Abstract
Background
The prevalence of pulmonary hypertension is unusually high in Taiwanese patients with end-stage renal disease. Thrombosis of hemodialysis grafts is common and pulmonary embolism has been reported after endovascular thrombectomy. The aim of this study was to evaluate the relationship between pulmonary hypertension and endovascular thrombectomy of hemodialysis grafts.
Methods
One hundred and ten patients on hemodialysis via arteriovenous grafts were enrolled in our study. The mean pulmonary artery pressure (PAP) was measured by right heart catheterization. Clinical information was collected by review of medical records. Comorbid cardiopulmonary disease was evaluated by echocardiography and chest X-ray. The history of patient vascular access thrombosis was reviewed from database, hemodialysis records, and interviews with staff at hemodialysis centers.
Results
Fifty-two participants (47%) had pulmonary hypertension diagnosed by right heart catheterization. There was no difference in the number of thrombectomy procedures between patients with and without pulmonary hypertension. Based on multivariate analysis, the number of prior endovascular thrombectomy procedures did not correlate with mean PAP (F-value = 1.10, p = 0.30) nor was it associated with pulmonary hypertension (odds ratio = 0.92, p = 0.17).
Conclusions
Prior endovascular arteriovenous graft thrombectomies were not associated with pulmonary hypertension or increased mean PAP in end-stage renal disease patients on maintenance hemodialysis.
Keywords: Graft, Hemodialysis, Pulmonary hypertension, Thrombectomy, Thrombosis
INTRODUCTION
In end-stage renal disease (ESRD) patients on regular hemodialysis (HD), vascular access thrombosis is a common complication contributing to increased health care costs. The acute thrombosis rate is higher in patients with arteriovenous grafts (AVGs) compared to patients with arteriovenous fistulas (AVFs). In the past, surgical thrombectomy was performed to salvage acutely occluded AVGs. In recent years, an endovascular approach has been widely adopted. The advantages of the endovascular approach include a shorter operative duration, immediate resumption of daily dialysis sessions, better patient tolerance, and lower total costs. Although the thrombi within thrombosed grafts are fragmented, macerated, and aspirated, it is not possible to capture all the thromboemboli before they enter the central circulation, in comparison with surgical thrombectomy.1
Four case series reported iatrogenic pulmonary embolism diagnosed by scintigraphy after endovascular thrombectomy procedures.2-5 In addition, pulmonary hypertension is prevalent among the ESRD patients,6 and chronic thromboembolic events are a well-known cause of pulmonary hypertension.7 Controversy remains, however, regarding the cumulative effects of repeat iatrogenic pulmonary embolism on pulmonary circulation after endovascular thrombectomy in ESRD patients.2,3 This retrospective study was conducted to investigate the impact of multiple endovascular thrombectomies on the prevalence of pulmonary hypertension in ESRD patients.
MATERIALS AND METHODS
Study design
Beginning in January 2010, a prospective observation study to investigate pulmonary hypertension in hemodialysis patients was undertaken in our hospital. Patients on maintenance hemodialysis, at either our hospital’s hemodialysis unit or nearby hemodialysis centers, were eligible for enrollment. Patients with suspected shunt malfunction were evaluated prior to angiography, based on one or more of the following findings: clinical signs of decreased bruits or thrills, reduction in flow rate, or increased venous pressure during dialysis. Patients entered the evaluation process if they met the following inclusion criteria: (1) the patient was on regular hemodialysis for six months; (2) the hemodialysis was performed via synthetic AVG; and (3) the patient had a stable dry weight without clinical evidence of fluid overload (such as lung edema/congestion based on chest radiograph or peripheral edema on physical examination) for at least two weeks. Patients were excluded if graft thrombosis had occurred within the past two weeks or acute cardiovascular events (including decompensating heart failure or acute coronary syndrome) had occurred within the past three months.
