Abstract
Background
Hepatic recurrence after resection of colorectal liver metastasis (CLM) occurs in 50% of patients during follow-up, with 2.8% to 13.9% presenting with surgical margin recurrence (SMR). The aim of this study is to analyze factors that related to SMR in patients with CLM undergoing hepatectomy.
Methods
Demographics, clinical and survival data of patients who underwent hepatectomy were identified from a prospectively maintained, institutional review board (IRB)-approved database between 2000 and 2012. Statistical analysis was performed using univariate Kaplan Meier and Cox proportional hazard model.
Results
There were 85 female and 121 male patients who underwent liver resection for CLM. An R0 resection was performed in 157 (76%) patients and R1 resection in 49. SMR was detected in 32 patients (15.5%) followed up for a median of 29 months (range, 3–121 months). A half of these patients had undergone R1 (n=16) and another half R0 resection (n=16). Tumor size, preoperative carcinoembryonic antigen (CEA) level and margin status were associated with SMR on univariate analysis. On multivariate analysis, a positive surgical margin was the only independent predictor of SMR. The receipt of adjuvant chemotherapy did not affect margin recurrence. SMR was an independent risk factor associated with worse disease-free (DFS) and overall survival (OS).
Conclusions
This study shows that SMR, which can be detected in up to 15.5% of patients after liver resection for CLM, adversely affects DFS and OS. The fact that a positive surgical margin was the only predictive factor for SMR in these patients underscores the importance of achieving negative margins during hepatectomy.
Keywords: Colorectal liver metastasis (CLM), surgical margin recurrence (SMR), liver resection
Introduction
In 2013, 142,000 new patients with colorectal cancer were diagnosed in the United States (1). Although, the incidence and mortality of colorectal cancer have been declining over the recent years, about 10–25% of these patients present with synchronous liver metastasis; while another 20–50% will develop metachronous liver metastases during the course of their disease (2-6). Liver resection is the only potentially curative treatment in these patients, resulting in 5-year survival rates of 32% to 58% (2,3,6-11). However, hepatic recurrence occurs in 50% of patients during follow-up, with 2.8% to 13.9% presenting with surgical margin recurrence (SMR) (6,11-15). SMR is not reported consistently in the hepatectomy literature. Furthermore, there are very few studies focusing on the incidence and predictors of SMR (12,14,15).
The aim of this study is to define the incidence and identify predictors of SMR after hepatectomy in patients with colorectal liver metastasis (CLM).
Materials and methods
Patients who underwent liver resection for CLM between April 2000 and April 2012 were identified from a prospectively maintained, institutional review board (IRB)-approved database. The details of the surgical technique were described previously (16). An intraoperative ultrasound was performed in all procedures, whereas frozen section was not performed routinely to assess margins. An R0 resection was defined as no malignant cells seen at surgical margin on final pathology.
A major hepatic resection was defined as three or more liver lobe resection according to Brisbane International Hepato-Pancreato-Biliary Association (IHPBA) classification (17). The patients were followed up with abdominopelvic and chest CTs quarterly for the first 2 years and then biannually. The scans were reviewed by independent radiologists. An SMR was defined as a recurrence seen along the resection line on follow-up imaging. Magnetic resonance imaging (MRI) and positron emission tomography (PET) scans were not obtained routinely in follow-up.
Demographic, clinical, and survival data were assessed with the univariate Kaplan–Meier analysis. Those parameters with a significance of P<0.1 on Kaplan–Meier univariate analyses were entered into a multivariate Cox proportional hazards model. Continuous data are presented as mean ± SEM. A P value of <0.05 was accepted for statistical significance.
Results
There were a total of 206 patients with a mean age of 62.1±11.2. Eighty-five patients were female and 121 male. The average tumor size was 3.8 cm and number of tumors 1.7. Thirty-five percent (n=73) of the procedures were major and 65% (n=133) minor hepatectomies. An R0 resection was performed in 157 patients (76%) and R1 resection in 49 (24%). The patients were followed up for a median of 29 months (range, 3–121 months). An SMR was detected in 32 patients (15.5%), with an incidence of 32.6% (n=16) after an R1, and 10% (n=16) after an R0 resection (P<0.0001). Surgical margin width was <5 mm in 60 patients, 5–10 mm in 48, >10 mm in 43 and unknown in 6. In these subgroups, SMR was detected in 11%, 9%, 10%, and 0%, respectively. Demographic, clinical, intraoperative and oncologic parameters are summarized in Tables 1,2.
