Abstract
Purpose
These experiments were done to determine if mitochondria of macular retinal pigment epithelium (RPE) change with age in rhesus monkeys (Macaca mulatta). Mitochondria are the main instigators of oxidative stress which has often been considered to play a role in the pathogenesis of age related macular degeneration (AMD). Any pathological changes in the mitochondria of aging macular RPE, the main target of AMD, would be a clue to the pathogenesis of this common retinal degeneration which afflicts both monkey and man.
Methods
Transmission electron microscopy was used to identify mitochondria and determine their appearance, their density per unit area of RPE cytoplasm and their length. The eyes of seven monkeys, 1, 2, 6.5, 23, 26, 27 and 35 years of age, were studied. Measurements were kept separate for the basal, middle and apical third of each cell. The basal third of the macular RPE had many more mitochondria than the middle third and the apical third was almost devoid of mitochondria.
Results
Mitochondrial number decreased and length increased with age. The increasing length was associated with an unusual clustering of mitochondria into parallel arrays of elongated mitochondria with their long axis orthogonal to the basal membrane of the cell, structures not described before in RPE.
Conclusions
Mitochondrial elongation is associated with metabolic and/or oxidative stress implying that age is producing stress in macular RPE. The increasing clustering of very elongated mitochondria suggests that pathological changes are occurring in mitochondrial organization with age. These changes support the hypothesis that age related mitochondrial dysfunction could play a role in the pathogenesis of AMD.
Keywords: retina, macula, epithelium, monkey, mitochondria, morphology, electron microscopy
Introduction
The pathogenesis of age related macular degeneration (AMD) is not well understood. It is a common disorder in aging humans as well as non-human primates. The major target of AMD is the RPE and aging of this cell layer plays a key role in the disease. Many investigators proposed that oxidative stress may be the cause or initiator of AMD [1–5]. Oxidative stress is produced mainly by mitochondria which release reactive oxygen species as byproducts in the formation of energy-rich adenosine tri-phosphate. Reactive oxygen species can damage molecules in the mitochondria as well as in other RPE organelles leading to dysfunction and degeneration. It is therefore of interest to examine whether and how mitochondria change in aging RPE. Mitochondria are dynamic organelles continuously undergoing fusion (elongation) and fission (shortening) [6–8]. The balance between these two opposing processes shapes the mitochondria and reflects the metabolic status of the cell [9]. Fusion allows mitochondria to compensate for one another’s defects by sharing components, which can maintain energy output in the face of stress but if a certain threshold of damage occurs, mitochondria are eliminated by autophagy. Fission regulates morphology, facilitates mitochondrial trafficking and segregates the most seriously damaged mitochondria to preserve the health of the mitochondrial network [6, 8]. In this paper we examine how morphology, number and length of mitochondria in the macula RPE change with age in the rhesus monkey, an animal with a retina very similar to that of man.
Materials and Methods
All procedures were approved by the Institutional Animal Care and Use Committee of the Oregon Health and Science University and the NIH primate center in Poolesville, Maryland and conformed to the Guide for the Care and Use of Laboratory Animals (8th edition, 2011). After euthanasia, eyes from seven rhesus monkeys Macaca mulatta, 1, 2, 6.5, 23, 26, 27, and 35 years of age, were removed within minutes and fixed by 4% paraformaldehyde + 0.45 % glutaraldehyde. Diffusion of fixative was facilitated by piercing the eye with an 18-gauge needle at the limbus and injecting 0.5–1 ml of fixative into the vitreous. After a week or longer in fixative, the eyes were washed with a balanced salt solution and dissected with the aid of a surgical microscope. A square segment was cut out that contained the macula with the fovea at its center. After removal of the sclera, the choroid and attached retina were post fixed with 2% osmium tetroxide, dehydrated and embedded in epon. The epon block was sectioned and the sections were mounted on grids, stained with uranyl acetate and lead citrate and examined with an electron microscope (JEOL 1200). Digital photographs were viewed on a large screen monitor and examined in conjunction with Photoshop (Adobe, Mountain View, CA), which allowed adjustments of brightness, contrast and magnification to facilitate distinguishing organelles. The number and maximal length of 12 to 24 epithelial cells were measured per eye. Measurements were kept separate for the basal, middle and apical thirds of each cell. However, we only present measurements of the basal and middle thirds because mitochondria were rare in the apical third. The Student t-test was used to determine statistically significant differences.
