Abstract
Background
In-stent restenosis following the insertion of conventional drug-eluting stent has become an extremely serious problem due to coating techniques, with polymer matrices used to bind biological ingredients to the stent surface. However, several studies have indicated that new pro-healing technique could prevent stent thrombosis that can be caused by conventional drug-eluting stents.
Methods
A novel method of attaching anti-CD34 antibodies directly on the porous surface of a 316L stainless steel bare metal stent was developed in this study, which achieved both high stability of attached anti-CD34 antibodies on the metal stent surface and high antibody activity for stem cell capture.
Results
The in vitro and in vivo experimental results indicated that the new stent with directly coupled anti-CD34 antibodies can efficiently enhance stent endothelialization.
Conclusions
This study indicates that we have developed a unique method of attaching anti-CD34 antibodies directly on the porous surface of a 316L stainless steel bare metal stent, which provides a novel polymer-free approach for developing pro-healing stents.
Keywords: Adsorption, Endothelialization, Nano-porous surface, Self-assembly
INTRODUCTION
Drug-eluting stents induce incomplete and/or delayed endothelialization that can lead to stent thrombosis. Thus, their increased use around the globe has raised significant concerns by clinicians as they decide whether DESs are appropriate for their patients.1-6 A pro-healing approach has been proposed to facilitate endothelialization, which is achieved by eluting eNOS/VEGF/curcumin/epigallocatechin gallate to promote endothelial cell proliferation7-11 or to capture endothelial progenitor cells (EPCs) from blood using anti-CD34 antibodies or Arg-Gly-Asp peptides.1,7,8,12 EPCs could differentiate into endothelial cells, facilitate stent endothelialization, and decrease in-stent restenosis.13,14 A biological stent (OrbusNeich, Hong Kong, China) has been developed with anti-CD34 antibodies covalently attached on the metal stent surface covered with a biocompatible polymer matrix. Clinical trials have indicated that the stent significantly reduced stent thrombosis. However, the decrease of in-stent restenosis has been significantly compromised because of certain negative effects of the polymer matrix. 1,15
The polymer matrix is essential for protein immobilization on the metal surface because chemical coupling reactions use active groups, such as -CHO, -NH2 or -COOH.16-19 Previous studies have developed various methods to improve the hemocompatibility of polymer coatings, including the use of a novel poly-1, 8-octanediol-co-citric acid polymer incorporated with vascular endothelial growth factor20 and coated with plasma derivatives, such as tropoelastin,21 heparin-collagen multilayer,22 and plasma polymerized n-butyl methacrylate.23,24
Moreover, researchers have indicated that the microparticles of stainless steel can adsorb protein and then induce the aggregation of antibodies that remain active on the metal surface.25 Thus, the present study proposes that a stable adsorption of antibodies directly on the stent surfaces at a density comparable with immobilizing antibodies on polymer matrices can be achieved, and a relatively low amount of EPCs from the high velocity of coronary blood can be effectively captured to avoid the potential negative effects of polymers and to retain antibody activity fully, which could be lost during the chemical coupling reaction.16,18,19 In addition, the polymer-free approach will greatly simplify the stent manufacturing process.
This study has for the first time developed a method of attaching anti-CD34 antibodies directly on the porous surface of a 316L stainless steel bare metal stent (BMS). The new method achieves both high stability of attached anti-CD34 antibodies on the metal stent surface and high antibody activity for stem cell capture. The in vitro and in vivo experimental results indicate that the new stent with directly coupled anti-CD34 antibodies could enhance stent endothelialization as well as avoid the negative effects of the polymer matrix.
MATERIALS AND METHODS
Materials
BMS (316L stainless steel) were obtained from Lepu Medical Technology Co. Ltd. (Beijing, China). Mouse anti-human CD34 monoclonal antibody was sourced from Abcam (Cambridge, MA, USA), and 48 measurements were taken for each group. Fluorescein isothiocyanate-labeled goat anti-mouse monoclonal immunoglobulin (FITC-IgG) and horseradish peroxidase conjugated goat anti-mouse immunoglobulin (HRP-IgG) were from BD Biosciences (San Jose, CA, USA). Moreover, the 4′-6-diamidino-2-phenylindole (DAPI), Roswell Park Memorial Institute (RPMI) 1640, 4′,6-diamidino-2-phenylindole, diaminobenzidine, and tetramethylbenzidine (TMB) were from Amresco (Solon, OH, USA). KG-1a cells were obtained from American Type Culture Collection (No. CCL-246.1, ATCC, USA), which is a type of leukemia cell line. Most of the KG-1a cells are CD34+, and the KG-1a cell lines tend to be a subpopulation of cancer stem cell-like cells.