Table 1. Demographic, clinical and perioperative data of study patients (n=206).
| Parameter | N |
|---|---|
| Age | 62.1±11.2 |
| Gender (F/M) | 85/121 |
| ASA status | 2.7±0.5 |
| Primary tumor (colon/rectum) | 129/77 |
| Type of metastasis (synchronous/metachronous) | 79/127 |
| Liver involvement (unilobar/bilobar) | 178/28 |
| Number of liver metastases | 1.7±1.1 |
| Tumor size (cm) | 3.8±2.3 |
| Preoperative CEA level (ng/mL) | 80.6±321.1 |
| Type of resection (minor/major) | 133/73 |
| Estimated blood loss (mL) | 604±709 |
| Blood transfusion, n (%) | 29 (14%) |
| Operative time (min) | 261±103 |
| Margin status (R0/R1) | 157/49 |
| Complications | 60 (29%) |
| ICU stay | 62 (30%) |
| Hospital stay (day) | 6.5±4.0 |
Continuous data are presented as mean ± standard error of the mean. F, female; M, male; ASA, American Society of Anesthesiologist score; CEA, carcinoembryonic antigen; ICU, intensive care unit.
Table 2. Oncological parameters in the study groups.
| Parameters | N (%) |
|---|---|
| Preoperative chemotherapy | 166 (80.0) |
| Postoperative chemotherapy | 120 (58.0) |
| Surgical margin recurrence | 32 (15.5) |
| New liver recurrence | 62 (30.0) |
| New extrahepatic disease | 76 (36.8) |
On univariate analysis, factors affecting SMR were tumor size (P<0.0001), preoperative carcinoembryonic antigen (CEA) level (P<0.0001), and margin status (P<0.0001). On multivariate analysis, a positive surgical margin was the only independent predictor of SMR. A positive surgical margin was associated with a 3.6 fold-increased risk of SMR in follow-up (P=0.0007, 95% CI: 1.7–7.4). There were 166 (80%) patients who received neoadjuvant chemotherapy. Surgical margin clearance was similar in patients who received and did not receive neoadjuvant chemotherapy (23% vs. 24%). The receipt of adjuvant chemotherapy did not affect SMR, either.
Figure 1 shows the development of SMR for both the R0 and R1 resections in a Kaplan Meier format. After an R0 and R1 resection, 5-year disease-free survival (DFS) was 19% and 11%, respectively (P=0.318) and 5-year overall survival (OS) 54% and 51% (P=0.363), respectively. SMR was an independent predictor of worsened DFS (P<0.0001) and OS (P=0.009) (Tables 3,4).
Figure 1.
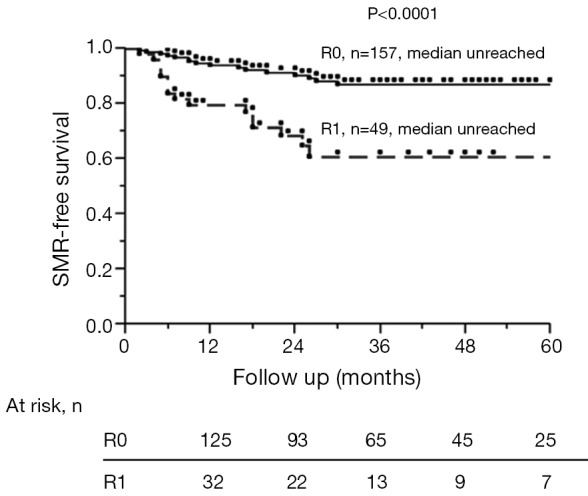
Kaplan-Meier SMR-free survival in patients who underwent R0 and R1 resections. SMR, surgical margin recurrence.
Table 3. Univariate Kaplan-Meier and multivariate cox proportional hazard model for disease-free survival (DFS).
