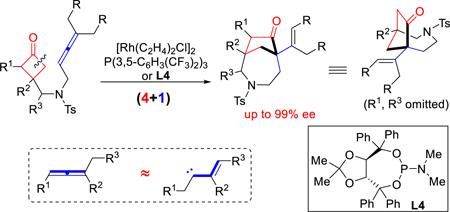
Abstract
Herein we describe a rhodium-catalyzed (4+1) cyclization between cyclobutanones and allenes, which provides a distinct [4.2.1]-bicyclic skeleton containing two quaternary carbon centers. The reaction involves C–C activation of cyclobutanones and employs allenes as a one-carbon unit. A variety of functional groups can be tolerated, and a diverse range of polycyclic scaffolds can be accessed. Excellent enantioselectivity can be obtained, which is enabled by a TADDOL-derived phosphoramidite ligand. The bridged bicyclic products can be further functionalized or derivatized though simple transformations.
Graphical abstract
1. INTRODUCTION
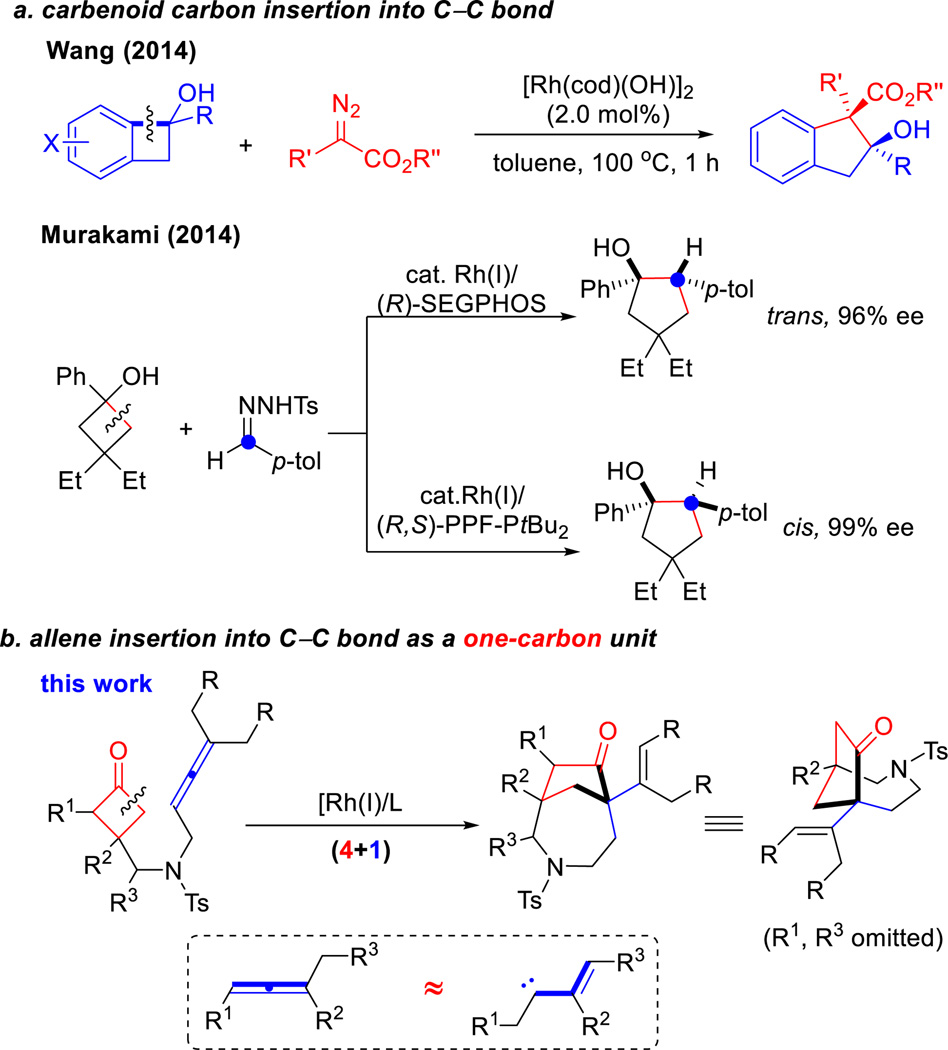
Complementary to cycloaddition reactions, transition metal-catalyzed C–C σ-bond cleavage of cyclic compounds followed by 2π-insertion offers a distinct strategy to access complex fused and bridged systems.1,2 Given the particular challenge for constructing bridged scaffolds,3 approaches have emerged based on C–C activation of four-membered ring ketones. In 1984, Liebeskind2a reported a Co-mediated intramolecular insertion of an alkyne into a benzocyclobutendione for preparing a bridged benzoquinone, and demonstrated its usage in the total synthesis of nanaomycin A. Exemplary work by Murakami and Ito described catalytic carboacylations of cyclobutenones with aryl alkenes for building [3.2.1] and [2.2.2] bicyclic compounds through rhodium (2002) and nickel catalysis (2006) respectively,2b,4 which can also be achieved asymmetrically.5 To circumvent the undesired decarbonylation pathway, we recently developed an intramolecular Rh–catalyzed Jun’s cofactor-assisted (4+2) coupling between cyclobutanones and alkenes to access various [3.3.1]-bridged products.6 In contrast to the rapid developments of alkene insertion into C–C σ-bonds, the corresponding process with allenes has not yet been reported. This would be a highly valuable transformation because an additional olefin moiety would be installed in the product serving as a handle for further functionalization. In this article, we describe an unexpected discovery of an intramolecular coupling between cyclobutanones and trisubstituted allenes leading to an unusual (4+1)7 (instead of (4+2)) transformation. This method provides various [4.2.1] and [3.2.1] bicycles and, particularly, a rapid entry to azepane-bridged scaffolds that are difficult to access with conventional approaches.
Allenes are commonly employed as a coupling partner in catalytic cycloadditions.8 However, they generally serve as a two or three-carbon unit,8,9 and are seldom considered as a one-carbon component in these transformations.10 Recently, insertion of a carbenoid carbon into a C–C single bond of benzocyclobutenols and cyclobutanols were demonstrated by Wang11 and Murakami12 independently. Here we show allenes can formally serve as a vinyl carbenoid13 equivalent for an intramolecular one-carbon ring expansion.