Results
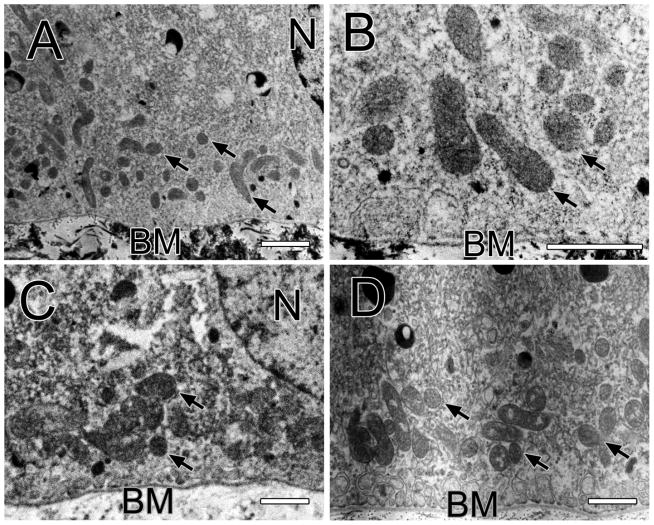
Figure 1A shows a macular RPE cell of the one-year old monkey. There are no lipofuscin bodies in the cytoplasm but mitochondria are abundant, most of which are circular but a few are longer. A large proportion is in the basal third of the cell; fewer are in the middle third and rarely in the apical third. Melanosomes are distinguishable from mitochondria by their much greater electron density. The darkness of melanosomes remains despite large increases in brightness of the digital image, while mitochondria virtually disappear with similar increases in brightness. Figure 1B shows the basal third of a macular RPE cell of the one-year old monkey at higher magnification to reveal the shape and density of the mitochondria more clearly. Figure 1C shows a macular epithelial cell of the two-year old monkey. These cells also lack lipofuscin bodies but have many melanosomes. Mitochondria are seen in the basal third of the cell between the nucleus and the basal membrane. Figure 1D shows the basal third of a macular RPE cell of the 6.5-year old monkey. Mitochondria both round and oval are seen. Lipofuscin bodies are now appearing in this older monkey.
Figure 1.
Figure 1A shows a macula RPE cell of the one-year old monkey with the basal membrane (BM) below and the apical side above as in all electron micrographs. Arrows point to mitochondria and a nucleus (N) is labeled. Bar, 1 μm in all panels. Figure 1B shows a magnified view of a macular RPE cell of the one-year old monkey. Several mitochondria are labeled with arrows. Bruch’s membrane (BM) is labeled. Figure 1C shows a macular RPE cell of the two-year old monkey. Many mitochondria (arrows) are located between the nucleus (N) and the basal membrane (BM) of the cell. Figure 1D shows the basal and middle third of a RPE cell of the 6.5-year old monkey. A group of mitochondria (arrows) are located just above the basal membrane.