Preparation of nano-porous on a 316L stainless steel BMS surface
An electrochemical method was used to produce etched pits on the surface of a 316L stainless steel BMS. Briefly, the BMS was pretreated in 20% HCl solution for 10 h at 20 °C, washed with 75% ethanol, dried in air, and then connected with positive electrode in 10% HCl solution for 10 min to prepare nanopores (current intensity, 0.2 A/cm2; frequency, 500 HZ). The titanium plate was connected with a negative electrode.
The average size and depth of pores of the 316L stainless steel BMS, after being washed with 75% ethanol for 15 min at 100 kHz ultrasonicator and dried in a stream of filtered air at room temperature, were observed and measured by a scanning electron microscope (SEM, S-4800, Hitachi High-Tech Corp., Tokyo, Japan).
Adsorption of anti-CD34 antibodies on metal stent surface
The nano-porous stents were incubated for 10 to 60 min at 37 °C in different buffers [0.1 mM citrate buffer saline, pH 4.5 or 0.1 mM phosphate buffered saline (PBS), pH 7.2 or 0.1 mM of carbonate sodium buffer, pH 9.6] containing various concentrations (10, 20, 50, 100, and 200) of mouse anti-human CD34 monoclonal antibody (Abcam, USA).
Analysis of attached antibodies
The amount of anti-CD34 antibodies on the nano-porous surface of BMS was analyzed using a fluorescence microscope and enzyme-linked immunosorbent assay (ELISA).
For fluorescence microscopic observation, the stents were blocked with bovine serum albumin (BSA) in 10 mM PBS (pH 7.2) for 24 h at 4 °C and washed three times with 10 mM PBS containing 0.1% Tween-20. The stents were then incubated with FITC-IgG (BD Biosciences, San Jose, CA, USA) diluted (1:500) in 10 mM PBS (pH 7.2) for 2 h at 37 °C, then rinsed three times with 10 mM PBS and examined on a fluorescence microscope (Olympus, Tokyo, Japan).
For ELISA, the stent surface was blocked with 10% BSA in 10 mM PBS, (pH 7.2) for 24 h at 4 °C and washed three times with 10 mM PBS containing 0.1% Tween-20. The stents were then incubated with HRP-IgG (BD Biosciences, USA) diluted (1:500) in 10 mM PBS (pH 7.2) for 1 h at 37 °C, washed three times, whereafter colorization solution was added containing 0.1 M of TMB and incubated for 15 min at 37 °C. The reaction was terminated with 0.5 mL of 1 M H2SO4, and the absorbance at 450 nm was measured with a microplate reader (Labsystems Diagnostics Oy, Vantaa, Finlan).
Open-circuit potential
Open-circuit potential was conducted using a PARATAT 2273 electrochemical impedance spectroscopy (Potentiostat, Princeton Applied Research. Ltd, USA) to investigate the change of properties of the BMS nano-porous surface upon antibody immobilization. Briefly, voltage of the BMS nano-porous surface was continuously measured after immersion in 0.01 mM PBS (pH 7.2) containing 100 ng/mL anti-CD34 antibodies. The voltages of BMS and anti-CD34 antibody-modified stents (CAMS) were also determined (CAMS: -0.082 ± 0.008 V; BMS: -0.031 ± 0.007 V). A working electrode with a platinum patch served as the counter electrode, and a saturated calomel electrode served as the reference electrode.
In vitro CD34+ cell-capturing activity of the CAMS
The stents were incubated in RPIM 1640 medium with 1 × 106/mL CD34+ KG-1a cells stained with DAPI under the flow velocity of 27 cm/s for 1 h in a coronary flow simulator26 to determine the in vitro cell-capturing activity of the CAMS. After being rinsed three times with 10 mM PBS, the stents were photographed using a fluorescence microscope (OLYMPUS BX41, Japan), and the adherent cell number was quantified using Anymicro Photo System (YuTian Technology, China).