2. RESULTS AND DISCUSSION
2.1 Rhodium-catalyzed intramolecular (4+1) cyclization between cyclobutanones and allenes
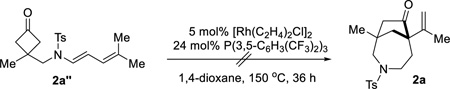
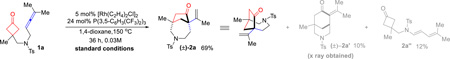
Allene 1a was employed as the initial substrate. Surprisingly, under the conditions previously optimized for the (4+2) addition with olefins (5 mol% [Rh(C2H4)2Cl]2, 24 mol% P(3,5-C6H3(CF3)2)3, and 100 mol% 3-methyl-2-aminopyridine), the expected 6-6 bridged bicycle ((±)-2a’) was only obtained in 8% yield; instead, an unusual 7-5 bridged molecule ((±)-2a) was isolated in 40% yield as the major product (Table 1, entry 2). This compound exhibits a number of interesting features: first, it has a [4.2.1] scaffold which should come from a (4+1) cyclization through cleavage of the cyclobutanone α C–C bond followed by insertion of the allene central carbon; second, it contains two quaternary carbon centers; third, it is rich in functional motifs including a ketone and an olefin that can offer further derivatization (vide infra, Scheme 3); lastly, the azepane moiety holds potential for pharmaceutical applications.14 Further optimization revealed that, in the absence of 3-methyl-2-aminopyridine but with 5 mol% of [Rh(C2H4)2Cl]2 and 24 mol% of P(3,5-C6H3(CF3)2)3, in 1,4-dioxane, compound (±)-2a can be formed in 69% yield (entry 1). The (4+2) addition ((±)-2a’, 10% yield) and allene isomerization (1,3-diene 2a’’, 12% yield) were observed as the minor reaction pathways.
Table 1.
Control Experimentsa
 | ||||
|---|---|---|---|---|
| Entry | Change from the standard conditions | Conversion | Yield of (±)-2a | Yield of (±)-2a' |
| 1 | none | >99% | 69% | 10% |
| 2 | with 100 mol% 3-methyl-2-aminopyridine | 85% | 40% | 8% |
| 3 | without [Rh(C2H4)2CI]2 | <1% | –d | – |
| 4 | without P(3,5-C6H3(CF3)2)3 | >99% | – | – |
| 5 | with 12 mol% P(3,5-C6H3(CF3)2)3 | >99% | 45% | 5% |
| 6 | with 48 mol% P(3,5-C6H3(CF3)2)3 | 60% | 35% | 7% |
| 7 | 12 mol% dppp instead of P(3,5-C6H3(CF3)2)3 | >99% | – | – |
| 8 | 24 mol% P(C6F5)3 instead of P(3,5-C6H3(CF3)2)3 | >99% | – | – |
| 9 | [Rh(cod)CI]2 instead of [Rh(C2H4)2CI]2 | >99% | 24% | 4% |
| 10 | [Rh(CO)2CI]2 instead of [Rh(C2H4)2CI]2 | >99% | 10% | – |
| 11 | [Rh(1,5-hexadiene)CI]2 instead of [Rh(C2H4)2CI]2 | >99% | 53% | 10% |
| 12 | THF instead of 1,4-dioxane | 50% | 29% | 3% |
| 13 | toluene instead of 1,4-dioxane | >99% | – | – |
| 14 | 130 °C instead of 150 °C | 37% | 27% | 6% |
| 15 | 110 °C instead of 150 °C | 21% | 19% | – |
| 16 | 110 °C but at 0.06 M | 15% | 14% | – |
| 17 | 110 °C but with 10 mol% [Rh(C2H4)2CI]2 and 48 mol% P(3,5-C6H3(CF3)2)3C |
>99% | 77% | 11% |
All yields and conversions were determined by crude 1H NMR using 1,1,2,2-tetrachloroethane as the internal standard.
The standard reactions were conducted with 1a (0.03 mmol), [Rh(C2H4)2Cl]2 (0.0015 mmol, 5 mol%), P(3,5-C6H3(CF3)2)3 (0.0072 mmol, 24 mol%), and 1,4-dioxane (1 mL) at 150 °C.
The reaction was run in 1,4-dioxane (2 mL) for 72 h.
Not determined.
Scheme 3.
Synthetic Applications
Control experiments were subsequently conducted to understand the role of each component (Table 1). In the absence of the Rh catalyst, no conversion was observed (entry 3). Without P(3,5-C6H3(CF3)2)3 the reaction gave full conversion, but led to a complex mixture of unidentifiable products (entry 4). Decreasing or increasing the ligand loading led to more decomposition or lower conversion (entries 5 and 6). P(3,5-C6H3(CF3)2)3 plays a key role in this reaction,15 and use of other ligands proved to be much less efficient. For example, bidentate ligands such as dppp, or a more electron-deficient monodentate phosphine, such as P(C6F5)3, only resulted in a complex mixture of unidentifiable products (entries 7 and 8). Other Rh(I) catalysts, such as [Rh(cod)Cl]2, [Rh(CO)2Cl]2, and [Rh(1,5-hexadiene)Cl]2 were less efficient and gave more isomerization products (such as 2a’’) (entries 9-11). Solvent effect was also surveyed: THF afforded the desired product albeit with a lower conversion (entry 12); in contrast, toluene gave complete decomposition of the starting material (entry 13). Decreasing the temperature to 130 °C or 110 °C resulted in lower conversion (entries 14 and 15). Interestingly, when the reaction was run at 110 °C, a higher selectivity for the (4+1) pathway was observed. Attempt to improve the conversion at 110 °C by increasing the reaction concentration was unsuccessful. It is likely that, according to a recent report by Wender and Houk,16 the reactivity of the Rh catalyst can be inhibited at high allene concentration, due to a competitive cyclometalation of the Rh with two allenes. Nevertheless, using a higher loading of the catalyst at a lower temperature (110 °C), 77% yield of the desired product ((±)-2a) was obtained with minimal olefin isomerization (entry 17).