The medical history, previous graft angiography, endovascular procedure reports, and hemodialysis records of each patient were reviewed, and physical examination was performed and recorded in the catheterization room. Echocardiography was performed on the day of enrollment or within one week if not applicable. The cardiologist interviewed patients during the angiography procedure. A simplified right heart study was performed after the diagnostic or therapeutic endovascular procedure was completed. A study nurse recorded all of the above information during the index catheterization room visit. The demographic data, comorbidities, current medication, graft characteristics, hemodialysis duration, and number of prior endovascular thrombectomies were obtained from review of the medical records, angiograms, hemodialysis records, computerized medical records, and during the catheterization room interview. Missing information was then collected by telephone contacts with the hemodialysis units during the review process. The flow chart of evaluation process was depicted in Figure 1.
Figure 1.
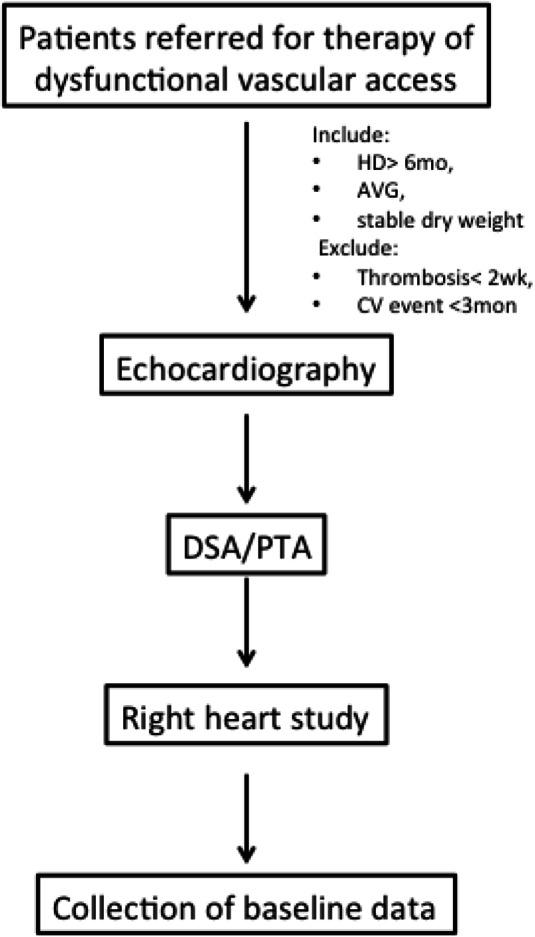
Flow chart of the evaluation process for the study participants. AVG, arteriovenous graft; CV, cardiovascular; DSA, digital subtraction angiography; HD, denotes hemodialysis; PTA, percutaneous transluminal angioplasty.
The original study was approved by the institutional review board of our hospital and each participant gave written informed consent before enrollment. The current study was a retrospective analysis of baseline data in the cohort, focusing on the relation between prior thrombectomy procedures and the prevalence of pulmonary hypertension. For analysis of a portion of data from the original study, a separate informed consent was waived from our institutional review board.
Endovascular thrombectomy procedures
The first short sheath (Terumo, Tokyo, Japan) was placed at the graft near the venous junction, toward the arterial anastomosis. After successfully crossing the arterial anastomosis, the arterial plug was dislodged by forceful withdrawal of the low-pressure inflated balloon. Thrombi dislodged within the access were aspirated via the sheath as much as possible. For large thrombus or wall-adherent thrombus, mechanical devicessuch as Arrow-Trerotola devices (Percutaneous thrombectomy device, Arrow International, Reading, PA, USA) were used to facilitate thrombus removal.8,9 Pharmacological lytic therapy was not used at our institution.
The second short sheath was placed at the graft near the arterial junction, toward the venous anastomosis. After successfully crossing to the central vein, balloon angioplasty of the outflow vein was performed if underlying stenosis was present.10 Stents or stent grafts were used only for vessel rupture during the study period. If not contraindicated, intravenous heparin (5000U) was also administered during the procedure. Anti-platelet agents were prescribed for three days after the procedure.