Nano-porous 316L stainless steel plates and anti-CD34 antibody-modified stainless steel plates were prepared as described above to further evaluate the cell-capturing activity and cell viability of the captured CD34+ cells on the metal surface. Human umbilical vein endothelial cells (HUVECs) were isolated from a newborn umbilical cord, cultured in RPIM 1640 medium, and then incubated at 37 °C in a humidified atmosphere of 5% CO2 as described previously. In excess of 40% of the HUVECs expressed CD34+, as determined by flow cytometry (BD FACS AriaTM SORP).27 The nano-porous 316L stainless steel plates and anti-CD34 antibody-modified stainless steel plates were incubated in RPIM 1640 medium containing 1 × 106/mL HUVECs at 1,000 rpm for 1 h at 37 °C. After being washed three times with 10 mM PBS, the plates were observed on an optical microscope (CKX41SF, OLYMPUS, Japan), and the adherent cell number was quantified using Anymicro Photo System (YuTian technology, China).
In vivo tests
In vivo tests were carried out by randomly implanting the BMS (n = 12) and CAMS (n = 12) in the right coronary artery, left anterior descending artery, and left circumflex artery of nine China miniature swine (batch number CEMPI20151132, male, 15-18 months of age, 27.5-30.2 kg; Chinese Experimental Miniature Pig Breeding Center, China Agricultural University, Beijing, China). Three pigs were killed 2 h and 48 h after stent implantation to evaluate the endothelium functions by SEM. Six pigs were killed after 7 d to quantify the stent endothelialization by a DAPI cell coverage scoring.
For SEM evaluation, the stented arteries were fixed in 3% glutardialdehyde for at least 1 h, dehydrated in a graded acetone series (30, 50, 70, 90, and 3 × 100%), and then critical-point-dried in CO2. The samples were fixed on specific stubs and sputter-coated with gold (SCD 030, Balzers Union) and then analyzed in a high-vacuum mode on a Quanta 200 SEM (FEI Company, Hillsboro, OH, USA).
For DAPI cell coverage scoring, the stented artery segments were extracted from pigs after 7 d and stained with DAPI for 1 h at 37 °C and then photographed on a fluorescence microscope (OLYMPUS BX41, Japan). The stent endothelialization scores were calculated as the averages of six independent scorings (n = 6) by the extent of the stent strut covered by endothelium as follows: 1) essentially no cells; 2) some interspersed cells; 3) localized cell density in certain areas; 4) consistent cell density covering most of the stent.28,29 Then the average score was calculated by different areas.
Statistical analysis
All results were expressed as mean ± SD of each sample, and each experiment was repeated independently three times. One-way analysis of variance was conducted using an OriginTM 8.0 program (MicroCal, USA) for data comparison. A value of p < 0.05 was considered significant. All measurements were assumed independent and normally distributed.
RESULTS
Preparation of nano-porous on 316L stainless steel BMS
An electrochemical process as earlier described was used to create etched pits on the surface of BMS (Figure 1A). The SEM results revealed that the pore size was 384.1 ± 160.6 nm wide and 127.7 ± 49.4 nm deep in micro scale.
Figure 1.
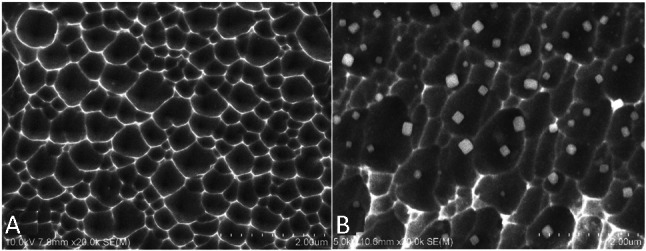
SEM images (20,000×) of the surface of bare metal stent (A) and anti-CD34 antibodies modified stent (B). The SEM results revealed that the pore size was 384.1 ± 160.6 nm wide and 127.7 ± 49.4 nm deep. SEM, scanning electron microscope.
