Abstract
Complex I is a multi-subunit enzyme of the respiratory chain with seven core subunits in its membrane arm (A, H, J, K, L, M, and N). In the enzyme from Escherichia coli the C-terminal ten amino acids of subunit K lie along the lateral helix of subunit L, and contribute to a junction of subunits K, L and N on the cytoplasmic surface. Using double cysteine mutagenesis, the cross-linking of subunit K (R99C) to either subunit L (K581C) or subunit N (T292C) was attempted. A partial yield of cross-linked product had no effect on the activity of the enzyme, or on proton translocation, suggesting that the C-terminus of subunit K has no dynamic role in function. To further elucidate the role of subunit K genetic deletions were constructed at the C-terminus. Upon the serial deletion of the last 4 residues of the C-terminus of subunit K, various results were obtained. Deletion of one amino acid had little effect on the activity of Complex I, but deletions of 2 or more amino acids led to total loss of enzyme activity and diminished levels of subunits L, M, and N in preparations of membrane vesicles. Together these results suggest that while the C-terminus of subunit K has no dynamic role in energy transduction by Complex I, it is vital for the correct assembly of the enzyme.
Keywords: Complex I, cross-linking, subunit interactions, Methanethiosulfonate, nuoK
Introduction
Respiratory Complex I is a multi-subunit, membrane-bound enzyme that couples NADH oxidation to reduction of ubiquinone, while transducing the free energy to drive proton translocation against a transmembrane electrochemical proton gradient (for recent reviews see (Hirst 2013; Verkhovskaya and Bloch 2013)). Structurally, the enzyme from E. coli contains thirteen conserved subunits that constitute a functional core, and are distributed between a membrane arm and a peripheral arm. The peripheral arm comprises subunits B, CD, E, F, G and I, and it contains a binding site for NADH and all of the prosthetic groups necessary for the transfer of electrons to a quinone substrate. The membrane arm comprises subunits A, H, J, K, L, M, and N. It interacts with the peripheral arm near the site of quinone reduction, and is likely to contain four sites of proton translocation. The mechanism of energy transduction between the two functionally-distinct arms is unknown.
Proton translocation through the membrane arm is thought to be mediated primarily by the three related subunits, named L, M and N in E. coli, which are also homologous to subunits from a multi-subunit, cation-proton antiporter (Ito et al. 1999). Subunit L differs from the related subunits M and N in that it has 2 additional TM (transmembrane) helices, which are connected by a long lateral helix that contacts subunits M, N and K (Efremov and Sazanov 2011). The role of the lateral helix has been tested by mutagenesis in several recent studies (Belevich et al. 2011; Steimle et al. 2011; Steimle et al. 2015; Steimle et al. 2012; Torres-Bacete et al. 2011). Proton translocation likely occurs through four pathways. Three of the pathways involve subunits N, M, and L. Each pathway appears to be formed by two offset vertical half-channels, connected by a horizontal channel near the middle of the membrane. Mutagenesis of residues along these pathways has confirmed their importance in enzyme activity (Amarneh and Vik 2003; Euro et al. 2008; Michel et al. 2011; Nakamaru-Ogiso et al. 2010; Sato et al. 2013; Torres-Bacete et al. 2007; Torres-Bacete et al. 2009). From the crystal structure of the entire Complex I (Baradaran et al. 2013), a fourth proton pathway (the E-channel) was proposed that is formed by the remaining bacterial membrane subunits A, H, J, and K. Subunit K has 100 amino acids and 3 TM helices, with the N-terminus localized to the periplasm, and the C-terminus, consisting of about 18 amino acids, in the cytoplasm. Residues 83–91 interact primarily with subunits N and J. Residues 92–100 interact primarily with subunits N and L. Residue Glu36 of TM2 in subunit K is thought to participate in the E-channel. It is found in the middle of the membrane in the half-channel that exits to the periplasm, along with Tyr58 of subunit J (Kao et al. 2005b; Kervinen et al. 2004; Torres-Bacete et al. 2012).
Recent work from this lab has shown that constraining the lateral helix of subunit L by a variety of different cross-links did not reduce enzyme activity or proton translocation (Zhu and Vik 2015). In this report we have tried a similar approach to test whether the C-terminus of subunit K has a dynamic role in the enzyme function of Complex I. In addition we have tested the effect of genetically deleting up to four amino acids from the C-terminus of K.
Materials and Methods
Materials
Materials were obtained as described in previous work (Zhu and Vik 2015). MTS (Methanethiosulfonate) cross-linking reagents, M3M (1,3-Propanediyl bismethanethiosulfonate), and M6M (1,6-Hexanediyl bismethanethiosulfonate) were from Toronto Research Chemicals (Toronto, Canada). The polyclonal antibodies against E. coli complex I subunits L and M were prepared by Affinity BioReagents (Golden CO USA). These antibodies were raised in rabbits against peptide QTYSQPLWTWMSVGD (corresponding to residues 58–72) in subunit L and peptide GKAKSQIASQELPGM (corresponding to residues 446–460) in subunit M, and were described previously (Michel et al. 2011; Zhu and Vik 2015). Subunit N was detected by the monoclonal rat anti-HA (high affinity) antibody from Roche. Polyclonal antibodies raised against oligopeptides from subunits A (Kao et al. 2004), F (Kao et al. 2004), G (Nakamaru-Ogiso et al. 2005), L-(NuoL-1) (Nakamaru-Ogiso et al. 2010) and K (Kao et al. 2005b) were a generous gift from T. Yagi and A. Matsuno-Yagi (Scripps Research Institute, La Jolla, CA USA). Oligonucleotides for mutagenesis and sequencing were synthesized by Eurofin Genomics (Huntsville, AL USA). DNA sequencing was performed in Lone Star Labs (Houston, TX USA).