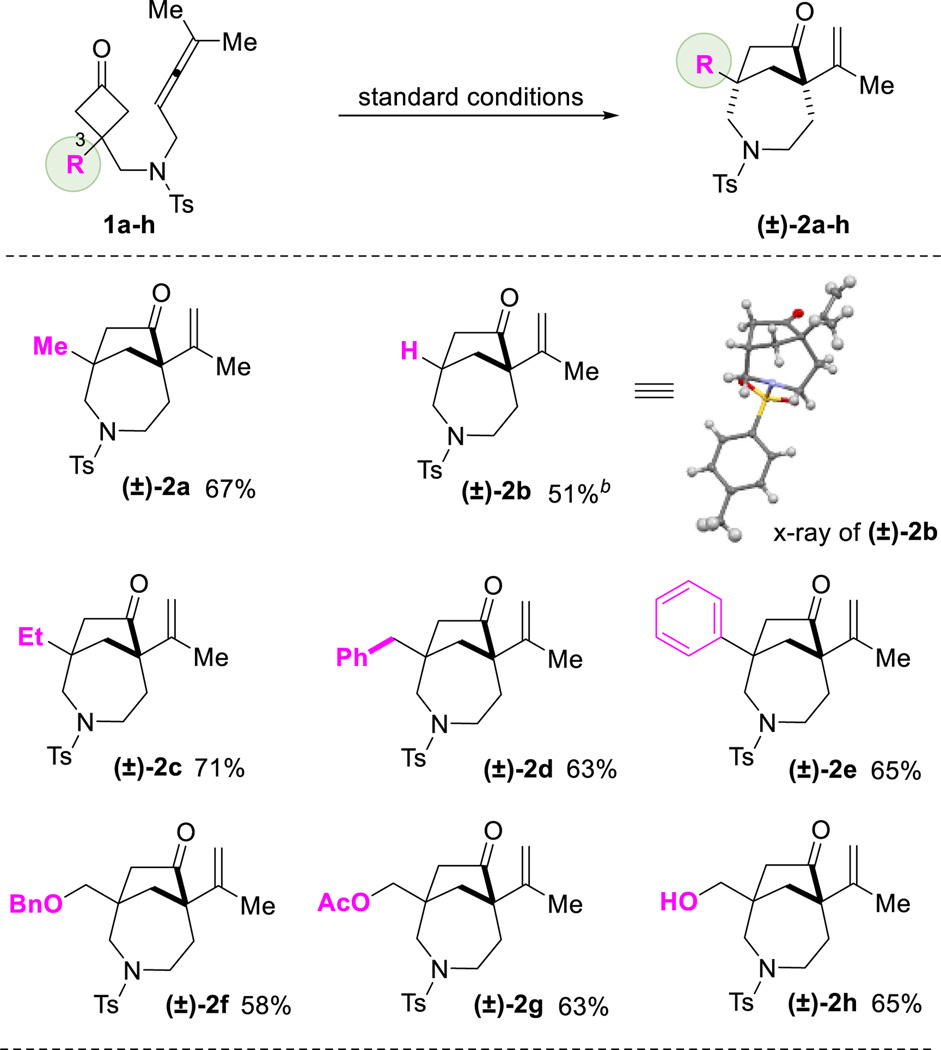
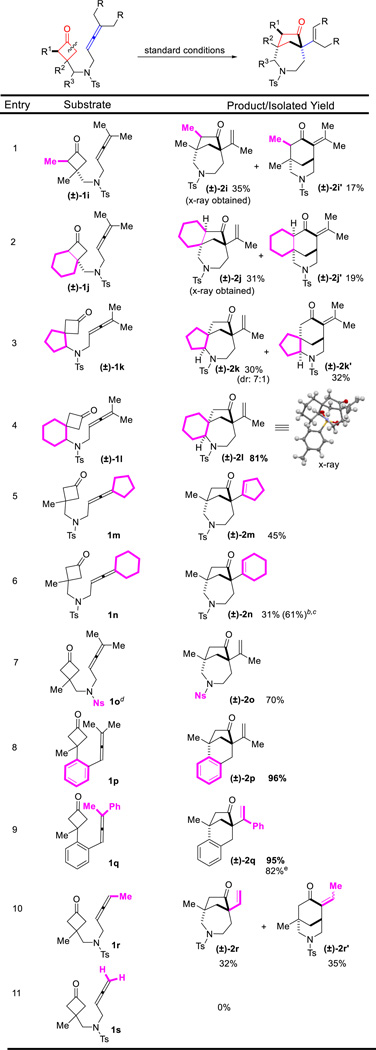
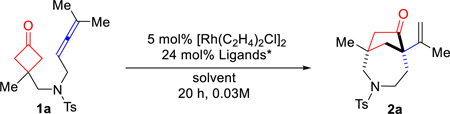
With the optimized conditions in hand, we next investigated the substrate scope. First, various substitutes at the C3 of cyclobutanones were found suitable for this reaction (Table 2). The substrate (1b) with a hydrogen substitution at the C3 position can potentially undergo β-hydrogen elimination after C–C cleavage of the cyclobutanone; however, it still afforded the desired product ((±)-2b). Besides methyl group ((±)-2a), other alkyls and aryl groups, such as ethyl ((±)-2c), benzyl ((±)-2d) and phenyl ((±)-2e) groups, were found compatible for this transformation. A high chemoselectivity was also observed: benzyl ethers ((±)-2f), esters ((±)-2g) and even unprotected primary alcohols ((±)-2h) can be tolerated.
Table 2.
Substrate Scope Ia
Each reaction was run on a 0.1 mmol scale using 5 mol% [Rh(C2H4)2Cl]2, 24 mol% P(3,5-C6H3(CF3)2)3, in 1,4-dioxane (3 mL) at 150 °C for 36 h. The (4+2) products were observed in 3–10% yield judged by crude 1H NMR. All yields are isolated yield.
The reaction was run with 10 mol% [Rh(C2H4)2Cl]2, 48 mol% P(3,5-C6H3(CF3)2)3, in 1,4-dioxane (8 mL) at 110 °C for 72 h.