Adsorption of anti-CD34 antibodies on BMS surface
In the present study, anti-CD34 antibodies were attached directly on the porous surface of BMS though adsorption of microparticles. Antibody particles (approximately 200 nm) were observed with SEM (Figure 1B) primarily on the ridge of the porous metal landform. Fluorescence observation revealed that the antibody-coated stents exhibited a strong fluorescence on the metal surface (Figure 2B) whereas the blank stents were dark (Figure 2A).
Figure 2.
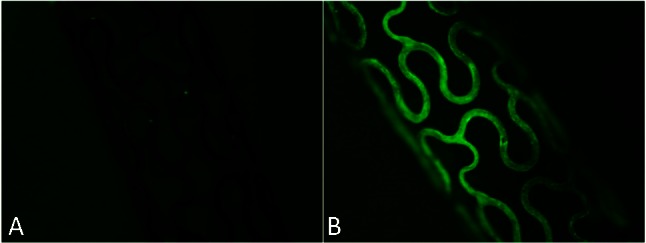
Fluorescence microscopic images (40×) of bare metal stent (A) and anti-CD34 antibodies modified stent (B). The antibody-coated stents exhibited a strong fluorescence on the metal surface (B) whereas the blank stents were dark (A).
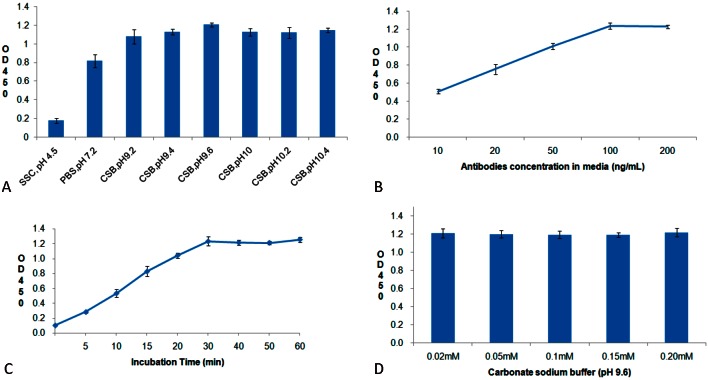
The determination of the amounts of attached anti-CD34 antibodies using ELISA showed the following: 1) the amounts of attached antibody increased significantly with the pH value of the media and stabilized in the range of pH 9-10 (Figure 3A); 2) the amounts of attached antibody increased with antibody concentrations in the media and stabilized at the antibody concentration of 100 ng/mL (Figure 3B); 3) the amounts of attached antibody stabilized after 30 min of incubation (Figure 3C); 4) the salt concentrations of the media did not affect antibody adsorption (Figure 3D).
Figure 3.
The effects of pH (A), antibody concentration (B), incubation time (C), and salt concentration (D) on the amounts of anti-CD34 antibodies attached on nano-porous surface bare metal stent.
The open-circuit potential (OCP) of BMS and CAMS were determined on a PARATAT 2273 EIS. The results showed that stent OCP slightly decreased with the incubation time in the presence of anti-CD34 antibodies and remained constant after 30 min; the constant OCP of CAMS was slightly lower than that of BMS (CAMS vs. BMS, -0.082 ± 0.008 V vs. -0.031 ± 0.007 V), which was obvious for the absolute value.
Characterization of anti-CD34 antibodies on the metal stent surface
The capacity of CAMS for capturing the EPCs was assessed in vitro on a coronary flow simulator under the flow velocity of 27 cm/s. The result showed that vast numbers of cells (63,500 ± 6,500 cells/mm2) of CD34+ positive KG-1a cells were captured on the stent surface, whereas only a small number of KG-1a cells were observed on the BMS surface (Figure 4). The number of cells increased with the amount of attached antibodies, and stabilized at the anti-CD34 antibody concentration of 100 ng/mL.
Figure 4.
Fluorescence microscopic images (40×) of CD34+ KG-1a cells stained with DAPI on bare metal stent (A) or anti-CD34 antibodies modified stent (B). Vast numbers of cells (63,500 ± 6,500 cells/mm2) of CD34+ positive KG-1a cells were captured on the stent surface with CAMs, whereas only a small number of KG-1a cells were observed on the BMS surface. BMS, bare metal stent; CAMs, anti-CD34 antibody-modified stent; DAPI, 4’-6-diamidino-2-phenylindole.
