Abstract
Background
Acute pulmonary embolism is a serious medical condition that has a substantial global impact. Inflammation plays a role in the pathophysiology and prognosis of acute pulmonary embolism (APE). The aim of the present study was to investigate the prognostic value of admission parameters for complete blood count (CBC) in APE.
Methods
A total of 203 patients who were hospitalized with diagnosed APE were retrospectively enrolled in the study. Clinical data, PESI scores, admission CBC parameters, neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were all recorded. The clinical outcomes of study subjects were determined by the reported patient 30-day mortality and long-term mortality.
Results
During a median follow-up period of 20 months [interquantile range 17], 34 subjects in the study population (17%) died. NLR and PLR levels were significantly higher in patients who died within the 30 days (n = 14) [9.9 (5.5) vs. 4.5 (4.1), p = 0.01 and 280 (74) vs. 135 (75), p = 0.01, respectively] and during the long-term follow-up (n = 20) [8.4 (2.9) vs. 4.1 (3.8), p = 0.01 and 153 (117) vs. 133 (73), p = 0.03, respectively] when compared to the patients that survived. In Cox regression analysis, age, systolic blood pressure, systolic pulmonary arterial pressure, PESI scores (HR 1.02 95%CI 1.01-1.04, p = 0.01), elevated levels of NLR (HR 1.13 95%CI 1.04-1.23, p = 0.01) and PLR (HR 1.002 95%CI 1.001-1.004, p = 0.01) were independently correlated with total mortality.
Conclusions
Admission NLR and PLR may have prognostic value in patients with APE.
Keywords: Mortality, Pulmonary embolism
INTRODUCTION
Acute Pulmonary Embolism (APE) is an emergent cardiovascular disease which usually develops secondary to deep vein thrombosis (DVT) and may cause mortality, especially in patients with poor hemodynamic status and comorbidities.1-3 The prognostic importance of several clinical and laboratory variables has been established in APE patients.4-6 In addition, elevated levels of biochemical markers such as troponin, brain natriuretic peptide (BNP), NT-proBNP, H-FABP (heart type fatty acid binding protein), myoglobin, and white blood cell (WBC) count have been shown to predict adverse events in APE.1,4-6 Immediate initiation of anticoagulation and/or thrombolytic therapy is vital to decrease patient mortality and morbidity rates. Laboratory parameters may be used to guide the therapy, especially in relatively stable patients.3-6
Inflammation has been shown to play an important role in venous thromboembolism, and in a meta-analysis it was observed that inflammation markers such as IL-6, IL-8, and monocyte chemotactic protein were involved in the disease’s pathogenesis.7,8 In recent studies, neutrophil-to-lymphocyte ratio, (NLR) and platelet-to-lymphocyte ratio (PLR) were shown to be better indicators of inflammation compared to WBC counts.7-13 The aim of this study was to determine the prognostic value of admission NLR and PLR measurements in patients with APE.
MATERIALS AND METHODS
Study population
Medical records of our institution were screened for patients who were diagnosed with APE and treated between January 2008 and January 2013. Initially, a total number of 241 subjects were recruited. The exclusion criteria were acute coronary syndrome within the previous 30 days, congestive heart failure, renal or hepatic failure, acute infectious disease, severe chronic obstructive pulmonary disease, active cancer, presence of another indication for long-term anticoagulation, haematological disorder using immunosuppressant medication, anemia (haemoglobin level < 13 g/dL in men and < 12 g/dL in women) and history of blood transfusion within the last three months. According to these criteria, 25 cases were excluded from further analysis.
To better evaluate long-term events, hospital records, telephone interviews and state-wide death registry database were used. Patients who were defined as passing due to secondary causes other than pulmonary embolism were also excluded from the study. This group included 5 cases of acute coronary syndrome, 3 cases of cerebrovascular accident, 2 cases of heart failure and 3 cases of non-cardiac death. Ultimately, a total of 203 patients were included in the study, which was approved by the local ethics committee.