Plasmids, mutagenesis, and growth
Plasmids pUC19-L'MN, 6.57 kb, (Michel et al. 2011) and pUC19-L'MN-SpeI, 6.62 kb, (Zhu and Vik 2015) were used for construction of cysteine mutants K581C (nuoL) and T292C (nuoN), respectively. Both plasmids contain a truncated gene for L and full length genes for subunits M and N. They differ only in that pUC19 L'MN-SpeI contains a unique SpeI endonuclease site downstream of the nuoN gene, and nuoN has HA and His tags that are identical to those in the expression vector pBA400. The SpeI restriction site is essential for moving mutations at the 3' end of nuoN from the plasmid in which they are constructed to the plasmid for expression, pBA400-SpeI. Plasmid pUC19-LMN (Michel et al. 2011) was used for construction of the G100stop and the R99C mutations in nuoK. It is 8.10 kb in size and contains full-length genes for subunits L, M, N and the 3' end of nuoK. During the course of this project plasmid pUC19-I'JKL' became available, and was used for construction of the three other nuoK mutations, E97stop, M98stop, and R99stop. This plasmid was produced by ligating a 2.57 kb KpnI-PstI fragment from pAJW105 (Prüss et al. 1994) to pUC19 that had been digested with the same enzymes. The resulting plasmid, 5.23 kb, contains truncated forms of nuoI and nuoL, and full-length nuoJ and nuoK. The four truncation mutants of the K subunit were constructed by replacing each of the last four codons with TGA stop codons. The K581C (nuoL) mutation was transferred to the expression vector pBA400 (Amarneh et al. 2006), which contains the entire nuo operon, using PstI and AscI. The T292C (nuoN) mutation was transferred to the expression vector pBA400-SpeI, which differs from pBA400 only by a unique SpeI endonuclease site downstream of nuoN, using AscI and SpeI, however, expression of nuo genes appears to be reduced (Zhu and Vik 2015). This is likely due to effects on the mRNA, such as stability. Mutations constructed in K were transferred to the expression vector, pBA400, using restriction enzymes BsrGI and PstI. The double mutants were constructed by the sequential transfer of single mutations. The double mutants were transformed into strain BA14, which carries a chromosomal deletion for the entire nuo operon (Amarneh et al. 2006). Each of the double mutants was grown on M63 minimal media agar plates with acetate as sole carbon source (see below) to compare the growth of mutants relative to the wild type. Acetate plates contained 1.36% KH2PO4, 0.2% (NH4)2SO4, 0.05% FeSO4 • 7H2O, 1.5% Agar, 0.02% MgSO4, 0.001% vitamin B1 and 0.2% potassium acetate, pH 7.0. Growth was evaluated by visual inspection after 48–72 hours at 37°C. Growth yield measurements were made in M63 minimal salt media supplemented with succinate. Cultures were first grown overnight at 37°C in M63 media supplemented with 0.2% succinate as a carbon source, and then used to inoculate 20 mL of M63 media supplemented with 0.1% succinate. Cultures were shaken at approximately 250 rpm at 37°C and absorbance measurements were taken at A600 every hour for a period of eight hours, or until the absorbance did not further increase.
Membrane preparation, Cross-linking, and Immunoblotting
Membrane preparations were made from mutant and wild type E. coli cells as described previously (Amarneh et al. 2006; Michel et al. 2011; Zhu and Vik 2015). For cross-linking experiments (Zhu and Vik 2015), TMG-Acetate buffer (50 mM Tris•Acetate, 5 mM magnesium acetate, 10% glycerol, pH 7.5) was used. For cross-linking a solution of 25 mM MTS reagent was made in dimethyl sulfoxide and used immediately. Membrane vesicles in TMG-Acetate buffer were treated with 250 μM MTS reagent and incubated at 4°C for 10 min. The reaction was stopped by the addition of 20 mM Na2EDTA at 4°C for 15 min. Membrane vesicles were incubated with an equal volume of sample buffer for 1 hour before analyzing by SDS electrophoresis. Samples of 40 μg of membrane protein were run on 12% acrylamide gels at 150 V for 1 hour and then were transferred to PVDF membrane for immunoblotting as described previously (Zhu and Vik 2015). The PVDF membrane was incubated with rabbit custom antibodies diluted 1:5000 for subunit L or M, 1:10,000 for subunits A, F, G, L (nuoL-1) K, or rat anti-HA serum, diluted 1:5000 for subunit N at room temperature for at least 2 hours.
Enzyme Assays
All activity assays were conducted using deamino-NADH, according to the methods described previously (Zhu and Vik 2015). In brief, deamino-NADH oxidase activity assays were started with 0.25 mM deamino-NADH (extinction coefficient 6.22 mM−1 cm−1) and the absorbance was monitored at 340 nm for 2 minutes. Proton translocation assays were conducted by measuring the fluorescence quenching of the acridine dye ACMA (9-amino 3-chloro 2-methoxy acridine) as a ΔpH indicator using excitation and emission wave lengths of 410 and 490 nm, respectively. Both deamino-NADH oxidase activity assays and proton translocation assays were conducted by using 150 μg/ml protein membrane in the same buffer (50 mM MOPS, 10 mM MgCl2, pH 7.3) at room temperature. The 1 μM uncoupler FCCP (carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone) from a 1 mM ethanol stock was added to eliminate the buildup of a proton gradient during NADH oxidase assays. For proton translocation assays, ACMA was added to 1 μM, while other concentrations were the same as for oxidase assays. Ferricyanide reductase assays were conducted as previously described (Kao et al. 2005a). The assays were started with 80 μg/ml protein membrane and 0.15 mM deanimo-NADH.