The capturing capacities for HUVECs of plain and antibody-modified stainless steel BMS were compared to confirm the activity of antibody coating. The results (Figure 5) showed that the anti-CD34 antibody-modified stainless steel surface captured HUVECs of 148 ± 26 cells/mm2, which was about six times more efficient than the plain surface (24 ± 8 cells/mm2).
Figure 5.
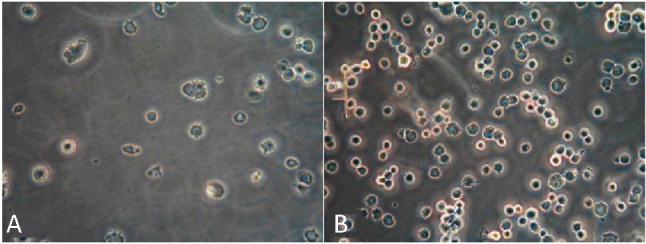
Optical microscope images (200×) of HUVECs on stainless steel plates with anti-CD34 antibodies (A) compared with those on plates without anti-CD34 antibodies (B). The anti-CD34 antibody-modified stainless steel surface captured HUVECs of 148 ± 26 cells/mm2, which was about six times more efficient than the plain surface (24 ± 8 cells/mm2). HUVECs, human umbilical veinendothelial cells.
In vivo evaluations
In animal tests, the antibody-modified stents collected many cells on the stent surface within 2 h after implantation. CD34 expressed selectively on human stem cells (HSC), progenitor cells (PC) and vascular endothelial cells which takes about 0.15% of the peripheral blood cells, so the captured CD34 positive cells mostly were endothelial cells, and they were smaller than the mesothelial cells and lined up tightly. Therefore, they were specifically a thin layer of epithelium, by a layer composed of flat cells. At 48 h, the endothelial cells covered most of the stent surface. In contrast, the BMS collected considerably fewer cells 2 h and 48 h after implantation (Figure 6). At seven days, the endothelialization scores of CAMS were significantly higher than those of BMS (CAMS vs. BMS, 3.88 ± 0.12 vs. 3.27 ± 0.14, p < 0.05, Figure 7).
Figure 6.
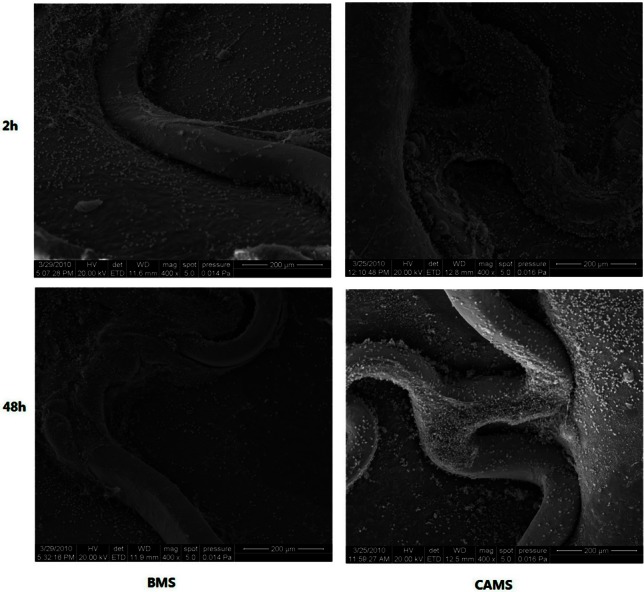
SEM images (400×) of porcine coronary arteries at 2, 48 hours after implantation of bare metal stent (BMS) and anti-CD34 antibodies modified stent (CAMS). The BMS collected much fewer cells than CAMS 2 h and 48 h after implantation. SEM, scanning electron microscope.
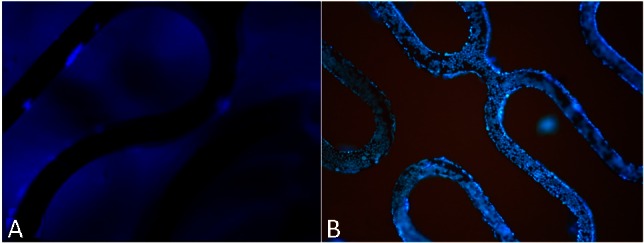
Figure 7.