Definitions
We used the revised Geneva and Wells clinical prediction scales which were recommended in the guidelines on the diagnosis and management of acute pulmonary embolism and clinical probability for APE was established (low, medium and high) for each case.1 Also, all patients’ original PESI scores which included 11 parameters (age, male sex, cancer, chronic heart failure, chronic pulmonary disease, pulse rate, systolic blood pressure, respiratory rate, temperature, altered mental status, arterial oxyhaemoglobin saturation) were calculated for clinical risk stratification.1 Definite diagnosis was made by combining these scores with D-dimer levels, echocardiographic assessment and multi-slice spiral computed tomography (CT) imaging. Deep venous thrombosis diagnosis was made with lower extremity venous Doppler ultrasonography. Obesity was defined as a body mass index over 30 m2/kg; hypertension (HT) was defined as use of antihypertensive drugs or initial blood pressure over 140/90 mmHg; diabetes mellitus (DM) was defined as use of antidiabetic drugs or fasting plasma glucose levels of > 126 mg/dL and hyperlipidemia was defined as total serum cholesterol levels > 240 mg/dL. Additionally, smoking status was defined as current use of tobacco. Lastly, hypotension was defined as a systolic blood pressure < 90 mmHg or a pressure drop of more than 40 mmHg for longer than 15 minutes if not caused by a new-onset arrhythmia, hypovolemia or sepsis.1
Laboratory analysis
According to routine hospital protocol, venous blood samples were taken from the antecubital vein during the patients’ emergency admission and collected in calcium EDTA tubes. Blood counts were studied by an auto-analyser (Cell-dyn 3700, Abbott Laboratories, Abbott Park, IL USA) within 30 minutes after blood sampling. The results of other routine biochemical laboratory parameters, such as maximal troponin-I, BNP, and D-dimer levels were collected by using the hospital’s electronic database. The NLR was calculated by dividing the neutrophil count by the lymphocyte count, and PLR was calculated by dividing the platelet count by the lymphocyte count. In addition, consecutive international normalized ratio (INR) measurements after hospital discharge were screened to establish the effectiveness of oral anticoagulation therapy, and the mean INR measurements were included in the analysis.
Echocardiography and computed tomography imaging
A complete echocardiographic study was performed using the Vivid 3 Ultrasound System (General Electric, Norway) during the initial evaluation of the patients. Right ventricular (RV) dimensions were measured from the apical 4-chamber view in diastole at the mid-ventricular level. Right ventricular dilation was defined by RV dimension > 3.3 cm.14 McConnell’s sign was defined as the presence of segmental wall motion abnormality restricted to the RV free wall and normal contractility of the RV apex.15 Systolic pulmonary arterial pressure was calculated by adding trans-tricuspid pressure gradient to mean right atrial pressure, which was estimated from the inferior vena cava diameter and motion during respiration as follows: mean right atrial pressure was estimated to be 5 mm Hg if there was complete collapse of a normal diameter inferior vena cava during inspiration, 10 mm Hg if a normal diameter inferior vena cava collapse was > 50%, 15 mm Hg if a dilated inferior vena cava collapsed by > 50% with inspiration, and 20 mm Hg if there was no visible evidence of dilated inferior vena cava collapse.14
We performed multi-slice spiral CT in the radiology clinic, using the pulmonary embolism protocol (field of view: 35 cm, section thickness: 3 mm, contrast material volume: 135 mL, contrast material injection rate: 4 mL/sec). Diagnosis of APE was established in case of a complete or partial luminal filling defect in the main pulmonary artery or its branches.
Medications
Enoxaparin administration was commenced for all patients with a dose of 1 mg/kg twice a day immediately after the diagnosis of APE was ascertained. Thrombolytic treatment (t-PA) was provided by an intravenous infusion of 100 mg for 2 hours in patients with hemodynamic instability. Oral warfarin therapy was started for all patients on the day of admission except for cases treated with t-PA who had warfarin treatment 24 hours after the therapy. The patients continued oral anticoagulation therapy for at least three months after discharge and INR levels were checked monthly. The duration of oral warfarin therapy was left to the discretion of the primary physician.