Results
Cross-linking of K to L or N
Previous work revealed that cross-linking the lateral helix of subunit L to either or both subunit M or N did not reduce the enzyme activity of Complex I, or its rate of proton translocation (Zhu and Vik 2015). Using a similar approach, we attempted to construct cross-links between R99C of subunit K and either K581C in the lateral helix of subunit L, or T292C of subunit N, as illustrated in Fig. 1. The double cysteine mutations were constructed and transferred to a nuo expression vector, pBA400 or pBA400-SpeI, and then used to transform the nuo deletion strain BA14. Membrane vesicles were prepared and assayed for NADH oxidase activity using deamino-NADH as substrate, and the results are shown in Table 1. Both the K581C + R99C double mutant and the T292C + R99C double mutant are indistinguishable from the wild-type version of their host plasmids as measured by deamino-NADH oxidase activity. In Fig. 2 the rates of proton translocation by the two double mutants are shown to be identical to that of their respective wild types.
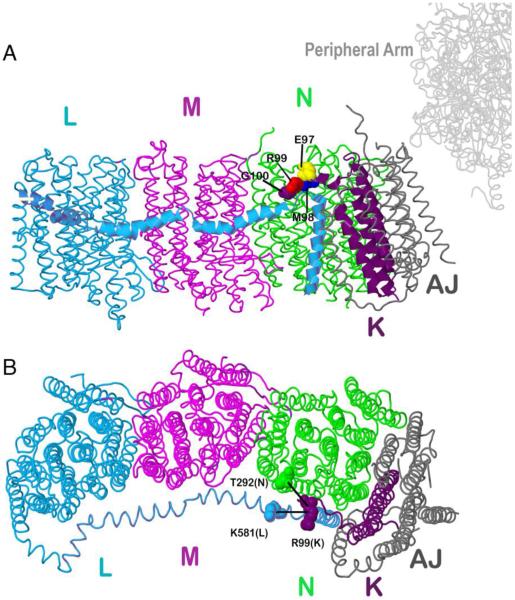
Fig. 1.
Schematic view of the membrane arm of Complex I showing the sites of cross-linking and stop codon substitutions. Subunit L is colored blue, subunit M magenta, subunit N green, subunit K purple and subunits A and J are colored gray. A. Residues G100 (purple), R99 (red), M98 (blue) and E97 (yellow) are showed in space-filling. B. Two cross-links between subunit K and N or L are also indicated. The image was developed from Protein Data Bank file 3rko (16). The view is from the cytoplasm. The peripheral arm would be to the right. The crystal form of this membrane arm from E. coli is lacking subunit H, but overall its structure is very similar to that of the entire Complex I structure determined from Thermusthermophilus (PDB file 4hea) (Baradaran et al. 2013).
Table 1.
| Mutationa | Deamino-NADH oxidase activityb (nmoles/mg protein/min) | % Deamino-NADH oxidase activityc |
|---|---|---|
| pBA400 | 165±48 (15)c | 100 |
| K581C+R99C | 160±12 (2) | 97 |
| pBA400-SpeI | 95±8 (5) | 100 |
| T292+R99C | 97±8 (2) | 102 |
K581C+R99C was made in the background of pBA400, T292+R99C was made in the background of pBA400-SpeI
Activity was measured in membrane preparations as described in “Methods”. The mean, standard error and (number of measurements) are shown.
Activity is expressed as a percentage of the wild type rate.
Fig. 2.
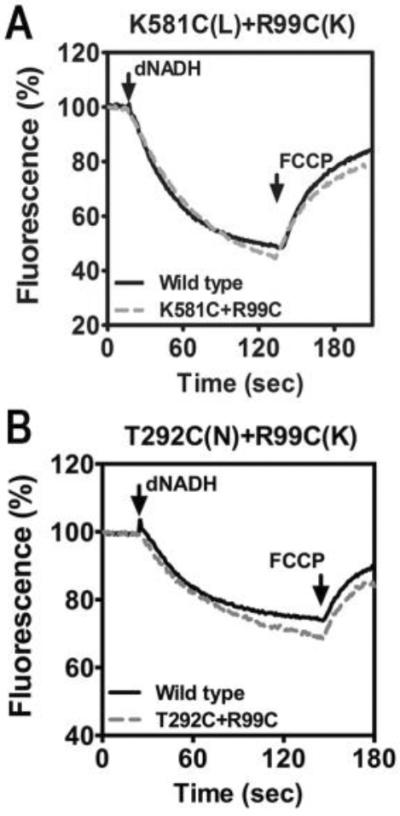
Proton translocation rates of two double cysteine substitutions. The reactions were initiated with deamino-NADH (dNADH) to 250 mM final concentration. The fluorescence of ACMA (1 mM) was followed for several minutes. The uncoupler FCCP was added (1 mM) to collapse the generated proton gradient. A. K581C (nuoL) + R99C (nuoK). B. T292C (nuoN) + R99C (nuoK). In each panel the wild type strain is shown for comparison. The traces shown are representative of 2–3 experiments.