| Variable | No. of patients | Median survival (months) | Univariate, P value | Multivariate | |
|---|---|---|---|---|---|
| HR (95% CI) | P value | ||||
| Age | 0.793 | – | – | ||
| ≤65 | 120 | 15 | |||
| >65 | 86 | 20 | |||
| Gender | 0.836 | – | – | ||
| Female | 85 | 18 | |||
| Male | 121 | 17 | |||
| ASA status | 0.010 | ||||
| I-II | 56 | 24 | |||
| III-IV | 150 | 15 | 1.5 (1.0–2.3) | 0.044 | |
| Primary tumor | 0.08 | ||||
| Colon | 129 | 16 | 1.1 (0.6–1.1) | 0.479 | |
| Rectum | 77 | 21 | |||
| Metastases type | 0.031 | ||||
| Synchronous | 79 | 14 | 1.5 (1.0–2.1) | 0.019 | |
| Metachronous | 127 | 20 | |||
| No. of metastases | 0.027 | ||||
| ≤3 | 187 | 18 | |||
| >3 | 19 | 12 | 1.8 (0.9–3.1) | 0.071 | |
| Tumor size | 0.837 | – | – | ||
| <3 | 82 | 15 | |||
| 3–5 | 78 | 21 | |||
| >5 | 46 | 19 | |||
| Tumor location | 0.476 | – | – | ||
| Unilobar | 178 | 18 | |||
| Bilobar | 28 | 14 | |||
| Margin status | 0.318 | – | – | ||
| R0 | 157 | 19 | |||
| R1 | 49 | 14 | |||
| CEA (ng/mL) | 0.067 | ||||
| ≤10 | 98 | 21 | |||
| >10 | 108 | 15 | 1.4 (1.0–2.0) | 0.038 | |
| Resection type | 0.146 | – | – | ||
| Major | 73 | 15 | |||
| Minor | 133 | 21 | |||
| Preop chemotherapy | 0.141 | – | – | ||
| Yes | 166 | 16 | |||
| No | 40 | 34 | |||
| Postop chemotherapy | 0.997 | – | – | ||
| Yes | 120 | 16 | |||
| No | 86 | 18 | |||
| Surgical margin recurrence | <0.0001 | ||||
| Yes | 32 | 6.5 | 3.0 (1.9–4.5) | <0.0001 | |
| No | 174 | 22 | |||
| Blood transfusion | 0.227 | – | – | ||
| Yes | 29 | 15 | |||
| No | 177 | 18 | |||
Parameters showing a statistical significance P<0.10 on univariate analysis were entered into the multivariate model. HR, hazard ratio; CI, confidence interval; ASA, American Society of Anesthesiologist score; CEA, carcinoembriyogenic antigen.
Table 4. Univariate Kaplan-Meier and multivariate cox proportional hazard model of overall survival (OS).
| Variable | No. of patients | Median survival (months) | Univariate, P value | Multivariate | |
|---|---|---|---|---|---|
| HR (95% CI) | P value | ||||
| Age | 0.446 | – | – | ||
| ≤65 | 120 | 43 | |||
| >65 | 86 | 38 | |||
| Gender | 0.263 | – | – | ||
| Female | 85 | 63 | |||
| Male | 121 | 67 | |||
| ASA status | 0.142 | – | – | ||
| I-II | 56 | 64 | |||
| III-IV | 150 | 50 | |||
| Primary tumor | 0.919 | – | – | ||
| Colon | 129 | 60 | |||
| Rectum | 77 | 62 | |||
| Metastases type | 0.397 | – | – | ||
| Synchronous | 79 | 60 | |||
| Metachronous | 127 | 63 | |||
| No. of metastases | 0.890 | – | – | ||
| ≤3 | 187 | 63 | |||
| >3 | 19 | – | |||
| Tumor size | 0.417 | – | – | ||
| <3 | 82 | 68 | |||
| 3–5 | 78 | 64 | |||
| >5 | 46 | 45 | |||
| Tumor location | 0.386 | – | – | ||
| Unilobar | 178 | 63 | |||
| Bilobar | 28 | – | |||
| Margin status | 0.363 | – | – | ||
| R0 | 157 | 63 | |||
| R1 | 49 | 67 | |||
| CEA (ng/mL) | 0.0002 | ||||
| ≤10 | 98 | 87 | |||
| >10 | 108 | 43 | 2.2 (1.4–3.5) | 0.0003 | |
| Resection type | 0.866 | – | – | ||
| Major | 73 | 60 | |||
| Minor | 133 | 67 | |||
| Preop chemotherapy | 0.352 | – | – | ||
| Yes | 166 | 60 | |||
| No | 40 | 67 | |||
| Postop chemotherapy | 0.941 | – | – | ||
| Yes | 120 | 63 | |||
| No | 86 | 56 | |||
| Surgical margin recurrence | 0.003 | ||||
| Yes | 32 | 38 | 2.0 (1.1–3.3) | 0.010 | |
| No | 174 | 64 | |||
| Blood transfusion | 0.101 | – | – | ||
| Yes | 29 | 37 | |||
| No | 177 | 63 | |||
Parameters showing a statistical significance P<0.10 on univariate analysis were entered into the multivariate model. HR, hazard ratio; CI, confidence interval; ASA, American Society of Anesthesiologist score; CEA, carcinoembriyogenic antigen.