Echocardiography
Transthoracic echocardiography was performed on a mid-week non-dialysis day if the patient demonstrated stable dry weight without graft thrombosis during the preceding two weeks. An ultrasound machine equipped with a standard 2.5 MHz sector probe (Philips, IE33, Andover, MA, USA) was used. The patients were placed in a left lateral position and the echocardiography was performed during quiet respiration. Standard views with 2-dimensional, M-mode, and Doppler studies were obtained. Echocardiography images and animations were stored for off-line analysis. The average values of three consecutive cardiac cycles were obtained. Two experienced cardiologists (who performed at least 60 echocardiograms per month) performed all studies. Two independent cardiologists were blinded to the medical history and catheterization results of each patient. A cardiologist who was blinded to the echocardiography and catheterization results evaluated the images for tricuspid regurgitation (TR) volume, tricuspid regurgitation pressure gradient (TRPG), and image quality. Only data of good image quality and significant TR volume were considered reliable. TRPG measurements made using poor quality images were removed during the review process.
Right heart catheterization
A simplified right heart catheterization procedure was performed after the echocardiogram. A 6-Fr 5 cm sheath (Terumo, Tokyo, Japan) was placed in the venous limb of the hemodialysis graft. A 5-Fr 110 cm pigtail catheter was then introduced using a 0.035-inch guide wire (Glide Wire, Terumo, Tokyo, Japan). Under fluoroscopy, the pigtail catheter was advanced along the hemodialysis vascular access into the central veins, the right atrium, the right ventricle, and finally the main pulmonary artery. Serial pressure measurements were performed during advancement and pullback from the main pulmonary artery.
Definitions
Pulmonary hypertension was defined as a mean pulmonary artery pressure (PAP) of 25 mmHg or more, according to the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines.11 Active smoking status was defined as having smoked within the 6 months prior to enrollment. Ischemic heart disease was defined by abnormal treadmill results, abnormal thallium stress imaging, > 50% stenosis of at least one major coronary artery on coronary angiography, evidence of acute coronary syndrome or myocardial infarction (troponin I elevation and ischemic changes on electrocardiogram), history of coronary intervention (balloon angioplasty or stenting), or history of coronary artery bypass surgery. Systolic LV dysfunction was defined as LVEF less than 45% as measured by echocardiography or LV ventriculography. Valvular heart disease was defined as stenosis or regurgitation of at least moderate severity. Upon review of the medical records, connective tissue disease was confirmed based on a rheumatologic diagnosis which included systemic lupus erythematous, systemic sclerosis, or Sjögren’s syndrome.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation if they were normally distributed. Those variables not normally distributed were expressed as median and 25-75% interquartile range. Categorical variables were expressed as numbers and percentages. The independent t-test and Fisher’s test were used to compare groups with and without pulmonary hypertension for continuous variables and categorical variables, respectively. Univariate logistic regression analysis was used to evaluate predictors of pulmonary hypertension. To compare the independent association between thrombectomy procedures and pulmonary hypertension, the number of thrombectomy procedures were tested in multivariate logistic analysis, including all co-factors with p < 0.05 at univariate analysis. Comparisons among groups having different numbers of thrombectomies were made usingthe Chi-square or the Kruskal-Wallis tests, as appropriate. Spearman’s correlation test was used for non-parametric continuous variables (mean PAP and number of thrombectomy procedures). Tests were considered significant if the p-value was < 0.05. All statistical analyses were performed using R software (R version 3.1.0, 2014-04-10, with packages Hmisc, Gmisc, and Greg).
RESULTS
A total of 110 patients with ESRD on regular hemodialysis were enrolled in the study. All 110 patients underwent successful right heart catheterization via AVG. The mean age of the participants was 68 (±12) years. The mean hemodialysis duration at enrollment was 29 (11-61) months (median and interquartile range). Among the 110 participants, 42 (38%) patients had a history of ischemic heart disease, nine (8%) had heart failure, 58 (53%) had valvular heart disease (valvular stenosis or regurgitation of at least moderate severity), and five (5%) had COPD. The characteristics of patients were stratified according to the presence or absence of pulmonary hypertension, as shown in Table 1.