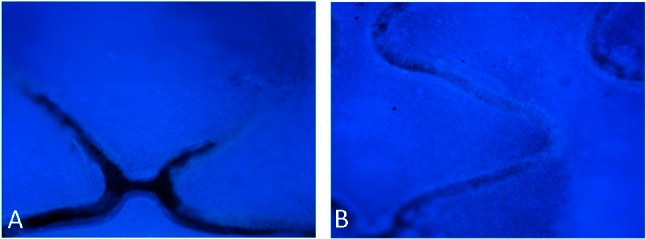
DAPI staining images (40×) of endothelialization on bare metal stent (A) and anti-CD34 antibodies modified stent (B) at 7 days after implantation. After seven days, the endothelialization scores of CAMS were significantly higher than those of BMS (CAMS vs BMS, 3.88 ± 0.12 vs. 3.27 ± 0.14, p < 0.05). BMS, bare metal stent; CAMs, anti-CD34 antibody-modified stent; DAPI, 4’-6-diamidino-2-phenylindole
DISCUSSION
In the development of pro-healing stents, the attachment of effective biological ingredients to the BMS surface is a critical problem.23,30,31 In the present study, we developed a simple method to attach antibodies directly on the stent metal surface that was pretreated by electrochemical etched pits on the surface (Figure 1A). Under optimal conditions (pH 9-10, 100 ng/mL of anti-CD34 antibodies, and 30 min of incubation time), the density of attached anti-CD34 antibodies on the stent surface of 200 ± 25 ng/cm2 was routinely obtained, indicating a consistent application of antibodies onto the stent (Figure 3).
Both in vitro and in vivo experimental data revealed that CAMS are effective in capturing CD34+ cells: 1) the antibody-coated stainless steel surface was about six times more efficient than the plain metal surface for capturing CD34+ HUVECs (Figure 5); 2) the antibody-modified stents could collect over 60,000/mm2 of CD34+ KG-1a cells (Figure 6), which was higher than that of the stent described previously (10,000 cells/mm2 to 30,000 cells/mm2);1,32 and 3) the CAMS collected substantially more cells after implantation (Figure 6) and exhibited greatly enhanced endothelialization compared with the BMS (Figure 7). All these results indicated that our polymer-free antibody-modified stents function as efficiently as, or may even be superior to polymer-based stents.
The adsorption mechanism of antibodies by stent metal surface is not fully understood. Nonetheless, several indicators were observed: 1) the salt concentrations had no effect on antibody immobilization (Figure 3), suggesting that the antibody does not bind by electric charge attraction or does not form any salt bond with metal surface; 2) the antibodies were observed with SEM as approximately 200 nm size particles primarily on the ridge of the porous metal landform (Figure 1B), suggesting that IgG may assemble/aggregate on the porous metal surface; 3) the change of voltage of the metal stent upon antibody adsorption (about 0.06 V) revealed a weak binding energy (about 6 kJ/ mol), suggesting the lack of significant change of surface chemical components. Therefore, the adsorption of antibodies on the stent surface may be similar to the heterogeneous nucleation and aggregation of antibodies on stainless steel nanoparticles.25 Thus, the nano texture of the metal surface induced the assembly/aggregation of the antibody to the metal surface, thereby achieving a physical adsorption with high affinity. Nevertheless, the details on the mechanism remain to be further studied.
CONCLUSIONS
In conclusion, we have described a new simple method of adsorption of anti-CD34 antibody on 316L stainless steel stents with enough density and stability. The in vitro and in vivo tests confirmed that the new anti-CD34 antibodies stent facilitated endothelialization. The present study would provide a novel polymer-free approach for developing pro-healing stents with the merits of avoiding any potential negative effects of polymers, retaining a higher antibody activity, and simplifying the stent manufacturing process.
Acknowledgments
The present study was supported by NNSFC (#30770581 and 20971008), the Research Fund for the Doctoral Program of Higher Education of China, and Lepu Medical Technology. We also thank our valuable partner Yongqiang Chen for his efforts in accomplishing this work, and Huagang Zhu, Xingsheng Yang and Yongbin Li for their excellent work in the animal experiment of this study.