Discussion
This study documents the incidence of SMR after liver resection in patients with CLM and analyzes the possible predictive factors. The incidence of R0 resection in our series (76%) is similar to that reported in the literature (4,11,13-15,18). This reflects our aggressive utilization of resection in the treatment of CLM. In this cohort of patients who had intense follow-up after hepatectomy with regular office visits and imaging studies, the incidence of SMR was 15.5%. A positive surgical margin was the only independent predictor of SMR. Furthermore, SMR was found to adversely affect both DFS and OS. The current study is one of the few reports in the literature critically analyzing SMR after hepatectomy and documenting an associated worse DFS and OS (11,12,19).
Hepatic resection is the only curative treatment for CLM, but recurrence develops in 50% of patients during follow-up, with up to 13.9% of recurrences occurring at the surgical margin (6,11-15). There is no consensus about the impact of positive surgical margin on SMR. Some authors have reported that an increased incidence of SMR after an R1 resection, whereas others have not shown a difference between R1 and R0 resection (12,14,15,18,20). Among these studies, only one multicenter study, by Pawlik et al., analyzed parameters that may affect SMR (12). In this report, a positive surgical margin was the only factor that affected SMR. The results of the current study are similar, with tumor size, CEA and margin status being associated with SMR in univariate analysis and margin status being the only independently predictor on multivariate analysis.
Although, the goal of a hepatic resection for CLM is to obtain negative margins, this is not possible in 7.6% to 27% of the patients (3,4,8,10,12,15,18). Larger tumor size, bilobar involvement, intraoperative blood transfusion, higher CEA levels and the presence of >3 tumors were reported to be risk factors for an R1 resection (11,18,21). In the current series, the incidence of a positive surgical margin was 24%. The effect of a positive surgical margin on survival is controversial. Some authors have reported worse DFS or OS with a positive surgical margin; whereas others have not (3,4,9,11-13,15,18,19,22). Recently Sadot et al. has reported a study of 2,368 patients who underwent liver resection for CLM from The Memorial Sloan Kettering Cancer Center. In this study it was shown that a larger surgical margin width was associated with better OS. Patients with surgical margin clearance of 1 mm or more had a better OS compared patients with submillimeter surgical margin. Patients with submillimeter surgical margin also had a better OS than patients with positive surgical margin (23). In the current study, the width of the negative surgical margin didn’t affect DFS or OS but, SMR was found to negatively affect both DFS and OS. To the best of our knowledge, this association has not been analyzed or reported in the literature. Although some studies have suggested that R1 resections are still associated with long-term survival (6,13,15,18), our results show that every effort should be made for an R0 resection during liver resection.
The effect of chemotherapy on the development of SMR after an R0 or R1 resection is controversial. In one study, the receipt of adjuvant chemotherapy did not affect the SMR rate after an R0 or R1 resection (20). Eveno et al. has reported on 86 patients who underwent hepatectomy for CLM. An R0 resection was achieved in 73% and R1 resection 27%. Although the 5-year OS and DFS were not different between the two groups, intrahepatic recurrences and SMR were more frequent in the R1 group (52% vs. 27%, respectively) (15). In the present study, neo-adjuvant or adjuvant chemotherapy did not affect the incidence of SMR.
The limitations of the current study are the retrospective nature of data collection and the fact that multiple surgeons were involved in the procedures. Nevertheless, it critically analyzes an important issue in liver resection for CLM, SMR, for which there are scant data in the literature.