Table 1. Characteristics of participants stratified by the presence or absence of pulmonary hypertension.
| PH present (n = 52) | PH absent (n = 58) | p-value | |
| Demographics | |||
| Age (years) | 68 ± 12 | 68 ± 12 | 0.7 |
| Female gender | 36 (69%) | 27 (47%) | 0.02 |
| Smoking history | 2 (4%) | 12 (21%) | 0.009 |
| Hemodialysis duration (mos) | 29 (10-52) | 23 (14-66) | 0.9 |
| Comorbidities | |||
| Hypertension | 30 (58%) | 34 (59%) | 0.99 |
| Diabetes mellitus | 27 (52%) | 32 (55%) | 0.85 |
| Ischemic heart disease | 23 (44%) | 19 (33%) | 0.24 |
| Dyslipidemia | 17 (33%) | 13 (22%) | 0.28 |
| Systolic LV dysfunction | 7 (13%) | 2 (3%) | 0.08 |
| Valvular heart disease | 28 (54%) | 30 (52%) | 0.85 |
| Cerebrovascular accident | 5 (10%) | 11 (19%) | 0.19 |
| COPD | 2 (4%) | 3 (5%) | 0.99 |
| Connective tissue disease | 2 (4%) | 0 (0%) | 0.22 |
| Medications | |||
| Anti-platelet | 11 (21%) | 13 (22%) | 0.99 |
| Anti-coagulant | 0 (0%) | 3 (5%) | 0.25 |
| ACEi/ARB | 4 (8%) | 4 (7%) | 0.99 |
| Beta-blocker | 2 (4%) | 7 (12%) | 0.17 |
| Calcium channel blocker | 2 (4%) | 5 (9%) | 0.44 |
| Graft characteristics | |||
| Shunt age (mos) | 25 (8-41) | 16 (9-34) | 0.54 |
| Right arm graft | 9 (17%) | 13 (22%) | 0.63 |
| Upper arm graft | 16 (31%) | 15 (26%) | 0.67 |
| Straight graft | 21 (40%) | 18 (31%) | 0.33 |
| Number of thrombectomies | 1 (0-3) | 1 (0-3) | 0.46 |
| Echocardiographic parameters | |||
| RVD (mm) | 7 ± 7 | 7 ± 6 | 0.82 |
| LVIDd (mm) | 46 ± 9 | 44 ± 6 | 0.9 |
| LVIDs (mm) | 30 ± 9 | 28 ± 6 | 0.79 |
| IVS (mm) | 12 ± 2 | 12 ± 2 | 0.38 |
| PW (mm) | 11 ± 2 | 12 ± 2 | 0.47 |
| LV mass (gm) | 254 ± 108 | 249 ± 75 | 0.63 |
| LVEF (%) | 62 ± 13 | 65 ± 9 | 0.52 |
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; HD, hemodialysis; IDd, internal diameter in diastole; IDs, internal diameter in systole; IVS, interventricular septum; LV, left ventricle; LVEF, LV ejection fraction; PAP, pulmonary artery pressure; PH, pulmonary hypertension, PW, posterior wall; RVD, right ventricular diameter.
Pulmonary hypertension was diagnosed by right heart catheterization in 52 (47%) patients. The mean systolic, diastolic, and PAPs for these 52 patients were 45 (±9), 16 (±6), and 30 (±6) mmHg, respectively. In 58 (53%) patients without pulmonary hypertension, the mean systolic, diastolic, and PAPs were 30 (±5), 9 (±4), and 18 (±4) mmHg, respectively. The percentage of female patients was greater in the pulmonary hypertension group compared with the group without pulmonary hypertension (69% vs. 47%, p = 0.02). The pulmonary hypertension group had less active smokers than the group without pulmonary hypertension (4% vs. 21%, p = 0.009). More patients with systolic heart failure were noted in the pulmonary hypertension group compared with the group without pulmonary hypertension but the difference did not reach significance (13% vs. 3%, p = 0.08). There were no differences in the echocardiographic parameters between those patients with and without pulmonary hypertension (Table 1).
No difference in the total number of prior throm-bectomy procedures was noted in the group with pulmonary hypertension compared with the group without pulmonary hypertension (Table 1). When participants were stratified into groups by the number of thrombectomy procedures (0, 1, 2-5, and > 5 times), the mean PAPs were 24.1, 24.1, 23.8, and 22.5 mmHg, respectively. No significant differences in mean PA pressures were found among these four groups (p = 0.91, Figure 2). The prevalence of pulmonary hypertension among the groups (0, 1, 2-5, and > 5 times) was 48.6%, 55.6%, 41.9%, and 41.2%, respectively. There was no significant difference in the prevalence of pulmonary hypertension among the four groups (p = 0.71, Figure 3). In addition, there was no correlation between the number of prior thrombectomy procedures and mean PAP (R = -0.02, p = 0.82, Figure 4).