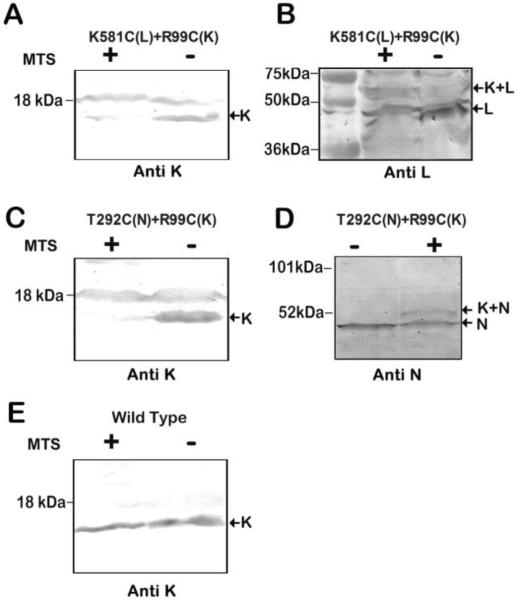
Membrane vesicles containing the double mutant K581C + R99C were treated with the bi-functional MTS cross-linking reagent M3M (6.5 Å span) (Loo and Clarke 2001), and the activity measurements were repeated. As shown in Fig. 3 there was little or no effect on NADH oxidase activity, or on the rate of NADH-driven proton translocation. Identical results were obtained with the double mutant T2921C + R99C, using the longer cross-linker M6M (10.4 Å span). Little or no effect was seen on NADH oxidase activity or on the rate of proton translocation. Cross-linking of subunits L and K was assessed by immunoblots, and the results are shown in Fig. 4. In general, the yield of such cross-linking reactions can be indicated by the extent of disappearance of bands for the individual subunits, and the appearance of a larger-sized band for the cross-linked product. In Fig. 4A and 4B, cross-linking results of the K581C + R99C double mutant are shown. As shown in panel 4A, the protein band for subunit K disappeared after treatment with M3M, but the K-L cross-linked product did not appear. The K antibody was raised against a C-terminal peptide of subunit K, so it is likely that a cross-link through residue 99 interfered with the binding of the antibody. The 18 kDa band is an artifact. In panel 4B, evidence for the K-L cross-linked product is shown by using antibody to L (nuoL-1). The band for L in the treated-lane has diminished, and a new band at higher molecular weight has appeared. In Fig. 4C and 4D, the results of cross-linking the T292C + R99C double mutant are shown. In panel 4C, using the K antibody for detection, the disappearance of K is apparent after treatment with M6M to generate cross-linking. In panel 4D, a low-yield cross-linked product could be confirmed by an HA antibody, which recognized the tagged N subunit. At the same time, the band for subunit N has diminished. Prior work has shown that some antibodies, even polyclonal ones, will fail to recognize a subunit in a cross-linked product, and so it can be difficult to determine an accurate yield (Zhu and Vik 2015). In panel E, the wild type is shown with (+) and without (−) M3M treatment, which can be seen to have no effect on the detection of subunit K.
Fig. 3.
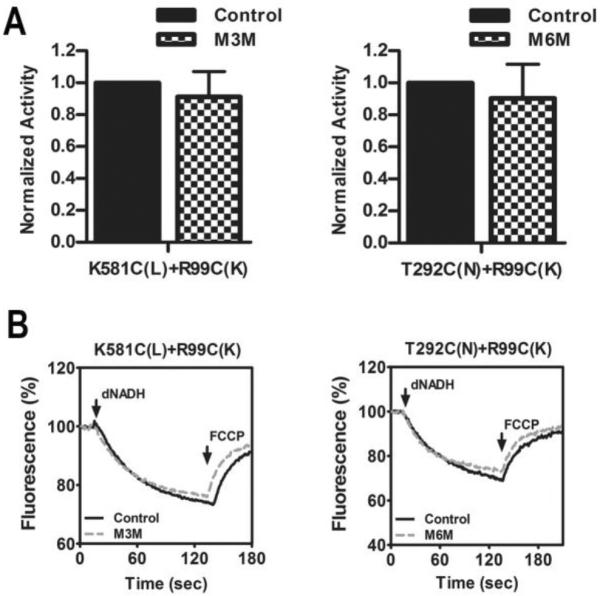
Cross-linking between subunit K and the lateral helix of subunit L, or subunit N using MTS reagents. (A) The effect of MTS treatment on the deamino-NADH oxidase activity of the two mutants is shown. Results are the means and standard errors of at least 4 measurements from at least 2 membrane preparations. (B) Proton translocation assays of the two mutants, with and without treatment with an MTS reagent. The fluorescence quenching of ACMA is initiated by deamino-NADH and is reversed by addition of FCCP.
Fig. 4.
Cross-linking results of mutants K581C (nuoL) + R99C (nuoK) and T292C (nuoN) + R99C (nuoK). (A, B) Representative immunoblots of membrane samples from K581C (nuoL) + R99C (nuoK) are shown, with and without treatment with M3M to promote cross-link formation. The cross-link between subunit L and K was generated by the MTS reagent M3M. In panel A antibody against K was used, and with MTS treatment, the band largely disappears. The 18 kDa band is an artifact. No cross-linked product was visible. In panel B antibody against L (nuoL-1) was used, and upon MTS treatment the L band diminishes, and a new, larger MW band appears, indicated by the arrow, K+L. (C, D) Representative immunoblots of membrane samples from T292C (nuoN) + R99C (nuoK) are shown, with and without treatment with the MTS reagent M6M to promote cross-link formation. In panel C antibody against K was used, and upon MTS treatment, the band for K disappears, but no cross-linked product was seen. The 18 kDa band is an artifact. In panel D antibody against N (HA epitope) was used, and upon MTS treatment, the band for N diminishes and a larger MW band appears, indicated by the arrow K+N. (E) Immunoblot of membrane samples from wild type is shown, with and without treatment with MTS reagent M3M. The antibody used was against subunit K.