Conclusions
An R1 resection is a risk factor for SMR, which adversely affects DFS and OS. Therefore, every effort should be made to achieve a negative margin during liver resection for CLM.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. 10.3322/caac.21166 [DOI] [PubMed] [Google Scholar]
- 2.Morris EJ, Forman D, Thomas JD, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg 2010;97:1110-8. 10.1002/bjs.7032 [DOI] [PubMed] [Google Scholar]
- 3.Rees M, Tekkis PP, Welsh FK, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247:125-35. 10.1097/SLA.0b013e31815aa2c2 [DOI] [PubMed] [Google Scholar]
- 4.de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg 2009;250:440-8. [DOI] [PubMed] [Google Scholar]
- 5.Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist 2008;13:51-64. 10.1634/theoncologist.2007-0142 [DOI] [PubMed] [Google Scholar]
- 6.Inoue Y, Hayashi M, Komeda K, et al. Resection margin with anatomic or nonanatomic hepatectomy for liver metastasis from colorectal cancer. J Gastrointest Surg 2012;16:1171-80. 10.1007/s11605-012-1840-7 [DOI] [PubMed] [Google Scholar]
- 7.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21. 10.1097/00000658-199909000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konopke R, Kersting S, Makowiec F, et al. Resection of colorectal liver metastases: is a resection margin of 3 mm enough?: a multicenter analysis of the GAST Study Group. World J Surg 2008;32:2047-56. 10.1007/s00268-008-9629-2 [DOI] [PubMed] [Google Scholar]
- 9.Hayashi M, Inoue Y, Komeda K, et al. Clinicopathological analysis of recurrence patterns and prognostic factors for survival after hepatectomy for colorectal liver metastasis. BMC Surg 2010;10:27. 10.1186/1471-2482-10-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mavros MN, de Jong M, Dogeas E, et al. Impact of complications on long-term survival after resection of colorectal liver metastases. Br J Surg 2013;100:711-8. 10.1002/bjs.9060 [DOI] [PubMed] [Google Scholar]
- 11.Tranchart H, Chirica M, Faron M, et al. Prognostic impact of positive surgical margins after resection of colorectal cancer liver metastases: reappraisal in the era of modern chemotherapy. World J Surg 2013;37:2647-54. 10.1007/s00268-013-2186-3 [DOI] [PubMed] [Google Scholar]
- 12.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 2005;241:715-22, discussion 722-4. 10.1097/01.sla.0000160703.75808.7d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodingbauer M, Tamandl D, Schmid K, et al. Size of surgical margin does not influence recurrence rates after curative liver resection for colorectal cancer liver metastases. Br J Surg 2007;94:1133-8. 10.1002/bjs.5762 [DOI] [PubMed] [Google Scholar]
- 14.Muratore A, Ribero D, Zimmitti G, et al. Resection margin and recurrence-free survival after liver resection of colorectal metastases. Ann Surg Oncol 2010;17:1324-9. 10.1245/s10434-009-0770-4 [DOI] [PubMed] [Google Scholar]
- 15.Eveno C, Karoui M, Gayat E, et al. Liver resection for colorectal liver metastases with peri-operative chemotherapy: oncological results of R1 resections. HPB (Oxford) 2013;15:359-64. 10.1111/j.1477-2574.2012.00581.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsinberg M, Tellioglu G, Simpfendorfer CH, et al. Comparison of laparoscopic versus open liver tumor resection: a case-controlled study. Surg Endosc 2009;23:847-53. 10.1007/s00464-008-0262-9 [DOI] [PubMed] [Google Scholar]
- 17.Strasberg SM, Phillips C. Use and dissemination of the brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg 2013;257:377-82. 10.1097/SLA.0b013e31825a01f6 [DOI] [PubMed] [Google Scholar]
- 18.de Haas RJ, Wicherts DA, Flores E, et al. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg 2008;248:626-37. [DOI] [PubMed] [Google Scholar]
- 19.Mbah NA, Scoggins C, McMasters K, et al. Impact of hepatectomy margin on survival following resection of colorectal metastasis: the role of adjuvant therapy and its effects. Eur J Surg Oncol 2013;39:1394-9. 10.1016/j.ejso.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 20.Ayez N, Lalmahomed ZS, Eggermont AM, et al. Outcome of microscopic incomplete resection (R1) of colorectal liver metastases in the era of neoadjuvant chemotherapy. Ann Surg Oncol 2012;19:1618-27. 10.1245/s10434-011-2114-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Are C, Gonen M, Zazzali K, et al. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg 2007;246:295-300. 10.1097/SLA.0b013e31811ea962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei AC, Greig PD, Grant D, et al. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol 2006;13:668-76. 10.1245/ASO.2006.05.039 [DOI] [PubMed] [Google Scholar]
- 23.Sadot E, Groot Koerkamp B, Leal JN, et al. Resection margin and survival in 2368 patients undergoing hepatic resection for metastatic colorectal cancer: surgical technique or biologic surrogate? Ann Surg 2015;262:476-85; discussion 483-5. 10.1097/SLA.0000000000001427 [DOI] [PMC free article] [PubMed] [Google Scholar]