Figure 2.
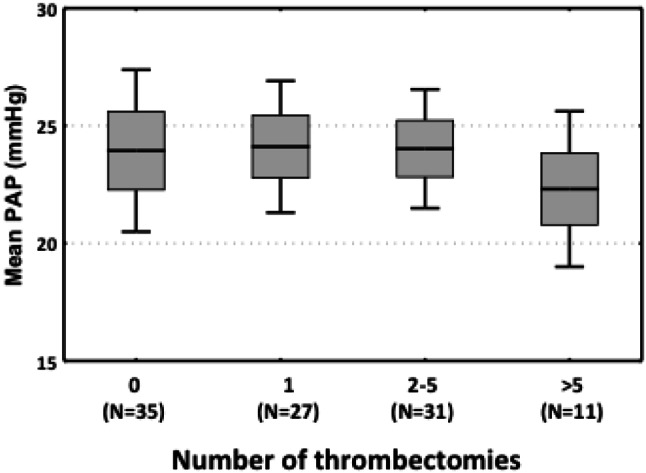
Distribution of mean pulmonary artery pressure (PAP) stratified by number of thrombectomies.
Figure 3.
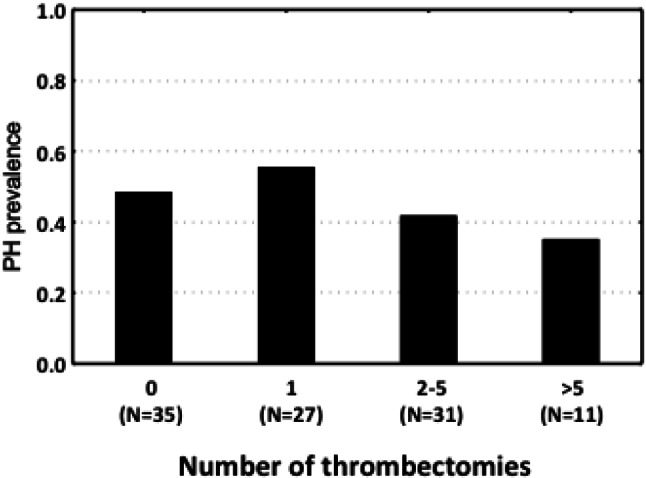
Prevalence of pulmonary hypertension (PH) stratified by number of thrombectomies.
Figure 4.
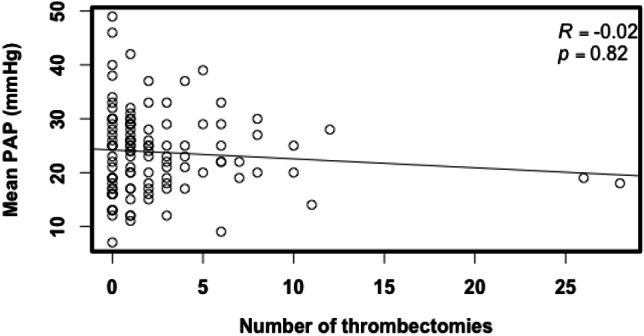
Scatter plot of mean pulmonary artery pressure (PAP) versus number of thrombectomies.
In the univariate logistic regression model (Table 2), female gender increased the odds of pulmonary hypertension (OR = 2.58, p = 0.02). Smoking decreased the odds of pulmonary hypertension (OR = 0.15, p = 0.02). The actual number of prior thrombectomy procedures was not significantly associated with pulmonary hypertension.
Table 2. Logistic regression for pulmonary hypertension using univariate and multivariate models.
| OR | 95% CI | p-value | |
| Univariate analysis | |||
| Female gender | 2.58 | 1.18 to 5.65 | 0.02 |
| Smoking history | 0.15 | 0.03 to 0.72 | 0.02 |
| Number of thrombectomies | 0.94 | 0.85 to 1.04 | 0.25 |
| Multivariate analysis | |||
| Female gender | 2.1 | 0.89 to 4.95 | 0.09 |
| Smoking history | 0.22 | 0.04 to 1.13 | 0.07 |
| Number of thrombectomies | 0.92 | 0.81 to 1.04 | 0.17 |
CI, confidence interval; OR, odds ratio.