In general, α-substituted cyclobutanones are challenging substrates for C–C activation due to the steric hindrance around the carbonyl group. To our delight, using allene as the coupling partner, α-methyl substituted cyclobutanone (1i) still provided the desired 7-5 bridged bicycle in 35% isolated yield as a single diastereomer, along with 17% of the (4+2) product. The x-ray structure of product (±)-2i clearly indicated that the less hindered C–C bond was selectively activated. In addition, the substrate with a fused cyclohexane ring (1j) exhibited similar reactivity and selectivity as 1i, leading to a complex tricycle system. Substrates 1k and 1l, containing a spirocyclic center, were also viable for this transformation. Interestingly, they showed vast different selectivity for the (4+1) versus (4+2) cyclization. While the cyclopentane-spiro substrate did not differentiate these two pathways, the cyclohexane one gave almost complete selectivity for the (4+1) product; the 6-7-5 fused/bridged scaffold ((±)-2l) was isolated in 81% yield. It is likely that the substrate conformation plays an important role in the (4+1) vs (4+2) selectivity. Not surprisingly, the (4+1) cyclization became more sluggish when increasing the steric bulk on the allene substituents. For example, cyclopentyl or cyclohexyl-fused allenes showed lower reactivity but still provided the desired products ((±)-2m and (±)-2n). Changing the protecting group on the nitrogen from Ts to 2-nitrobenzenesulfonyl (Ns) did not affect the reactivity ((±)-2o).17 Furthermore, substrates with an arene (all-carbon) linkage (1p and 1q) were found to be remarkable, providing the desired 6-5 bridged bicycle ((±)-2p and (±)-2q) in 96% and 95% yields respectively. In particular, substrate 1q with a phenyl/methyl-substituted allene (unsymmetrical allenes) can be efficiently transformed to the desired [3.2.1] bridged product. 1,3-Di-substituted allene, such as substrate 1r, can also react under the standard conditions to afford the desired 7-5 bridged bicycle, albeit with a poor (4+1) vs (4+2) selectivity. The exact reason for such a selectivity remains to be defined. In contrast, use of mono-substituted allene substrates, e.g. 1s, mainly resulted in a complex of dimeric products, which is consistent with the previous observation by Wender and Houk.16
2.2 Rhodium-catalyzed enantioselective intramolecular (4+1) cyclization between cyclobutanones and allenes for constructing chiral azepane-based scaffolds
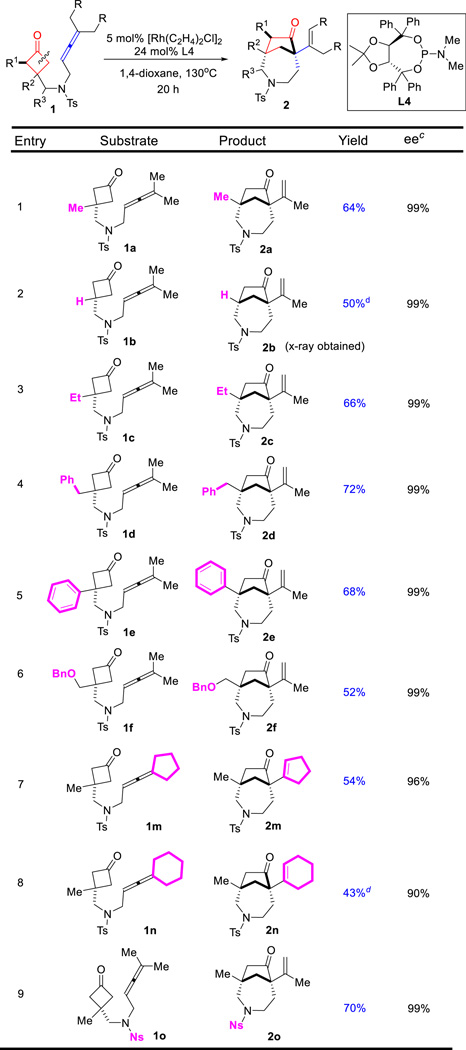
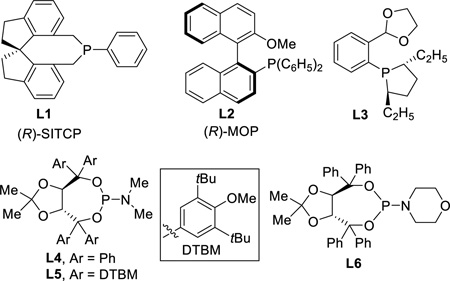
Optically pure azepane-based scaffolds are expected to be attractive for pharmaceutical applications.14 Next we continued to explore the enantioselective intramolecular coupling between cyclobutanones and allenes. Cyclobutanone 1a was again employed as the model substrate (Table 4). Given the inefficiency of bidentate ligands in this transformation (vide supra, Table 1), a range of chiral monodentate phosphine ligands were examined first. While (R)-SITCP showed a promising level of enantioselectivity, only 21% yield was observed (entry 1). On the other hand, the (R)-MOP and ligand L3 proved to be not effective (entries 2 and 3). Fruitful results were obtained when TADDOL-derived phosphoramidites18 were surveyed; in particular, excellent enantioselectivity was obtained with L4 ligand (entry 4). Surprisingly, the reaction is highly sensitive to the nitrogen substitution on the phosphine; replacing of the dimethylamino group with a 4-morpholinyl group (L6) led to no desired product (entry 6). After further examination of the solvent and temperature effects, we found 68% yield with 99% ee can be afforded with 5 mol% [Rh(C2H4)Cl]2 and 24 mol% of L4 in 1,4-dioxane at 130 °C (entry 11), which was used as the standard conditions for investigating the substrate scope. It is worthy to note that the enantioselective reaction can also be run at 110 °C with reasonably good yields (entries 10 and 12).
Table 4.
Selected Optimization for the Enantioselective Reactiona
| Entry | Ligand | Solvent | T[°C] | Yieldb | eec |
|---|---|---|---|---|---|
| 1 | L1 | 1,4-dioxane | 150°C | 21% | −60% |
| 2 | L2 | 1,4-dioxane | 150°C | 19% | 10% |
| 3 | L3 | 1,4-dioxane | 150°C | 0% | --e |
| 4 | L4 | 1,4-dioxane | 150°C | 54% | 98% |
| 5 | L5 | 1,4-dioxane | 150°C | 40% | 95% |
| 6 | L6 | 1,4-dioxane | 150°C | 0% | -- |
| 7 | L4 | THF | 150°C | 20% | -- |
| 8 | L4 | Toluene | 150°C | trace | -- |
| 9 | L4 | PhCI | 150°C | trace | -- |
| 10 | L4 | 1,4-dioxane | 110°C | 60% | 99% |
| 11 | L4 | 1,4-dioxane | 130°C | 68% | 99% |
| 12d | L4 | 1,4-dioxane | 110°C | 77% | 99% |
 | |||||
 | |||||
The reactions were conducted with 1a (0.03 mmol), [Rh(C2H4)2Cl]2 (0.0015 mmol, 5 mol%), ligands (0.0072 mmol, 24 mol%), and solvent (1 mL) for 20 h.
All yields and conversions were determined by crude 1H NMR using 1,1,2,2-tetrachloroethane as the internal standard.
Determined by HPLC analysis using a chiral stationary phase.
The reactions were conducted with 1a (0.03 mmol), [Rh(C2H4)2Cl]2 (0.003 mmol, 10 mol%), ligands (0.0144 mmol, 48 mol%), and solvent (2 mL) at 110°C for 36 h.