C-terminal truncation mutants in K
To further probe the possible importance of the C-terminal amino acids of subunit K a series of deletions were constructed in the gene for the 100-residue protein. The codons for residues Gly100, Arg99, Met98 and Glu97 were replaced individually with stop codons, generating truncations of 1, 2, 3, and 4 amino acids at the C-terminus. The mutations were moved to the low-copy expression vector pBA400 and used to transform the nuo deletion strain BA14. The growth yields of the resulting strains were measured in succinate minimal medium, since a decreased growth yield in a minimal medium can be indicative of a faulty Complex I (Kervinen et al. 2006). The results are shown in Table 2. The strain with the G100stop mutation, lacking a single amino acid, grew nearly as well as the wild type, while the other three strains grew only slightly better than the nuo deletion strain BA14. These results were consistent with NADH oxidase assays of membrane vesicles which showed that the G100stop mutant had 83% of the wild type rate, while the other three were near the background rate (Table 2). Similarly, measurements of the rates of NADH-driven proton translocation showed that G100stop was nearly as active as the wild type, while the other three mutants had rates below the background level (Fig. 5). Ferricyanide reductase assays also showed that the activity of the G100stop mutant was nearly the same as wild type, as seen in Table 2. The other three mutants showed rates of ferricyanide reductase that were about half of the wild type rate, suggesting that Complex I assembled poorly, or was unstable, resulting in reduced amounts of the peripheral arm attached to the membrane.
Table 2.
| Mutation | Deamino-NADH oxidase activitya (nmoles/mg protein/min) | % Deamino-NADH oxidase activityb | Ferricyanide reductase activitya (nmoles/mg protein/min) | % Ferricyanide reductase activityb | % Growth Yield (n=3) |
|---|---|---|---|---|---|
| WT | 165±48 (15) | 100% | 1690±120 (3)c | 100% | 100 |
| G100stop | 137± (2) | 83% | 1602±173 (3) | 95% | 97±1 (4) |
| R99stop | 13.3±1 (3) | 8% | 949±16 (3) | 56% | 78±3 (4) |
| M98stop | 20±3 (2) | 12% | 1042±46 (3) | 62% | 74±1 (4) |
| E97stop | 14±0.15 (2) | 8.5% | 970±80 (3) | 57% | 73±1 (4) |
| BA14 | 18±3 (3) | 11% | 84±2 (3) | 5% | 71±1 (3) |
Activity was measured in membrane preparations as described in “Methods”. The mean, standard error and (number of measurements) are shown.
Activity is expressed as a percentage of the wild type rate.
Results from Michel et al. (2011)
Fig. 5.
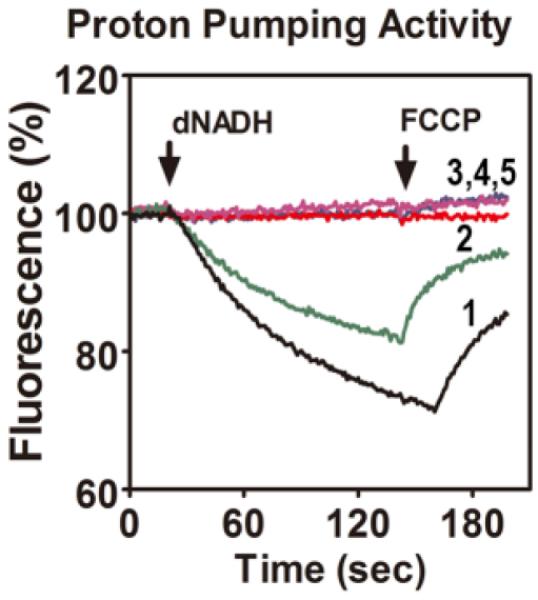
Proton translocation rates of the four stop codon substitutions in nuoK. The reactions were initiated with deamino-NADH (dNADH) to 250 μM final concentration. The fluorescence of ACMA (1 mM) was followed for several minutes. After about 2 minutes, the uncoupler FCCP was added (1 mM) to collapse the generated proton gradient. The wild type strain is shown for comparison (black, trace 1). The G100stop is shown in green, trace 2. The traces for R99stop, M98stop, and E97stop were identical (traces 3,4,5), and lacked any proton pumping activity. The traces shown are representative of 2–3 experiments.
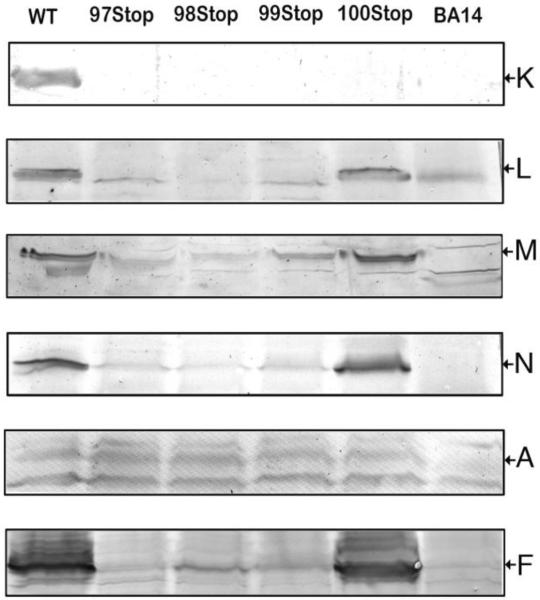
This issue was further explored by immunoblots of membrane vesicles, as shown in Fig. 6. The membrane samples were probed with antibodies to subunit A, F, K, L, M and N (tagged with an HA epitope). The antibody used is indicated at the right of the blots, and the position of the band for each subunit is indicated by the arrow. Since the antibody to subunit K was raised against an oligopeptide corresponding to the C-terminus, it was not surprising that none of the deletion mutants were recognized. Using the L antibody, bands were identified only in the wild type and the G100stop mutant. The faint bands are artifacts, as indicated by its presence in the null strain (right lane). Similar results were obtained using the M antibody or the HA antibody for subunit N, in which a strong band appeared in the 100stop lane, but trace amounts of the subunits also seemed to be visible for R99stop, M98stop, and E97stop. Subunit A is another membrane arm subunit that is found in proximity to subunit K. Using the A antibody, similar bands were identified in the wild type and all of the mutants, except the deletion strain BA14. Bands just above and below the band for A are artifacts. The presence of peripheral arm subunits F and G was also probed for. Using the F antibody, strong bands were identified only in the wild type and the G100stop mutant, but lower levels of F appeared in the lanes of the other mutants, similar to the results of M and N. Similar results were obtained using the G antibody (results not shown). The results support a partially assembled Complex I in the 97, 98, and 99 mutants. To control for the possibility that translation downstream of the mutations was blocked, due to coupled translation, a second plasmid, pUC19-LMN, was introduced into the cells containing the stop mutants. After induction with IPTG, the genes for L, M and N should be expressed. Immunoblots showed a high level of expression of M, but there was no corresponding increase in NADH oxidase activity. This suggested that low levels of subunits L, M and N are not likely to explain the lack of Complex I activity.