In the multivariate logistic regression model (Table 2), gender (OR = 2.10, p = 0.09), smoking (OR = 0.22, p = 0.07), and number of prior thrombectomy procedures (OR = 0.92, p = 0.17) failed to reach significance.
DISCUSSION
Pulmonary hypertension is a progressive pulmonary disease with high morbidity and mortality which can result from heart disease, lung disease, or systemic disease. The prevalence of pulmonary hypertension in ESRD patientsis unusually high. Pulmonary hypertension is also an independent predictor of mortality in ESRD patients.12 In one study, pulmonary hypertension was found in 40% of ESRD patients on maintenance hemodialysis via arteriovenous access.6
An accurate etiological diagnosis is essential in the treatment of pulmonary hypertension. However, the pathogenesis of pulmonary hypertension in ESRD patients is unclear. ESRD patients, especially those with prosthetic grafts, are prone to repeat vascular access thrombosis. In addition, there have been concerns that thrombectomy-related pulmonary emboli may result in pulmonary hypertension. A number of scintigraphic studies have documented the presence of silent pulmonary embolism after thrombectomy procedures.2-5 Up to 15-20% of patients with an acute pulmonary embolism will have incomplete resolution of scintigraphic perfusion defects after the embolic event.13,14
Chronic thromboembolic pulmonary hypertension (CTEPH) may occur in patients after acute pulmonary embolic events. Although the incidence of CTEPH is relatively low, distinct medical conditions present in hemodialysis patients may predispose them to the development of CTEPH. A substantial portion of ESRD patients with grafts will undergo multiple thrombectomy procedures, as demonstrated in our study. In addition, uremic patients frequently have multiple cardiovascular risk factors or co-morbid cardiopulmonary diseases which predispose them to the development of pulmonary embolism.15 Patients with cardiopulmonary disease have also demonstrated a decreased clearance of pulmonary emboli.16 Finally, anti-coagulation therapy is usually not prescribed after thrombectomy procedures, as opposed to that given to patients with pulmonary embolism or deep vein thrombosis. Accordingly, the relationship between repeat endovascular thrombectomies and pulmonary hypertension requires clarification.
In this study, we found a high prevalence of pulmonary hypertension in ESRD patients undergoing hemodialysis. Based on univariate logistic regression analysis, female gender and smoking status were positively associated with pulmonary hypertension. After multivariate logistic regression analysis, none of these factors, including the number of prior thrombectomy procedures, was related to pulmonary hypertension. This implies that when confounding factors were considered together, no single factor contributed to the development of pulmonary hypertension in ESRD patients. The cause of pulmonary hypertension in ESRD patients may be multi-factorial and future studies are needed to clarify its characteristics and etiology.
Our study was the first to use right heart catheterization to obtain the PAP.17 All previous studies of uremic pulmonary hypertension have used only echocardiography to estimate the PAP.6,12,17 Although Doppler velocity measurements of tricuspid regurgitation correlate well with PAP, a large regurgitation volume is needed for accurate measurement. In addition, non-invasive estimation of right atrial pressure is challenging and often inaccurate. As a consequence, non-invasive measurement of PAP is not always feasible and over- or underestimation of PAP may occur in up to 40% of individuals, rendering the diagnosis of pulmonary hypertension unreliable.18
In our study, the number of prior thrombectomy procedures was not associated with the presence of pulmonary hypertension. However, the number of prior thrombectomy procedures in our study population was variable. When covariates were added to the multivariate analysis, the number of prior thrombectomies still had no impact on the presence of pulmonary hypertension or PAP. Our result provided evidence that pulmonary hypertension in ESRD patients was not related to the number of prior thrombectomy procedures.