Not determined. (R)-SITCP = (11aR)-(+)-5,6,10,11,12,13-Hexahydro-5-phenyl-4H-diindeno[7,1-cd:1,7-ef]phosphocin, (R)-MOP = (R)-(+)-2-(Diphenylphosphino)-2′-methoxy-1,1′-binaphthyl.
With the optimized conditions in hand, the generality of this enantioselective (4+1) cyclization was next studied for constructing chiral [4.2.1] scaffolds (Table 5). To our delight, a range of substrates with different substitutes at the C3 position of cyclobutanones gave almost perfect enantioselectivity (Table 5). In particular, the substrate (1b) with a hydrogen substitution at the C3 position still provided the desired product (2b) with 99% ee. Increasing the steric bulkiness on the allene substituents diminished the yields, which is consistent with the observation in the racemic studies (vide supra, Table 3); nevertheless, excellent enantioselectivity can still be obtained. For example, cyclopentyl and cyclohexyl-fused allenes gave 96% ee and 90% ee separately (entries 7 and 8). In addition, use of 2-nitrobenzenesulfonyl (Ns) as the nitrogen protecting group did not affect the reactivity or the enantioselectivity (entry 9).
Table 5.
Substrate Scope of the Enantioselective (4+1) Cy clization for Preparing Chiral Azepane Scaffoldsa,b
Each reaction was run on a 0.1 mmol scale using 5 mol% [Rh(C2H4)2Cl]2, 24 mol% ligand L4, in 1,4-dioxane (3 mL) at 130 °C for 20 h. Unless otherwise mentioned, single diastereomers were isolated and the (4+2) products were observed in 3–10% yield judged by crude 1H NMR.
The absolute configurations of the (4+1) products were assigned based on the analogy to the one of compound 2b, which was determined by heavy-atom X-ray crystallography.
Determined by HPLC analysis using a chiral stationary phase.
With 10 mol% [Rh(C2H4)2Cl]2, 48 mol% ligand L4, in 1,4-dioxane (6 mL) at 110 °C for 36 h.
Table 3.
Substrate Scope IIa
Each reaction was run on a 0.1 mmol scale using 5 mol% [Rh(C2H4)2Cl]2, 24 mol% P(3,5-C6H3(CF3)2)3, in 1,4-dioxane (3 mL) at 150°C for 36 h. Unless otherwise mentioned, single diastereomers were isolated and the (4+2) products were observed in 3–10% yield judged by crude 1H NMR.
With 10 mol% [Rh(C2H4)2Cl]2, 48 mol% P(3,5-C6H3(CF3)2)3, in 1,4-dioxane (8 mL) at 110 °C for 72 h.
Number in parentheses is the yield based on recovered starting material.
Ns: 2-nitrobenzenesulfonyl.
2.5 mol% [Rh(C2H4)2Cl]2, 12 mol% P(3,5-C6H3(CF3)2)3 was used.
2.3 Mechanistic studies of (4+1) cyclization between cyclobutanones and allenes
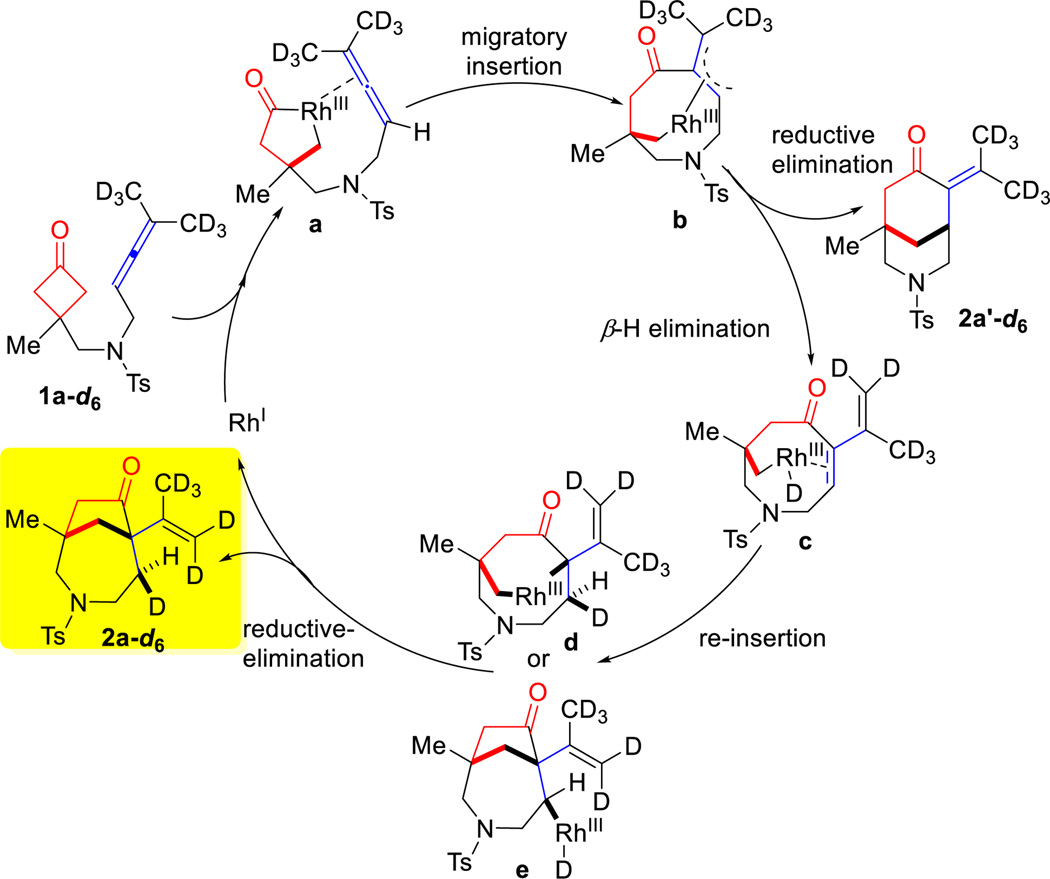
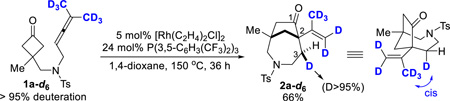
To gain mechanistic information about this (4+1) cyclization, the following two experiments were conducted. First, since 1,3-diene 2a’’ was found as the major by-product in this cyclization, one natural question is whether (±)-2a came from allene 1a or the isomerized diene (2a’’). When 2a’’ was treated under the standard coupling conditions, no (4+1) product was observed (Eq. 1). This result suggests that the (4+1) product directly comes from the allene precursor. Second, a deuterium-labeled substrate (1a-d6) was synthesized, in which two deuterated-methyl groups were installed on the allene. Treatment of allene 1a-d6 under the standard reaction conditions led to the desired product in 66% isolated yield with greater than 95% deuteration at the C3 position. According to the 2D NMR and NOESY experiments, the C3 deuterium is cis to the alkenyl side chain.