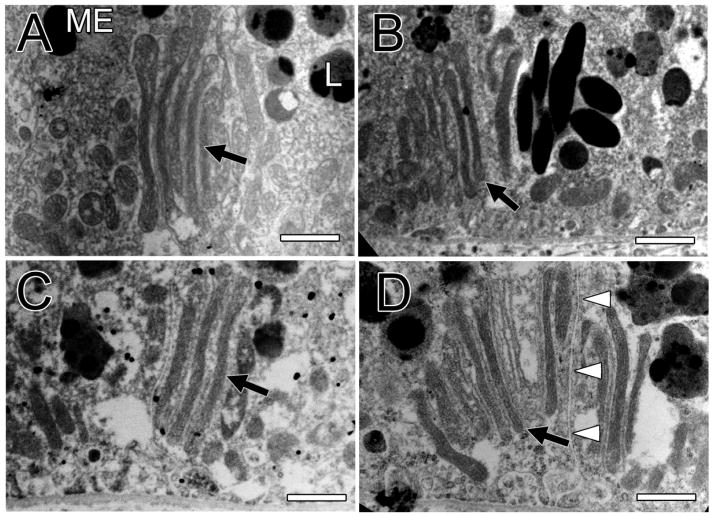
Figure 2A, also from the 6.5-year old monkey, reveals a unique mitochondrial structure not seen in the younger monkeys. It is composed of a parallel array of very long, thin mitochondria. The ends of these long mitochondria are bulbous, which has been suggested to be a sign of dysfunction [10]. The long axis of the array is orthogonal to the basal membrane. Figure 2B shows another example of these unusual structures in the 26-year old monkey. Figure 2C shows another example of this clustering of thin, elongated mitochondria in the 35-year old monkey where these structures were more common. Figure 2D shows a clustering of ten elongated mitochondria that involves two neighboring RPE cells of the 35-year old monkey. Three of the mitochondria are in one cell and the other seven in the neighboring cell. Extracellular space separates the two RPE cells. The surrounding cytoplasm contains many lipofuscin bodies but few melanosomes in the macula of this old monkey. Melanosomes tend to be rare in elderly human [11, 12] and monkey [13] RPE.
Figure 2.
Figure 2A shows the basal third of a macular RPE cell of the 6.5-year old monkey. It reveals a group of five elongated mitochondria (arrow), all parallel to each other. The tips of these elongated mitochondria are bulbous and some resemble smaller, more normal looking mitochondria. A melanosome (ME) and a lipofuscin body (L) are labeled. The array of mitochondria are oriented orthogonally to the basal membrane. Bar, 1 μm in all panels. Figure 2B shows the basal third of a macular RPE cell of the 26-year old monkey. It is another example of a parallel array of long, thin mitochondria oriented orthogonally to the basal membrane of the cell (arrow). Smaller mitochondria are above the array. Figure 2C shows a similar array of four elongated mitochondria in the 35-year old monkey (arrow). Figure 2D shows a cluster of ten elongated mitochondria in parallel (arrow) in the 35-year old monkey. Three of the elongated mitochondria are located in one cell and the other seven in an adjacent cell; the membranes of these two cells and the extracellular space between them are labeled by white arrow heads. A magnified view of this unusual structure is shown in a Supplement.
Tables 1 and 2 show the mean mitochondrial density (mitochondria/μm2) and mitochondrial length (μm) at the basal and middle third of the cell, respectively. The youngest monkey has the highest and the oldest monkey the lowest mitochondrial density at both the basal and middle third of the cell. The youngest monkey has the shortest length at both the basal and middle third of the cell. This was statistically significant; p-values for the comparison of the one-year old with each of the other monkeys are shown in the tables.
Table 1.
Mitochondrial measurements (basal third of macular RPE)
| Age (years) | 1 | 2 | 6.5 | 23 | 26 | 27 | 35 |
|
| |||||||
| RPE cells (#) | 24 | 16 | 18 | 22 | 22 | 12 | 23 |
|
| |||||||
| Mt density (#/μm2) | 0.96 ± 0.42 | 0.37 ± 0.32 | 0.30 ± 0.10 | 0.26 ± 0.19 | 0.31 ± 0.14 | 0.22 ± 0.16 | 0.16 ± 0.08 |
| P (vs. 1-year) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
|
| |||||||
| Mt length (μm) | 0.57 ± 0.11 | 0.76 ± 0.16 | 1.06 ± 0.27 | 1.01 ± 0.30 | 0.88 ± 0.16 | 0.86 ± 0.13 | 1.27 ± 0.34 |
| P (vs. 1-year) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
Data are shown as average ± standard deviation.
Table 2.
Mitochondrial measurements (middle third of macular RPE)
| Age (years) | 1 | 6.5 | 23 | 26 | 27 | 35 |
|
| ||||||
| RPE cells (#) | 24 | 18 | 22 | 22 | 12 | 23 |
|
| ||||||
| Mt density (#/μm2) | 0.17 ± 0.18 | 0.04 ± 0.03 | 0.08 ± 0.05 | 0.03 ± 0.02 | 0.03 ± 0.02 | 0.01 ± 0.01 |
| P (vs. 1-year) | 0.001 | 0.023 | <0.001 | 0.001 | <0.001 | |
|
| ||||||
| Mt length (μm) | 0.76 ± 0.43 | 1.11 ± 0.28 | 1.27 ± 0.66 | 1.14 ± 0.40 | 2.00 ± 0.69 | 1.80 ± 0.88 |
| P (vs. 1-year) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
Data are shown as average ± standard deviation.