In a case-control study of ESRD patients who underwent echocardiography, Harp et al. studied patients’ systolic PAP following thrombectomy in 88 patients compared with 117 ESRD patients who did not receive prior thrombectomy. In their study, the prevalence of pulmonary hypertension among ESRD patients was high (42-52%), similar to our findings (47%). They also compared systolic PAP between groups with different pulmonary hypertension severity and found that prior thrombectomy was not significantly associated with pulmonary arterial hypertension. However, the diagnosis of pulmonary hypertension was obtained from an echocardiographic laboratory database where echocardiograms were performed as a routine investigation of comorbid cardiovascular disease. Thus, the condition of patients at the time of echocardiography was neither standardized nor clear enough for the diagnosis of pulmonary hypertension. In addition, their conclusion was greatly limited by the low number of prior thrombectomy procedures (an average of 1.5 thrombectomies per patient). ESRD patients, especially those with prosthetic grafts, are prone to repeat vascular access thrombosis throughout their lifetime. In consequence, the cumulative impact of repeat iatrogenic pulmonary emboli from thrombectomy procedures remains unknown.
In contrast, 48 patients in our study received more than two thrombectomies and 17 patients received more than five thrombectomies. In addition, the PAP was obtained during right heart catheterization, a much more accurate tool for the diagnosis of pulmonary hypertension. In consequence, our results provided the most robust evidence regarding the safety of endovascular thrombectomy in salvaging occluded AVGs.
At least four case series have documented acute pulmonary embolism after an endovascular thrombectomy procedure.2-5,19 After pulmonary embolism, incomplete resolution of pulmonary emboli was documented by scintigraphic perfusion scans.16 There was also evidence that chronic pulmonary thromboembolism was associated with pulmonary hypertension.20 Since iatrogenic pulmonary embolism has been reported by scintigraphic studies after endovascular thrombectomy, these emboli should have resulted in elevated PAP in our study. We postulated the following explanations for why this did not occur: (1) a thrombosed AVG would be detected earlier than that of a deep vein thrombosis in the lower limbs; (2) the volume of thrombi within a graft may be smaller than that within a deep vein thrombosis; (3) fresh clot would be more easily dissolved using heparin and balloon maceration during the endovascular thrombectomy procedure compared with deep vein thrombosis; and (4) the pulmonary circulation has intrinsic thrombolytic mechanisms which work better when thrombi are macerated as this process increases their surface to volume ratio.
Our study had several limitations, including its retrospective design and its small number of cases. In addition, it was conducted at only one center and the evaluation of right heart catheterizations was incomplete. During the data collection process, the true number of prior thrombectomies may have been underestimated. It is also difficult to determine the timing of onset of pulmonary hypertension from a retrospective aspect. Since the endovascular thrombectomy technique for thrombosed graft has evolved over decades, there are various methods to accomplish procedural success. Thus, our results may not be applicable to other methods.
CONCLUSIONS
Prior endovascular AVG thrombectomies were not associated with pulmonary hypertension or increased mean PAP in ESRD patients on maintenance hemodialysis.
Acknowledgments
This study was supported by grants from the National Taiwan University Hospital, Hsinchu Branch (DOH-99-HO-2022, 100-026-F, HCH101-12, HCH102-33, HCH 103-067).
DISCLOSURES
No conflict of interest to declare for all authors.