REFERENCES
- 1.Aoki J, Serruys PW, van Beusekom H, et al. Endothelial progenitor cell capture by stents coated with antibody against CD34: the HEALING-FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First in Man) Registry. J Am Coll Cardiol. 2005;45:1574–1579. doi: 10.1016/j.jacc.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 2.Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 3.Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007;115:1440–1455. doi: 10.1161/CIRCULATIONAHA.106.666800. [DOI] [PubMed] [Google Scholar]
- 4.James SK, Stenestrand U, Lindbäck J, et al. Long-term safety and efficacy of drug-eluting versus bare-metal stents in Sweden. N Engl J Med. 2009;360:1933–1945. doi: 10.1056/NEJMoa0809902. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien B, Carroll W. The evolution of cardiovascular stent materials and surfaces in response to clinical drivers: a review. Acta Biomater. 2009;5:945–958. doi: 10.1016/j.actbio.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Garg S, Serruys PW. Coronary stents: looking forward. J Am Coll Cardiol. 2010;56:S43–S78. doi: 10.1016/j.jacc.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Swanson N, Hogrefe K, Javed Q, Gershlick AH. In vitro evaluation of vascular endothelial growth factor (VEGF)-eluting stents. Int J Cardiol. 2003;92:247–251. doi: 10.1016/s0167-5273(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 8.McGuigan AP, Sefton MV. The influence of biomaterials on endothelial cell thrombogenicity. Biomaterials. 2007;28:2547–2571. doi: 10.1016/j.biomaterials.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan CJ, Tang JJ, Shao ZY, et al. Improved blood compatibility of rapamycin-eluting stent by incorporating curcumin. Colloids Surf B. 2007;59:105–111. doi: 10.1016/j.colsurfb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Han DW, Jung DY, Park JC, et al. Underlying mechanism for suppression of vascular smooth muscle cells by green tea polyphenol EGCG released from biodegradable polymers for stent application. J Biomed Mater Res A. 2010;95:424–433. doi: 10.1002/jbm.a.32870. [DOI] [PubMed] [Google Scholar]
- 11.Sharif F, Hynes SO, McCullagh KJ, et al. Gene-eluting stents: non-viral, liposome-based gene delivery of eNOS to the blood vessel wall in vivo results in enhanced endothelialization but does not reduce restenosis in a hypercholesterolemic model. Gene Ther. 2012;19:321–328. doi: 10.1038/gt.2011.92. [DOI] [PubMed] [Google Scholar]
- 12.Lin Q, Ding X, Qiu F, et al. In situ endothelialization of intravascular stents coated with an anti-CD34 antibody functionalized heparin-collagen multilayer. Biomaterials. 2010;31:4017–4025. doi: 10.1016/j.biomaterials.2010.01.092. [DOI] [PubMed] [Google Scholar]
- 13.Houtgraaf JH, Duckers HJ. Endothelial progenitor cell (EPC) capture to aid vascular repair following coronary stenting: a new frontier in stent technology? EuroIntervention. 2008;4:C67–C71. [PubMed] [Google Scholar]
- 14.Granada JF, Inami S, Aboodi MS, et al. Development of a novel prohealing stent designed to deliver sirolimus from a biodegradable abluminal matrix. Circ Cardiovasc Interv. 2010;3:257–266. doi: 10.1161/CIRCINTERVENTIONS.109.919936. [DOI] [PubMed] [Google Scholar]
- 15.Wendel HP, Avci-Adali M, Ziemer G. Endothelial progenitor cell capture stents--hype or hope? Int J Cardiol. 2010;145:115–117. doi: 10.1016/j.ijcard.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Ellingsen JE. A study on the mechanism of protein adsorption to TiO2. Biomaterials. 1991;12:593–596. doi: 10.1016/0142-9612(91)90057-h. [DOI] [PubMed] [Google Scholar]