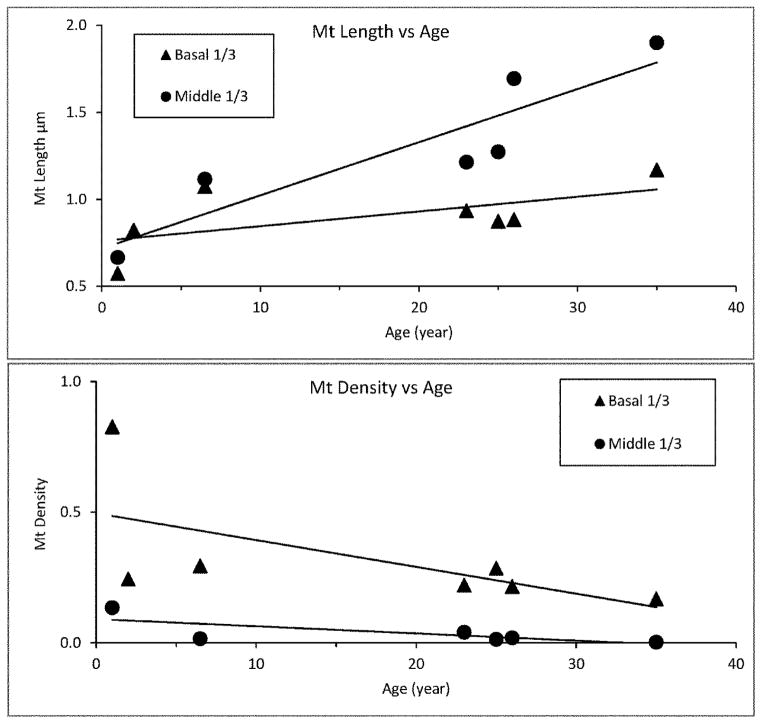
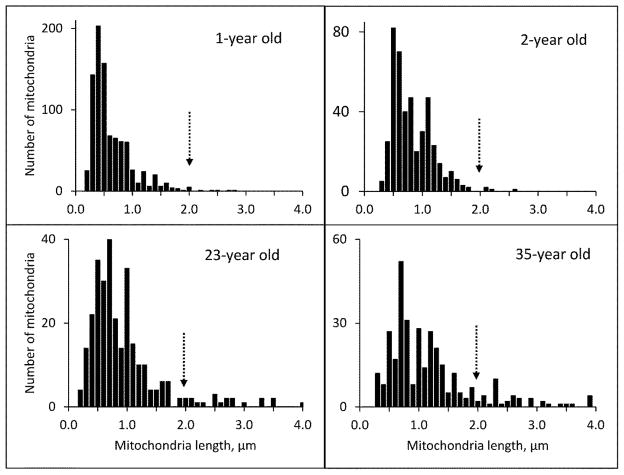
Figure 3 shows graphically that mitochondrial density decreases while length increases with age in both the basal and middle third of the cell. Figure 4 shows the frequency distribution of mitochondrial lengths for the two youngest and two of the older monkeys. The peak length for the younger monkeys is 0.5 μm; the peak length of the older monkeys is greater than 0.5 μm. But even more striking is the large number of mitochondria with lengths greater than 2 μm in the older monkeys and their virtual absence in the younger monkeys.
Figure 3.
Figure 3 shows a graphic relationship between mitochondrial length (μm) versus age (above) and density (mitochondria/μm2) versus age (below) for the basal and middle third of the RPE cell; it includes all of the mitochondria measured. The lines are the least square fits to the data.
Figure 4.
Figure 4 shows the frequency distribution of mitochondrial lengths for the youngest two monkeys (above) and two of the older monkeys (below). The peak length of the mitochondria in the younger monkeys is 0.5 μm compared to the mitochondria in the older monkeys that are greater than 0.5 μm. An arrow marks the length of 2 μm.