Fig. 6.
Immunoblots of membrane preparations from four stop codon substitutions in nuoK. Six independent blots were performed with each mutant, using antibodies against subunits K, L, M, N, A, and F. The antibodies used are indicated at the right of the blots. The arrows indicate the position of the band detected by the listed antibody. For comparison, the null strain, BA14, and the wild type strain, BA14/pBA400, were included in each blot. Each lane contained 40 μg of protein.
Discussion
Subunit K is composed of 3 TM helices and is an essential component of respiratory Complex I. The size and structure of the bacterial subunit K appears to be nearly identical to the bovine mitochondrial protein, ND4L (Vinothkumar et al. 2014). From the crystal structure of the T. thermophilus enzyme (Baradaran et al. 2013), subunit K was predicted to form part of a proton pumping unit, along with subunits H, J and A. It appears to contribute a conserved Glu residue in TM2 (Glu36 in E. coli) to the proton pathway. Mutagenesis has shown that this residue is likely to play a critical role in NADH-driven proton translocation (Kao et al. 2005b; Kervinen et al. 2004; Torres-Bacete et al. 2012). A second conserved Glu residue, in TM3, (Glu72 in E. coli) does not appear to be critical, but may be indirectly involved in function (Kao et al. 2005b; Kervinen et al. 2004).
Subunit K is homologous to MrpC, a component of the antiporter complex from Bacillus subtilis (Mathiesen and Hägerhäll 2003). MrpC does not contain either of the conserved Glu residues found in the Complex I subunits. It is predicted to have 3 TM helices, with a C-terminal extension of 15–20 amino acids beyond the third TM helix, similar to subunit K. In bacterial Complex I, the C-terminal extension of K has numerous interactions with the lateral helix of subunit L, as well as with subunits N and J. It is likely that Mrp and other antiporters also have a lateral helix, from sequence considerations (Virzintiene et al. 2013). We have recently provided evidence (Zhu and Vik 2015), through a cross-linking approach, that the lateral helix in the E. coli Complex I does not play a dynamic role in function. The results of others (Belevich et al. 2011; Steimle et al. 2015) also support that conclusion. Similarly, the results of the cross-linking approach presented in this report, suggest that the C-terminal extension of subunit K does not play a dynamic role in energy transduction or proton translocation. Cross-linking residue R99C of subunit K to either T292C of subunit N or to K581C of subunit L had no effect on activity. Since the cross-linking yield was low in each case, less than 50%, it is possible that restricting the C-terminus had a small effect on Complex I activity.
To test more directly the importance of the C-terminus of subunit K, genetic truncations of up to 4 residues were made. The side chains and backbone groups of these 4 residues make hydrogen bonds to residues of subunits J, L, and N. For example, according to the E. coli structure (Efremov and Sazanov 2011), Arg99 of subunit K contacts Glu587, Asn588, Gly589, Tyr590, and Tyr594, all in subunit L. Also, Met98 of subunit K contacts Arg151, Glu154, Ala155, Lys158, Asp229, Gln232, Gly233, and Arg296, all in subunit N. When the codons for residues 97–100 were replaced individually by a stop codon, the results fell into two groups. Truncation of the C-terminal residue, Gly100, resulted in a nearly wild type phenotype, in all respects. Truncation of 2, 3, or 4 residues resulted in a total loss of Complex I function. Reduced NADH:ferricyanide reductase activity indicated that Complex I assembly was impaired, resulting in decreased levels of the peripheral arm subunits necessary for reduction of ferricyanide. This was confirmed by immunoblots that indicated peripheral arm subunits F and G were greatly diminished in membrane preparations. Immunoblots also revealed that subunit L was no longer present in membrane preparations, and that M and N were greatly diminished. In contrast, subunit A appeared at the level of wild type, consistent with assembly of some of the subunits found at the junction of the membrane and peripheral arms. It is possible, but seems unlikely that subunit A could survive outside of a complex, since it has 3 TM spans that do not interact strongly with each other. As would be expected in these 3 mutants, NADH-driven proton translocation was absent, and NADH oxidase activity was at a background level.
Growth yield measurements were also consistent with a lack of Complex I function. In previous work (Kao et al. 2005b), a knockout of the nuoK gene resulted in a total loss of NADH:ferricyanide activity, and also the loss of other Complex I subunits from membrane preparations (see also (Erhardt et al. 2012)). That suggested a polar effect in transcription, or possibly translation, since all genes are transcribed in a single mRNA. The results presented here indicate a more deleterious effect on assembly and function of the complex. They suggest that the interactions of residue Arg99, and perhaps also Met98 and Glu97, from the C-terminus of subunit K are important for stabilizing a group of subunits, including K, L, and N. In the absence of these interactions, assembly of the complex is severely compromised
In conclusion, it does not appear that the C-terminus of K has a dynamic role in function. But several C-terminal residues of subunit K appear to make important interactions with other subunits in Complex I that are necessary for assembly.
Acknowledgements
This work was supported by grant R15GM099014 from the NIH. We thank the following students for technical assistance: April Wiseman, Caitlyn Le, Sarah Bruyere, Kayla Wilson, Emily Helm, Shivani Sharma, and Sarah Simmons. We also thank Alan J. Wolfe (Loyola University, Chicago IL USA) for providing plasmids.