 |
(1) |
 |
(2) |
Based on the above experiments and previous studies on transition metal-catalyzed allene-mediated cycloadditions,8a,9a,d,f,i,10a the following plausible catalytic cycle was proposed (Scheme 2). The reaction is expected to start with insertion of the RhI into the cyclobutanone α C–C bond2b,19 to give a five-membered rhodacycle (a) likely with the allene group coordinated to the rhodium. This is followed by migratory insertion to afford a π-allyl intermediate (b),8a,9d,10a which can undergo at least two different pathways. If direct reductive elimination occurs with intermediate b, the (4+2) product, [3.3.1]-bicycle 2a’-d6, would be generated. On the other hand, intermediate b can undergo β-hydrogen elimination to give enone species c. It is worthy to mention that similar sequences involving 2π-insertion followed by β-hydrogen elimination were proposed in Murakami19b and Bower’s20 studies. From intermediate c, either Rh–H (d) or Rh–C migratory insertion (e) followed by reductive elimination should result in the (4+1) product and regenerate the RhI catalyst. The observed stereochemistry of the transferred deuterium (2a-d6) is consistent with this proposed pathway.
Scheme 2.
Proposed Catalytic Cycle
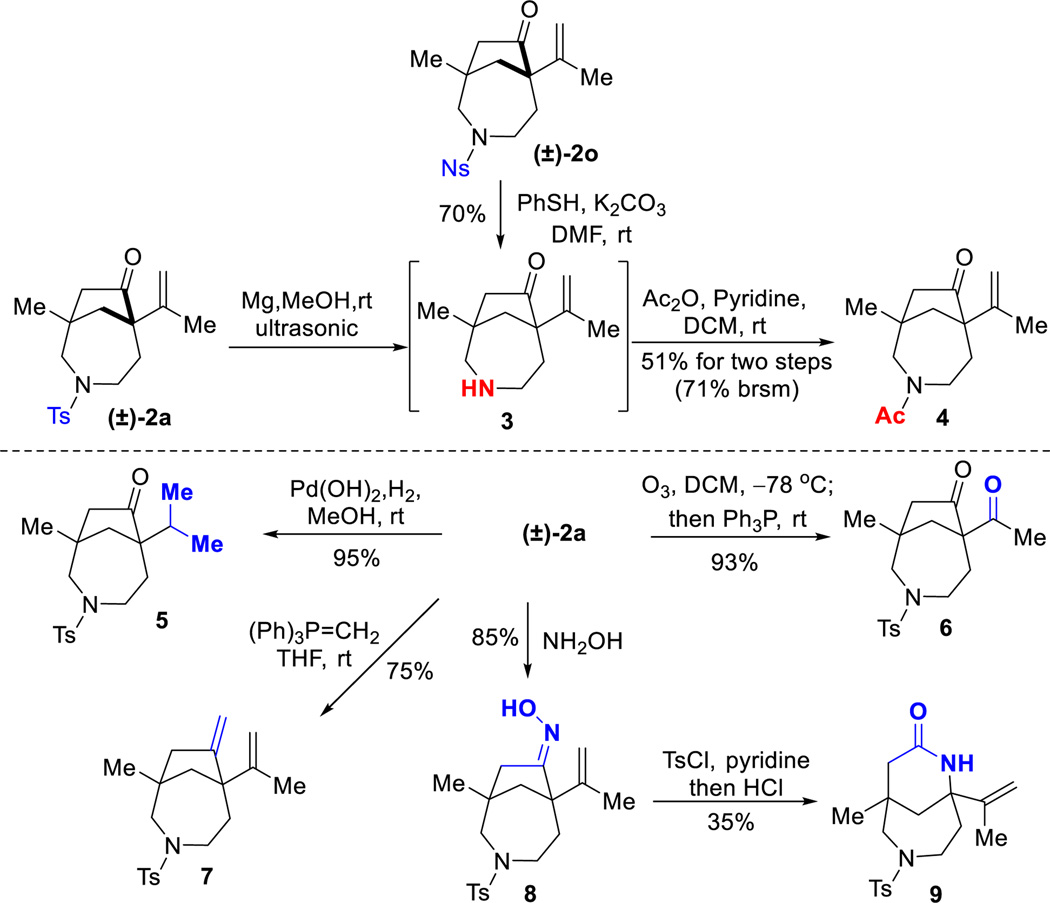
2.4 Derivatization of the (4+1) products
As expected, the 7-5 bridged bicyclic products can undergo a number of facile transformations to access other structures or functional groups (Scheme 3). First, while removal of sulfonyl protecting groups from nitrogen is often nontrivial, for this bridged system the Ts or Ns group can be smoothly removed. The olefin moiety can be efficiently reduced to the corresponding alkyl group (5) or oxidized to a ketone motif (6). The ketone group can be rapidly converted to the corresponding exo-cyclic olefin (7) via Wittig reaction or an oxime (8) through condensation. A subsequent Beckmann rearrangement with oxime 8 can be adopted to give ring-expanded bridged lactams (9).
3. CONLUSIONS
In summary, a rhodium-catalyzed (4+1) cyclization between cyclobutanones and allenes was developed, leading to a distinct [4.2.1]-bicyclic skeleton, containing two quaternary carbon centers. The reaction involves C–C activation of cyclobutanones and employs allenes as a one-carbon unit. A variety of functional groups can be tolerated, and a diverse range of bridged/fused scaffolds can be accessed. In addition, a highly enantioselective version of this transformation can also be achieved. This new method is expected to offer a new rapid entry to nitrogen-containing polycyclic systems, and is potentially useful for streamlining the synthesis of complex bioactive compounds. Detailed mechanistic study (e.g. why the (4+1) is favored) is ongoing.
4. EXPERIMENTAL SECTION
General conditions for the Rh-catalyzed (4+1) cyclization between cyclobutanones and allenes
In a nitrogen filled glove box, a 8 mL vial was charged with [Rh(C2H4)2Cl]2 (1.95 mg, 0.005 mmol, 5 % mmol) and tris[3,5-bis(trifluoromethyl)phenyl]phosphine (16.0 mg, 0.024 mmol, 24% mmol). A solution of the cyclobutanone substrate (0.1 mmol) in 1,4-dioxane (3 mL) was added, and then the vial was capped. After stirring at room temperature for 5 minutes, the solution was maintained at 150 °C for 36 h. The reaction was removed from the glove box and concentrated under reduced pressure. The residue was purified by silica gel chromatography (iodine chamber was used to visualize the location of the sample on the TLC plate).
