin CH, Lin MS, Lin YH, Chen IM, Lin PR, Cheng CY, Tsai MC. Pharmacology. 2003;67:202. doi: 10.1159/000068402.
- 11.(a) Ittel SD, Johnson LK. Chem. Rev. 2000;100:1169. doi: 10.1021/cr9804644. [DOI] [PubMed] [Google Scholar]; (b) Milios CJ, Stamatatos TC, Perlepes SP. Polyhedron. 2006;25:134. [Google Scholar]
- 12.(a) Xie J-H, Zhou Q-L. Acc. Chem. Res. 2008;41:581. doi: 10.1021/ar700137z. [DOI] [PubMed] [Google Scholar]; (b) Xie J-H, Zhu S-F, Zhou Q-L. Chem. Rev. 2011;111:1713. doi: 10.1021/cr100218m. [DOI] [PubMed] [Google Scholar]; (c) Fan B-M, Xie J-H, Li S, Wang L-X, Zhou Q-L. Angew. Chem. Int. Ed. 2007;46:1275. doi: 10.1002/anie.200603533. [DOI] [PubMed] [Google Scholar]; (d) Liu S, Xie J-H, Wang L-X, Zhou Q-L. Angew. Chem. Int. Ed. 2007;46:7506. doi: 10.1002/anie.200702491. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Shi S-L, Niu D, Liu P, Buchwald SL. Science. 2015;349:62. doi: 10.1126/science.aab3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.For examples of reductive cleavage of N–O bonds: (a) using samarium(II) iodide: Myers RM, Langston SP, Conway SP, Abell C. Org. Lett. 2000;2:1349. doi: 10.1021/ol0055162. (b) using ruthenium: Fukuzawa H, Ura Y, Kataoka Y. J. Organomet. Chem. 2011;696:3643. (c) using zinc or molybdenum: Atobe M, Yamazaki N, Kibayashi C. J. Org. Chem. 2004;69:5595. doi: 10.1021/jo049517+. Ren Z, Schulz JE, Dong G. Org Lett. 2015;17:2696. doi: 10.1021/acs.orglett.5b01098.
- 15.For reviews on C–N bond formations: Muci AR, Buchwald SL. Topics in Curr. Chem. 2002;219:131. Hartwig JF. Acc. Chem. Res. 1998;31:852. Wolfe JP, Wagaw S, Marcoux JF, Buchwald SL. Acc. Chem. Res. 1998;31:805. Bariwal J, Eycken EV. Chem. Soc. Rev. 2013;42:9283. doi: 10.1039/c3cs60228a. Beletskaya I, Cheprakov A. Coord. Chem. Rev. 2004;248:2337.
- 16. Januszkiewicz KR, Alper H. Organometallics. 1983;2:1055. and reference 8b.
- 17.Wardrop DJ, Basak A. Org. Lett. 2001;3:1053. doi: 10.1021/ol015626o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.