Footnotes
Conflict of interest The authors declare no conflict of interest.
References
- Amarneh B, De Leon-Rangel J, Vik SB. Construction of a deletion strain and expression vector for the Escherichia coli NADH:ubiquinone oxidoreductase (Complex I) Biochim Biophys Acta. 2006;1757:1557–1560. doi: 10.1016/j.bbabio.2006.08.003. doi:10.1016/j.bbabio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Amarneh B, Vik SB. Mutagenesis of subunit N of the Escherichiacoli complex I. Identification of the initiation codon and the sensitivity of mutants to decylubiquinone. Biochemistry. 2003;42:4800–4808. doi: 10.1021/bi0340346. doi:10.1021/bi0340346. [DOI] [PubMed] [Google Scholar]
- Baradaran R, Berrisford JM, Minhas GS, Sazanov LA. Crystal structure of the entire respiratory complex I. Nature. 2013;494:443–448. doi: 10.1038/nature11871. doi:10.1038/nature11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belevich G, Knuuti J, Verkhovsky MI, Wikstrom M, Verkhovskaya M. Probing the mechanistic role of the long alpha-helix in subunit L of respiratory Complex I from Escherichiacoli by site-directed mutagenesis. Mol Microbiol. 2011;82:1086–1095. doi: 10.1111/j.1365-2958.2011.07883.x. doi:10.1111/j.1365-2958.2011.07883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efremov RG, Sazanov LA. Structure of the membrane domain of respiratory complex I. Nature. 2011;476:414–420. doi: 10.1038/nature10330. doi:10.1038/nature10330. [DOI] [PubMed] [Google Scholar]
- Erhardt H, Steimle S, Muders V, Pohl T, Walter J, Friedrich T. Disruption of individual nuo-genes leads to the formation of partially assembled NADH:ubiquinone oxidoreductase (complex I) in Escherichiacoli. Biochim Biophys Acta. 2012;1817:863–871. doi: 10.1016/j.bbabio.2011.10.008. doi:10.1016/j.bbabio.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Euro L, Belevich G, Verkhovsky MI, Wikstrom M, Verkhovskaya M. Conserved lysine residues of the membrane subunit NuoM are involved in energy conversion by the proton-pumping NADH:ubiquinone oxidoreductase (Complex I) Biochim Biophys Acta. 2008;1777:1166–1172. doi: 10.1016/j.bbabio.2008.06.001. doi:10.1016/j.bbabio.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Hirst J. Mitochondrial complex I. Annu Rev Biochem. 2013;82:551–575. doi: 10.1146/annurev-biochem-070511-103700. doi:10.1146/annurev-biochem-070511-103700. [DOI] [PubMed] [Google Scholar]
- Ito M, Guffanti AA, Oudega B, Krulwich TA. mrp, a multigene, multifunctional locus in Bacillus subtilis with roles in resistance to cholate and to Na+ and in pH homeostasis. J Bacteriol. 1999;181:2394–2402. doi: 10.1128/jb.181.8.2394-2402.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao MC, Di Bernardo S, Nakamaru-Ogiso E, Miyoshi H, Matsuno-Yagi A, Yagi T. Characterization of the membrane domain subunit NuoJ (ND6) of the NADH-quinone oxidoreductase from Escherichia coli by chromosomal DNA manipulation. Biochemistry. 2005a;44:3562–3571. doi: 10.1021/bi0476477. doi:10.1021/bi0476477. [DOI] [PubMed] [Google Scholar]
- Kao MC, Di Bernardo S, Perego M, Nakamaru-Ogiso E, Matsuno-Yagi A, Yagi T. Functional roles of four conserved charged residues in the membrane domain subunit NuoA of the proton-translocating NADH-quinone oxidoreductase from Escherichia coli. J Biol Chem. 2004;279:32360–32366. doi: 10.1074/jbc.M403885200. doi:10.1074/jbc.M403885200. [DOI] [PubMed] [Google Scholar]
- Kao MC, Nakamaru-Ogiso E, Matsuno-Yagi A, Yagi T. Characterization of the membrane domain subunit NuoK (ND4L) of the NADH-quinone oxidoreductase from Escherichia coli. Biochemistry. 2005b;44:9545–9554. doi: 10.1021/bi050708w. doi:10.1021/bi050708w. [DOI] [PubMed] [Google Scholar]
- Kervinen M, et al. The MELAS mutations 3946 and 3949 perturb the critical structure in a conserved loop of the ND1 subunit of mitochondrial complex I. Hum Mol Genet. 2006;15:2543–2552. doi: 10.1093/hmg/ddl176. doi:10.1093/hmg/ddl176. [DOI] [PubMed] [Google Scholar]
- Kervinen M, Patsi J, Finel M, Hassinen IE. A pair of membrane-embedded acidic residues in the NuoK subunit of Escherichia coli NDH-1, a counterpart of the ND4L subunit of the mitochondrial complex I, are required for high ubiquinone reductase activity. Biochemistry. 2004;43:773–781. doi: 10.1021/bi0355903. doi:10.1021/bi0355903. [DOI] [PubMed] [Google Scholar]
- Loo TW, Clarke DM. Determining the dimensions of the drug-binding domain of human P-glycoprotein using thiol cross-linking compounds as molecular rulers. J Biol Chem. 2001;276:36877–36880. doi: 10.1074/jbc.C100467200. doi:10.1074/jbc.C100467200. [DOI] [PubMed] [Google Scholar]
- Mathiesen C, Hägerhäll C. The `antiporter module' of respiratory chain Complex I includes the MrpC/NuoK subunit - a revision of the modular evolution scheme. FEBS Lett. 2003;549:7–13. doi: 10.1016/s0014-5793(03)00767-1. [DOI] [PubMed] [Google Scholar]