Discussion
Our results indicate that the number of mitochondria in the macula RPE of the rhesus monkey decreases with age while mitochondrial length increases. Reduction in mitochondrial number was also found in aging human RPE [14]. A reduction in mitochondrial number with age is generally found in non-replicating cells [9]. Feher et al’s study of aging human RPE [14] found a decrease in mitochondrial area with age which does not seem to be in agreement with our finding of increased length. This may be due to a species difference. We are also studying monkeys 1 to 35 years in age while they were studying humans 40 to 90 years of age. They did not see these extraordinary clusters of thin elongated mitochondria. Did they miss them or is the monkey different? It is also possible that thinner mitochondria produce smaller areas than mitochondria of normal width. More research should clarify this discrepancy, perhaps using high resolution structured illumination microscopy.
Metabolic and oxidative stress are known to be associated with elongation of mitochondria [15]. Elongation can compensate for local deficiencies in smaller mitochondria. Mitochondrial elongation occurs in many senescent post-mitotic cells [16–19]. An increase of mitochondrial length by modulation of different mitochondrial fission and fusion factors confers resistance to apoptotic stimuli [20–22]. By contrast, fragmentation of mitochondria represents neuronal pathology [23]. Senescent endothelial cells in vitro develop elongated mitochondria that down regulates Fision 1 protein (Fis1) and Dynamin related protein1 (Drp1), two proteins that regulate mitochondrial fission and influence the expression of serine-threonine kinase PINK1 involved in Parkinson’s disease. Starvation causes mitochondrial elongation which is associated with a rapid increase in cAMP that activates protein kinase A, which in turn phosphorylates DRP1, keeping it in the cytosol that allows unopposed mitochondrial fusion. Such elongated mitochondria appear to be protected from autophagy and have a higher density of cristae, which promotes ATP production and survival of metabolically stressed cells [24]. Bax-interacting factor 1 (Bif-1) is a protein involved in regulating apoptosis, mitochondrial morphology, and autophagy. In post mitotic neurons, Bif-1 promotes viability and mitochondrial elongation [25].
Aging or dysfunctional cells can develop giant mitochondria [9] as found in muscle [16], cardiomyopathy [26, 27], riboflavin deficiency [28] and by forced senescence in culture [18, 19]. Giant mitochondria tend to be a sign of pathology [16]. Metabolic or oxidative stress of murine RPE results in the formation of large mitochondria produced by activation of the P13/AKT/mTor signaling pathway and mitigated in part by rapamycin [10].
The mitochondrial arrays that we encountered may be related to giant mitochondria and be a sign of pathology. The mitochondria of RPE may be deteriorating with age and especially in AMD. Feher et al. [14] reported disorganized mitochondrial cristae occurring with age in human RPE. Nordgard et al [29] found increased mitofilin in mitochondria of RPE from human donor eyes with AMD. Mitofilin stabilizes cristae; so its upregulation could be a compensatory response to mitochondrial dysfunction. Autophagy of damaged mitochondria may also be abnormal because there is a decreased number of autophagic vesicles in RPE from subjects with AMD compared to age matched controls [30]. There is accumulation of p62 protein normally eliminated by autophagy in AMD [31]. Insulin signaling decreases autophagy [32] so that increases in the insulin receptor subunit reported in AMD RPE [33] suggests that autophagy may be defective. Significant defects in mitochondrial DNA are also occurring in AMD [34, 35].
The unusual clustering of thin, elongated mitochondria has, to our knowledge, not been reported before. These structures may be a characteristic of monkey and not human RPE because they have not been found by others studying much older human RPE [11, 36, 37]. How these elongated mitochondrial arrays form is intriguing. It suggests that mitochondrial division may be occurring along the long axis of the mitochondrion. Elongation and clustering of thin mitochondria may be a morphometric indicator of aging RPE and perhaps aging itself [38].
Supplementary Material
Acknowledgments
We thank Krissty Brown for her assistance with electron microscopy, Julie Mattison from the National Institute on Aging for for providing the elderly monkeys and the Foundation Fighting Blindness and Research to Prevent Blindness, Inc. for their support.