REFERENCES
- 1.Bittl JA. Catheter interventions for hemodialysis fistulas and grafts. JACC Cardiovasc Interv. 2010;3:1–11. doi: 10.1016/j.jcin.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Smits HF, Van Rijk PP, Van Isselt JW, et al. Pulmonary embolism after thrombolysis of hemodialysis grafts. J Am Soc Nephrol. 1997;8:1458–1461. doi: 10.1681/ASN.V891458. [DOI] [PubMed] [Google Scholar]
- 3.Swan TL, Smyth SH, Ruffenach SJ, et al. Pulmonary embolism following hemodialysis access thrombolysis/thrombectomy. J Vasc Interv Radiol. 1995;6:683–686. doi: 10.1016/s1051-0443(95)71164-2. [DOI] [PubMed] [Google Scholar]
- 4.Kinney TB, Valji K, Rose SC, et al. Pulmonary embolism from pulse-spray pharmacomechanical thrombolysis of clotted hemodialysis grafts: urokinase versus heparinized saline. J Vasc Interv Radiol. 2000;11:1143–1152. doi: 10.1016/s1051-0443(07)61355-4. [DOI] [PubMed] [Google Scholar]
- 5.Petronis JD, Regan F, Briefel G, et al. Ventilation-perfusion scintigraphic evaluation of pulmonary clot burden after percutaneous thrombolysis of clotted hemodialysis access grafts. Am J Kidney Dis. 1999;34:207–211. doi: 10.1016/s0272-6386(99)70344-6. [DOI] [PubMed] [Google Scholar]
- 6.Yigla M, Nakhoul F, Sabag A, et al. Pulmonary hypertension in patients with end-stage renal disease. Chest. 2003;123:1577–1582. doi: 10.1378/chest.123.5.1577. [DOI] [PubMed] [Google Scholar]
- 7.Fedullo PF, Auger WR, Kerr KM, Rubin LJ. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2001;345:1465–1472. doi: 10.1056/NEJMra010902. [DOI] [PubMed] [Google Scholar]
- 8.Hung CW, Lai CL, Hsieh MY, et al. Endovascular declotting of wall-adherent thrombi in hemodialysis vascular access. Acta Cardiol Sin. 2014;30:128–135. [PMC free article] [PubMed] [Google Scholar]
- 9.Wen SC, Pu SY, Tsai KC, et al. AngioJet thrombectomy to salvage thrombosed native dialysis fistulas. Acta Cardiol Sin. 2011;27:101–108. [Google Scholar]
- 10.Hsieh MY, Lin L, Tsai KC, Wu CC. Radial artery approach to salvage nonmaturing radiocephalic arteriovenous fistulas. Cardiovasc Intervent Radiol. 2013;36:957–963. doi: 10.1007/s00270-012-0533-7. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Yigla M, Fruchter O, Aharonson D, et al. Pulmonary hypertension is an independent predictor of mortality in hemodialysis patients. Kidney Int. 2009;75:969–975. doi: 10.1038/ki.2009.10. [DOI] [PubMed] [Google Scholar]
- 13.Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257–2264. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 14.Becattini C, Agnelli G, Pesavento R, et al. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest. 2006;130:172–175. doi: 10.1378/chest.130.1.172. [DOI] [PubMed] [Google Scholar]
- 15.Tveit DP, Hypolite IO, Hshieh P, et al. Chronic dialysis patients have high risk for pulmonary embolism. Am J Kidney Dis. 2002;39:1011–1017. doi: 10.1053/ajkd.2002.32774. [DOI] [PubMed] [Google Scholar]
- 16.Wartski M, Collignon MA. Incomplete recovery of lung perfusion after 3 months in patients with acute pulmonary embolism treated with antithrombotic agents. THESEE Study Group. Tinzaparin ou Heparin Standard: Evaluation dans l'Embolie Pulmonaire Study. J Nucl Med. 2000;41:1043–1048. [PubMed] [Google Scholar]
- 17.Harp RJ, Stavropoulos SW, Wasserstein AG, Clark TW. Pulmonary hypertension among end-stage renal failure patients following hemodialysis access thrombectomy. Cardiovasc Intervent Radiol. 2005;28:17–22. doi: 10.1007/s00270-004-0223-1. [DOI] [PubMed] [Google Scholar]
- 18.Litwin SE. Noninvasive assessment of pulmonary artery pressures:moving beyond tricuspid regurgitation velocities. Circ Cardiovasc Imaging. 2010;3:132–133. doi: 10.1161/CIRCIMAGING.110.945121. [DOI] [PubMed] [Google Scholar]
- 19.Beathard GA, Welch BR, Maidment HJ. Mechanical thrombolysis for the treatment of thrombosed hemodialysis access grafts. Radiology. 1996;200:711–716. doi: 10.1148/radiology.200.3.8756920. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro A, Juhlin-Dannfelt A, Brodin LA, et al. Pulmonary embolism: relation between the degree of right ventricle overload and the extent of perfusion defects. Am Heart J. 1998;135:868–874. doi: 10.1016/s0002-8703(98)70048-1. [DOI] [PubMed] [Google Scholar]