- Michel J, DeLeon-Rangel J, Zhu S, Van Ree K, Vik SB. Mutagenesis of the L, M, and N subunits of Complex I from Escherichia coli indicates a common role in function. PLoS One. 2011;6:e17420. doi: 10.1371/journal.pone.0017420. doi:10.1371/journal.pone.0017420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamaru-Ogiso E, Kao MC, Chen H, Sinha SC, Yagi T, Ohnishi T. The membrane subunit NuoL(ND5) is involved in the indirect proton pumping mechanism of Escherichia coli complex I. J Biol Chem. 2010;285:39070–39078. doi: 10.1074/jbc.M110.157826. doi:10.1074/jbc.M110.157826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamaru-Ogiso E, Yano T, Yagi T, Ohnishi T. Characterization of the iron-sulfur cluster N7 (N1c) in the subunit NuoG of the proton-translocating NADH-quinone oxidoreductase from Escherichia coli. J Biol Chem. 2005;280:301–307. doi: 10.1074/jbc.M410377200. doi:10.1074/jbc.M410377200. [DOI] [PubMed] [Google Scholar]
- Prüss BM, Nelms JM, Park C, Wolfe AJ. Mutations in NADH:ubiquinone oxidoreductase of Escherichia coli affect growth on mixed amino acids. J Bacteriol. 1994;176:2143–2150. doi: 10.1128/jb.176.8.2143-2150.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Sinha PK, Torres-Bacete J, Matsuno-Yagi A, Yagi T. Energy transducing roles of antiporter-like subunits in Escherichia coli NDH-1 with main focus on subunit NuoN (ND2) J Biol Chem. 2013;288:24705–24716. doi: 10.1074/jbc.M113.482968. doi:10.1074/jbc.M113.482968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimle S, Bajzath C, Dorner K, Schulte M, Bothe V, Friedrich T. Role of subunit NuoL for proton translocation by respiratory complex I. Biochemistry. 2011;50:3386–3393. doi: 10.1021/bi200264q. doi:10.1021/bi200264q. [DOI] [PubMed] [Google Scholar]
- Steimle S, et al. Cysteine scanning reveals minor local rearrangements of the horizontal helix of respiratory complex I. Mol Microbiol. 2015;98:151–161. doi: 10.1111/mmi.13112. doi:10.1111/mmi.13112. [DOI] [PubMed] [Google Scholar]
- Steimle S, Willistein M, Hegger P, Janoschke M, Erhardt H, Friedrich T. Asp563 of the horizontal helix of subunit NuoL is involved in proton translocation by the respiratory complex I. FEBS Lett. 2012;586:699–704. doi: 10.1016/j.febslet.2012.01.056. doi:10.1016/j.febslet.2012.01.056. [DOI] [PubMed] [Google Scholar]
- Torres-Bacete J, Nakamaru-Ogiso E, Matsuno-Yagi A, Yagi T. Characterization of the NuoM (ND4) subunit in Escherichia coli NDH-1: conserved charged residues essential for energy-coupled activities. J Biol Chem. 2007;282:36914–36922. doi: 10.1074/jbc.M707855200. doi:10.1074/jbc.M707855200. [DOI] [PubMed] [Google Scholar]
- Torres-Bacete J, Sinha PK, Castro-Guerrero N, Matsuno-Yagi A, Yagi T. Features of subunit NuoM (ND4) in Escherichia coli NDH-1: Topology and implication of conserved Glu144 for coupling site 1. J Biol Chem. 2009;284:33062–33069. doi: 10.1074/jbc.M109.059154. doi:10.1074/jbc.M109.059154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Bacete J, Sinha PK, Matsuno-Yagi A, Yagi T. Structural contribution of C-terminal segments of NuoL (ND5) and NuoM (ND4) subunits of complex I from Escherichia coli. J Biol Chem. 2011;286:34007–34014. doi: 10.1074/jbc.M111.260968. doi:10.1074/jbc.M111.260968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Bacete J, Sinha PK, Sato M, Patki G, Kao MC, Matsuno-Yagi A, Yagi T. Roles of subunit NuoK (ND4L) in the energy-transducing mechanism of Escherichia coli NDH-1 (NADH:quinone oxidoreductase) J Biol Chem. 2012;287:42763–42772. doi: 10.1074/jbc.M112.422824. doi:10.1074/jbc.M112.422824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhovskaya M, Bloch DA. Energy-converting respiratory Complex I: on the way to the molecular mechanism of the proton pump. Int J Biochem Cell Biol. 2013;45:491–511. doi: 10.1016/j.biocel.2012.08.024. doi:10.1016/j.biocel.2012.08.024. [DOI] [PubMed] [Google Scholar]
- Vinothkumar KR, Zhu J, Hirst J. Architecture of mammalian respiratory complex I. Nature. 2014;515:80–84. doi: 10.1038/nature13686. doi:10.1038/nature13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virzintiene E, Moparthi VK, Al-Eryani Y, Shumbe L, Gorecki K, Hägerhäll C. Structure and function of the C-terminal domain of MrpA in the Bacillus subtilis Mrp-antiporter complex - the evolutionary progenitor of the long horizontal helix in complex I. FEBS Lett. 2013;587:3341–3347. doi: 10.1016/j.febslet.2013.08.027. doi:10.1016/j.febslet.2013.08.027. [DOI] [PubMed] [Google Scholar]
- Zhu S, Vik SB. Constraining the lateral helix of respiratory Complex I by cross-linking does not impair enzyme activity or proton translocation. J Biol Chem. 2015;290:20761–20773. doi: 10.1074/jbc.M115.660381. doi:10.1074/jbc.M115.660381. [DOI] [PMC free article] [PubMed] [Google Scholar]