Footnotes
Ethical approval:
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Compliance with Ethical Standards
Disclosure of potential conflicts of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Statement on the welfare of animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
All procedures were approved by the Institutional Animal Care and Use Committee of the Oregon Health and Science University and the NIH primate center in Poolesville, Maryland and conformed to the Guide for the Care and Use of Laboratory Animals (8th edition, 2011).
References
- 1.Cai J, Nelson KC, Wu M, Sternberg P, Jr, Jones DP. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 2.Frank RN. “Oxidative protector” enzymes in the macular retinal pigment epithelium of aging eyes and eyes with age-related macular degeneration. Trans Am Ophthalmol Soc. 1998;96:635–689. [PMC free article] [PubMed] [Google Scholar]
- 3.Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarrett SG, Boulton ME. Consequences of oxidative stress in age-related macular degeneration. Mol Aspects Med. 2012;33:399–417. doi: 10.1016/j.mam.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505:335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Picard M, Shirihai OS, Gentil BJ, Burelle Y. Mitochondrial morphology transitions and functions: implications for retrograde signaling? Am J Physiol Regul Integr Comp Physiol. 2013;304:R393–406. doi: 10.1152/ajpregu.00584.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terman A, Kurz T, Navratil M, Arriaga EA, Brunk UT. Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid Redox Signal. 2010;12:503–535. doi: 10.1089/ars.2009.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao C, Yasumura D, Li X, Matthes M, Lloyd M, Nielsen G, Ahern K, Snyder M, Bok D, Dunaief JL, LaVail MM, Vollrath D. mTOR-mediated dedifferentiation of the retinal pigment epithelium initiates photoreceptor degeneration in mice. J Clin Invest. 2011;121:369–383. doi: 10.1172/JCI44303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feeney-Burns L, Hilderbrand ES, Eldridge S. Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol Vis Sci. 1984;25:195–200. [PubMed] [Google Scholar]
- 12.Schmidt SY, Peisch RD. Melanin concentration in normal human retinal pigment epithelium. Regional variation and age-related reduction. Invest Ophthalmol Vis Sci. 1986;27:1063–1067. [PubMed] [Google Scholar]
- 13.Gouras P, Brown K, Ivert L, Neuringer M. A novel melano-lysosome in the retinal epithelium of rhesus monkeys. Exp Eye Res. 2011;93:937–946. doi: 10.1016/j.exer.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feher J, Kovacs I, Artico M, Cavallotti C, Papale A, Balacco Gabrieli C. Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol Aging. 2006;27:983–993. doi: 10.1016/j.neurobiolaging.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Mai S, Klinkenberg M, Auburger G, Bereiter-Hahn J, Jendrach M. Decreased expression of Drp1 and Fis1 mediates mitochondrial elongation in senescent cells and enhances resistance to oxidative stress through PINK1. J Cell Sci. 2010;123:917–926. doi: 10.1242/jcs.059246. [DOI] [PubMed] [Google Scholar]
- 16.Navratil M, Terman A, Arriaga EA. Giant mitochondria do not fuse and exchange their contents with normal mitochondria. Exp Cell Res. 2008;314:164–172. doi: 10.1016/j.yexcr.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Unterluggauer H, Hutter E, Voglauer R, Grillari J, Voth M, Bereiter-Hahn J, Jansen-Durr P, Jendrach M. Identification of cultivation-independent markers of human endothelial cell senescence in vitro. Biogerontology. 2007;8:383–397. doi: 10.1007/s10522-007-9082-x. [DOI] [PubMed] [Google Scholar]
- 18.Yoon YS, Yoon DS, Lim IK, Yoon SH, Chung HY, Rojo M, Malka F, Jou MJ, Martinou JC, Yoon G. Formation of elongated giant mitochondria in DFO-induced cellular senescence: involvement of enhanced fusion process through modulation of Fis1. J Cell Physiol. 2006;209:468–480. doi: 10.1002/jcp.20753. [DOI] [PubMed] [Google Scholar]