General Conditions for the Rh-catalyzed enantioselective C–C activation
In a nitrogen filled glove box, a 8 mL vial was charged with [Rh(C2H4)2Cl]2 (1.95 mg, 0.005 mmol, 5 % mmol) and L4 (12.9 mg, 0.024 mmol, 24% mmol). A solution of the cyclobutanone substrate (0.1 mmol) in 1,4-dioxane (3 mL) was added, and then the vial was capped. After stirring at room temperature for 5 minutes, the solution was maintained at 130 °C for 20 h. The reaction was removed from the glove box and concentrated under reduced pressure. The residue was purified by silica gel chromatography (iodine chamber was used to visualize the location of the sample on the TLC plate).
Supplementary Material
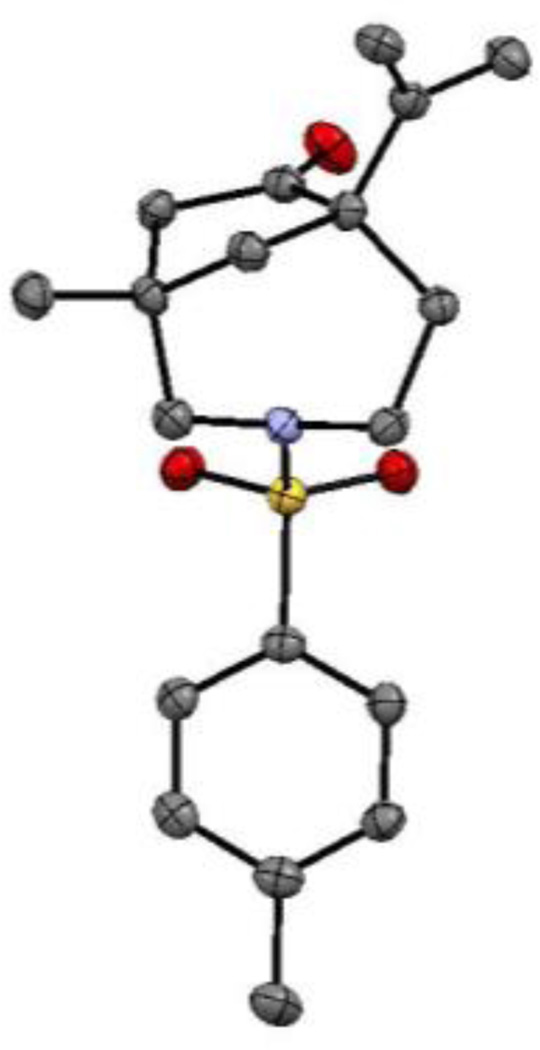
Figure 1.
Crystal structure of compound (±)-2a at 50% probability level. Hydrogen atoms are omitted for clarity.
Scheme 1.
Rhodium-catalyzed (4+1) cycloaddition initiated by C–C activation
Acknowledgments
We thank CPRIT, NIGMS (R01GM109054-01) and the Welch Foundation (F 1781) for research grants. G.D. is a Searle Scholar and Sloan fellow. We thank Dr. M. C. Young for proofreading the manuscript. We also thank Dr. V. Lynch and Dr. M. C. Young for X-ray structures. Johnson Matthey is acknowledged for a generous donation of Rh salts. Chiral Technologies is thanked for their generous donation of chiral HPLC columns.
Footnotes
ASSOCIATED CONTENT
Supporting Information Experimental procedures; spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interests.
REFERENCES
- 1.For selected reviews on C–C activation, see: Jones WD. Nature. 1993;364:676. Murakami M, Ito Y. Top. Organomet. Chem. 1999;3:97. Rybtchinski B, Milstein D. Angew. Chem., Int. Ed. 1999;38:870. doi: 10.1002/(SICI)1521-3773(19990401)38:7<870::AID-ANIE870>3.0.CO;2-3. Jun C-H. Chem. Soc. Rev. 2004;33:610. doi: 10.1039/b308864m. Satoh T, Miura M. Top. Organomet. Chem. 2005;14:1. Necas D, Kotora M. Curr. Org. Chem. 2007;11:1566. Crabtree RH. Chem. Rev. 1985;85:245. Ruhland K. Eur. J. Org. Chem. 2012:2683. Korotvicka A, Necas D, Kotora M. Curr. Org. Chem. 2012;16:1170. Seiser T, Saget T, Tran DN, Cramer N. Angew. Chem., Int. Ed. 2011;50:7740. doi: 10.1002/anie.201101053. Murakami M, Matsuda T. Chem. Commun. 2011;47:1100. doi: 10.1039/c0cc02566f. Dermenci A, Coe PW, Dong G. Org. Chem. Front. 2014;1:567. doi: 10.1039/c4qo00053f. (m) C–C bond activation. Dong G, editor. Topics in Current Chemistry. Vol. 346. Berlin: Springer-Verlag; 2014. Chen F, Wang T, Jiao N. Chem. Rev. 2014;114:8613. doi: 10.1021/cr400628s. Souillart L, Cramer N. Chem. Rev. 2015;115:9410. doi: 10.1021/acs.chemrev.5b00138.
- 2.For two seminal studies, see: South MS, Liebeskind LS. J. Am. Chem. Soc. 1984;106:4181. Murakami M, Itahashi T, Ito Y. J. Am. Chem. Soc. 2002;124:13976. doi: 10.1021/ja021062n.
- 3. Mei G, Liu X, Qiao C, Chen W, Li C-C. Angew. Chem., Int. Ed. 2015;54:1754. doi: 10.1002/anie.201410806. For related reviews, see: Kuwajiama I, Tanino K. Chem. Rev. 2005;105:4661. doi: 10.1021/cr040636z. Kim S, Winkler J. Chem. Soc. Rev. 1997;26:387. Ylijoki KEO, Stryker JM. Chem. Rev. 2013;113:2244. doi: 10.1021/cr300087g.