- 17.Goda T, Konno T, Takai M, et al. Biomimetic phosphorylcholine polymer grafting from polydimethylsiloxane surface using photo-induced polymerization. Biomaterials. 2006;27:5151–5160. doi: 10.1016/j.biomaterials.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 18.Shen Y, Wang G, Chen L, et al. Investigation of surface endothelialization on biomedical nitinol (NiTi) alloy: effects of surface micropatterning combined with plasma nanocoatings. Acta Biomater. 2009;5:3593–3604. doi: 10.1016/j.actbio.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Tosaka R, Yamamoto H, Ohdomari I, Watanabe T. Adsorption mechanism of ribosomal protein L2 onto a silica surface: a molecular dynamics simulation study. Langmuir. 2010;26:9950–9955. doi: 10.1021/la1004352. [DOI] [PubMed] [Google Scholar]
- 20.Xu H, Nguyen KT, Brilakis ES, et al. Enhanced endothelialization of a new stent polymer through surface enhancement and incorporation of growth factor-delivering microparticles. J Cardiovasc Transl Res. 2012;5:519–527. doi: 10.1007/s12265-012-9381-8. [DOI] [PubMed] [Google Scholar]
- 21.Yin Y, Wise SG, Nosworthy NJ, et al. Covalent immobilisation of tropoelastin on a plasma deposited interface for enhancement of endothelialisation on metal surfaces. Biomaterials. 2009;30:1675–1681. doi: 10.1016/j.biomaterials.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Lin Q, Yan J, Qiu F, et al. Heparin/collagen multilayer as a thromboresistant and endothelial favorable coating for intravascular stent. J Biomed Mater Res A. 2011;96:132–141. doi: 10.1002/jbm.a.32820. [DOI] [PubMed] [Google Scholar]
- 23.Yuan Y, Liu C, Yin M. Plasma polymerized n-butyl methacrylate coating with potential for re-endothelialization of intravascular stent devices. J Mater Sci Mater Med. 2008;19:2187–2196. doi: 10.1007/s10856-007-3319-8. [DOI] [PubMed] [Google Scholar]
- 24.Shahryari A, Azari F, Vali H, Omanovic S. The response of fibrinogen, platelets, endothelial and smooth muscle cells to an electrochemically modified SS316LS surface: towards the enhanced biocompatibility of coronary stents. Acta Biomater. 2010;6:695–701. doi: 10.1016/j.actbio.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Bee JS, Davis M, Freund E, et al. Aggregation of a monoclonal antibody induced by adsorption to stainless steel. Biotechnol Bioeng. 2010;105:121–129. doi: 10.1002/bit.22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berry JL, Santamarina A, Moore JE, Jr., et al. Experimental and computational flow evaluation of coronary stents. Ann Biomed Eng. 2000;28:386–398. doi: 10.1114/1.276. [DOI] [PubMed] [Google Scholar]
- 27.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonnema GT, Cardinal KO, Williams SK, Barton JK. An automatic algorithm for detecting stent endothelialization from volumetric optical coherence tomography datasets. Phys Med Biol. 2008;53:3083–3098. doi: 10.1088/0031-9155/53/12/001. [DOI] [PubMed] [Google Scholar]
- 29.Steigerwald K, Merl S, Kastrati A, et al. The pre-clinical assessment of rapamycin-eluting, durable polymer-free stent coating concepts. Biomaterials. 2009;30:632–637. doi: 10.1016/j.biomaterials.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Blindt R, Vogt F, Astafieva I, et al. A novel drug-eluting stent coated with an integrin-binding cyclic Arg-Gly-Asp peptide inhibits neointimal hyperplasia by recruiting endothelial progenitor cells. J Am Coll Cardiol. 2006;47:1786–1795. doi: 10.1016/j.jacc.2005.11.081. [DOI] [PubMed] [Google Scholar]
- 31.Nakazawa G, Granada JF, Alviar CL, et al. Anti-CD34 antibodies immobilized on the surface of sirolimus-eluting stents enhance stent endothelialization. JACC Cardiovasc Interv. 2010;3:68–75. doi: 10.1016/j.jcin.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Yang XS, Zhu HG, Yu ZJ, et al. The union of anti-CD34 antibody can improve the performance of drug-eluting stents. Cater Cardiovasc Interv. 2012;79:972–978. doi: 10.1002/ccd.23186. [DOI] [PubMed] [Google Scholar]