- 19.Zottini M, Barizza E, Bastianelli F, Carimi F, Lo Schiavo F. Growth and senescence of Medicago truncatula cultured cells are associated with characteristic mitochondrial morphology. New Phytol. 2006;172:239–247. doi: 10.1111/j.1469-8137.2006.01830.x. [DOI] [PubMed] [Google Scholar]
- 20.Jahani-Asl A, Cheung EC, Neuspiel M, MacLaurin JG, Fortin A, Park DS, McBride HM, Slack RS. Mitofusin 2 protects cerebellar granule neurons against injury-induced cell death. J Biol Chem. 2007;282:23788–23798. doi: 10.1074/jbc.M703812200. [DOI] [PubMed] [Google Scholar]
- 21.Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugioka R, Shimizu S, Tsujimoto Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem. 2004;279:52726–52734. doi: 10.1074/jbc.M408910200. [DOI] [PubMed] [Google Scholar]
- 23.Knott AB, Bossy-Wetzel E. Impairing the mitochondrial fission and fusion balance: a new mechanism of neurodegeneration. Ann N Y Acad Sci. 2008;1147:283–292. doi: 10.1196/annals.1427.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang DB, Uo T, Kinoshita C, Sopher BL, Lee RJ, Murphy SP, Kinoshita Y, Garden GA, Wang HG, Morrison RS. Bax interacting factor-1 promotes survival and mitochondrial elongation in neurons. J Neurosci. 2014;34:2674–2683. doi: 10.1523/JNEUROSCI.4074-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coleman R, Silbermann M, Gershon D, Reznick AZ. Giant mitochondria in the myocardium of aging and endurance-trained mice. Gerontology. 1987;33:34–39. doi: 10.1159/000212851. [DOI] [PubMed] [Google Scholar]
- 27.Tandler B, Dunlap M, Hoppel CL, Hassan M. Giant mitochondria in a cardiomyopathic heart. Ultrastruct Pathol. 2002;26:177–183. doi: 10.1080/01913120290076847. [DOI] [PubMed] [Google Scholar]
- 28.Tandler B, Erlandson RA, Smith AL, Wynder EL. Riboflavin and mouse hepatic cell structure and function. II. Division of mitochondria during recovery from simple deficiency. J Cell Biol. 1969;41:477–493. doi: 10.1083/jcb.41.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nordgaard CL, Karunadharma PP, Feng X, Olsen TW, Ferrington DA. Mitochondrial proteomics of the retinal pigment epithelium at progressive stages of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:2848–2855. doi: 10.1167/iovs.07-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitter SK, Song C, Qi X, Mao H, Rao H, Akin D, Lewin A, Grant M, Dunn W, Jr, Ding J, Bowes Rickman C, Boulton M. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy. 2014;10:1989–2005. doi: 10.4161/auto.36184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viiri J, Amadio M, Marchesi N, Hyttinen JM, Kivinen N, Sironen R, Rilla K, Akhtar S, Provenzani A, D’Agostino VG, Govoni S, Pascale A, Agostini H, Petrovski G, Salminen A, Kaarniranta K. Autophagy activation clears ELAVL1/HuR-mediated accumulation of SQSTM1/p62 during proteasomal inhibition in human retinal pigment epithelial cells. PLoS One. 2013;8:e69563. doi: 10.1371/journal.pone.0069563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Droge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Decanini A, Nordgaard CL, Feng X, Ferrington DA, Olsen TW. Changes in select redox proteins of the retinal pigment epithelium in age-related macular degeneration. Am J Ophthalmol. 2007;143:607–615. doi: 10.1016/j.ajo.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin H, Xu H, Liang FQ, Liang H, Gupta P, Havey AN, Boulton ME, Godley BF. Mitochondrial DNA damage and repair in RPE associated with aging and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:3521–3529. doi: 10.1167/iovs.10-6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terluk MR, Kapphahn RJ, Soukup LM, Gong H, Gallardo C, Montezuma SR, Ferrington DA. Investigating mitochondria as a target for treating age-related macular degeneration. J Neurosci. 2015;35:7304–7311. doi: 10.1523/JNEUROSCI.0190-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feeney L. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Invest Ophthalmol Vis Sci. 1978;17:583–600. [PubMed] [Google Scholar]
- 37.Hogan MJ, Alvarado JA, Weddell JE. Histology of the human eye: An atlas and textbook. Saunders; Philadelphia: 1971. [Google Scholar]
- 38.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.