- 4.Murakami M, Ashida S. Chem. Commun. 2006:643. doi: 10.1039/b611522e. [DOI] [PubMed] [Google Scholar]
- 5.(a) Souillart L, Parker E, Cramer N. Angew. Chem., Int. Ed. 2014;53:9640. doi: 10.1002/anie.201405834. [DOI] [PubMed] [Google Scholar]; (b) Souillart L, Cramer N. Angew. Chem., Int. Ed. 2014;53:3001. doi: 10.1002/anie.201311009. [DOI] [PubMed] [Google Scholar]; (c) Parker E, Cramer N. Organometallics. 2014;33:780. [Google Scholar]; (d) Liu L, Ishida N, Murakami M. Angew. Chem., Int. Ed. 2012;51:2485. doi: 10.1002/anie.201108446. [DOI] [PubMed] [Google Scholar]
- 6.Ko HM, Dong G. Nat. Chem. 2014;6:739. doi: 10.1038/nchem.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For a recent review of (4+1) transformation, see: Chen J-R, Hu X-Q, Lu L-Q, Xiao W-J. Chem. Rev. 2015;115:5301. doi: 10.1021/cr5006974.
- 8.For recent reviews, see: López F, Mascareñas JL. Chem. Soc. Rev. 2014;43:2904. doi: 10.1039/c4cs00024b. Yu S, Ma S. Angew. Chem., Int. Ed. 2012;51:3074. doi: 10.1002/anie.201101460. Croatt MP, Wender PA. Eur. J. Org. Chem. 2010:19. Kitagaki S, Inagaki F, Mukai C. J. Synth. Org. Chem., Jpn. 2009;67:618. Ma S. Aldrichimica Acta. 2007;40:91. Ma S. Chem. Rev. 2005;105:2829. doi: 10.1021/cr020024j. Bates RW, Satcharoen V. Chem. Soc. Rev. 2002;31:12. doi: 10.1039/b103904k.
- 9.For selected examples of allenes as a two or three-carbon unit in cata-lytic cycloadditions, see: Ma S, Lu P, Lu L, Hou H, Wei J, He Q, Gu Z, Jiang X, Jin X. Angew. Chem., Int. Ed. 2005;44:5275. doi: 10.1002/anie.200501350. Wender PA, Deschamps NM, Sun R. Angew. Chem., Int. Ed. 2006;45:3957. doi: 10.1002/anie.200600806. Wender PA, Croatt MP, Deschamps NM. Angew. Chem., Int. Ed. 2006;45:2459. doi: 10.1002/anie.200600300. Trillo B, López F, Gulίas M, Castedo L, Mascareñas JL. Angew. Chem., Int. Ed. 2008;47:951. doi: 10.1002/anie.200704566. Fujiwara Y, Fu GC. J. Am. Chem. Soc. 2011;133:12293. doi: 10.1021/ja2049012. Oonishi Y, Hosotani A, Sato Y. J. Am. Chem. Soc. 2011;133:10386. doi: 10.1021/ja203824v. Shu D, Li X, Zhang M, Robichaux PJ, Tang W. Angew. Chem., Int. Ed. 2011;50:1346. doi: 10.1002/anie.201006881. Evans PA, Negru DE, Shang D. Angew. Chem., Int. Ed. 2015;54:4768. doi: 10.1002/anie.201410857. Mei LY, Wei Y, Tang XY, Shi M. J. Am. Chem. Soc. 2015;137:8131. doi: 10.1021/jacs.5b02080.
- 10.For examples of allenes serving as a one-carbon component in catalytic cycloadditions, see: Casanova N, Seoane A, Mascareñas JL, Gulίas M. Angew. Chem., Int. Ed. 2015;54:2374. doi: 10.1002/anie.201410350. Szeto J, Sriramurthy V, Kwon O. Org. Lett. 2011;13:5420. doi: 10.1021/ol201730q. Kuppusamy R, Gandeepan P, Cheng C-H. Org. Lett. 2015;17:3846. doi: 10.1021/acs.orglett.5b01825.
- 11.Xia Y, Liu Z, Liu Z, Ge R, Ye F, Hossain M, Zhang Y, Wang J. J. Am. Chem. Soc. 2014;136:3013. doi: 10.1021/ja500118w. [DOI] [PubMed] [Google Scholar]
- 12.Yada A, Fujita S, Murakami M. J. Am. Chem. Soc. 2014;136:7217. doi: 10.1021/ja502229c. [DOI] [PubMed] [Google Scholar]
- 13.For selected reviews on vinyl carbenoids, see: Davies HML. Aldrichimica Acta. 1997;30:107. Davies HML. Curr. Org. Chem. 1998;2:463. For selected examples on vinyl carbenoid-insertion into C–H bonds, see: Wang X, Xu X, Zavalij PY, Doyle MP. J. Am. Chem. Soc. 2011;133:16402. doi: 10.1021/ja207664r. Lian Y, Davies HML. J. Am. Chem. Soc. 2011;133:11940. doi: 10.1021/ja2051155.
- 14.For selected drugs containing azepane structures, see: Mianserin, Imipramine hrdrochloride, Tolazamide, (−)-Galanthamine hydrobromide, Clomipramine hydrochloride, Azelastine hydrochloride, Amdinocillin, Meptazinol.
- 15.For selected recent examples of P(3,5-C6H3(CF3)2)3 serving as a key ligand for π-insertion into rhodacyclopentanones, see Shaw MH, Melikhova EY, Kloer DP, Whittingham WG, Bower JF. J. Am. Chem. Soc. 2013;135:4992. doi: 10.1021/ja401936c. Shaw MH, McCreanor NG, Whit-tingham WG, Bower JF. J. Am. Chem. Soc. 2015;137:463. doi: 10.1021/ja511335v. and ref 6
- 16.Hong X, Stevens MC, Liu P, Wender PA, Houk KN. J. Am. Chem. Soc. 2014;136:17273. doi: 10.1021/ja5098308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Other nitrogen protecting groups, such as PMB, TFA, and Bz, did not afford the desired product.
- 18.For selected reviews on TADDOL-derived phosphoramidites ligands, see: Feringa BL. Acc. Chem. Res. 2000;33:346. doi: 10.1021/ar990084k. Teichert JF, Feringa BL. Angew. Chem., Int. Ed. 2010;49:2486. doi: 10.1002/anie.200904948.
- 19.(a) Matsuda T, Shigeno M, Murakami M. Chem. Lett. 2006;35:288. [Google Scholar]; (b) Matsuda T, Fujimoto A, Ishibashi M, Murakami M. Chem. Lett. 2004;33:876. [Google Scholar]
- 20.Shaw MH, Croft RA, Whittingham WG, Bower JF. J. Am. Chem. Soc. 2015;137:8054. doi: 10.1021/jacs.5b05215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.