Statistical analyses
All data were presented as mean ± SD or a median (interquantile range) for parametric variables and as percentages for categorical variables. Continuous variables were checked for the normal distribution assumption using Kolmogorov-Smirnov statistics. Differences between survivors and non-survivors were evaluated using two-sample t-tests. Categorical variables were tested by Pearson’s χ2 test and Fisher’s exact test. Receiver operating curves were generated to define the cut-off values (upper left corner of ROC curve) of NLR and PLR for total mortality. Using these cut-off values of NLR and PLR levels, Kaplan-Meier estimates and curves were generated, and groups were compared using log-rank tests. Univariate and multivariate binary logistic regression analyses were performed to identify the independent predictors of short-term mortality. In addition, Cox regression analyses were used to investigate the univariable and multivariable predictors of total mortality during the study period. Forward stepwise multivariable regression models using parameters with p < 0.10 were created in logistic and Cox regression analyses. P-values were two-sided, and values < 0.05 were considered statistically significant. All statistical studies were carried out using the Statistical Package for Social Sciences software (SPSS 16.0 for Windows, SPSS Inc., Chicago, Illinois, USA).
RESULTS
During a follow-up period of 20 [17] months, 34 patients (16.7%) died, and 14 of the deceased subjects died within 30 days. Clinical, demographic and laboratory characteristics of the survived and deceased patients are summarized in Table 1 and Table 2. Between the two groups, significant differences were observed in terms of gender, frequency of DVT on admission, systolic blood pressure, neutrophil counts, lymphocyte counts, NLR, PLR, maximal troponin-I and BNP levels. However, hemoglobin, WBC count, platelet count, red cell distribution width, platelet distribution width, MPV, fasting glucose, urea, creatinine and maximum D-dimer levels were similar between the two groups.
Table 1. Comparison of demographic and clinic parameters and clinical outcome of the study population.
| Demographic and clinical features | Study population (n = 203) | Survivors (n = 169) | Non-survivors (n = 34) | p value* |
| Age, years | 65.8 ± 18.1 | 64.9 ± 18.2 | 70.4 ± 16.4 | 0.11 |
| Female gender, n (%) | 116 (57) | 91 (53) | 25 (73) | 0.04 |
| Hypertension, n (%) | 92 (45) | 81 (47) | 11 (32) | 0.11 |
| Diabetes mellitus, n (%) | 38 (19) | 28 (16) | 10 (29) | 0.08 |
| Smoking, n (%) | 38 (19) | 32 (18) | 6 (17) | 0.87 |
| Prior deep vein thrombosis, n (%) | 19 (9) | 17 (10) | 2 (5) | 0.54 |
| DVT on admission, n (%) | 58 (28) | 53 (31) | 5 (14) | 0.04 |
| CAD, n (%) | 36 (18) | 30 (17) | 6 (17) | 0.92 |
| COPD, n (%) | 20 (10) | 15 (8) | 5 (14) | 0.33 |
| Symptom duration, h | 66 [68] | 72 [68] | 54 [65] | 0.8 |
| Systolic BP, mmHg | 109.4 ± 20.8 | 111.5 ± 20.8 | 99.6 ± 17.8 | 0.01 |
| Respiratory rate | 15.6 ± 3.6 | 15.4 ± 3.3 | 16.3 ± 4.9 | 0.33 |
| Main pulmonary artery involvement, n (%) | 79 (39) | 60 (35) | 19 (55) | 0.03 |
| RV diastolic diameter, cm | 3.05 ± 0.53 | 3.01 ± 0.51 | 3.25 ± 0.46 | 0.02 |
| sPAP, mmHg | 55 ± 17 | 49 ± 11 | 78 ± 17 | 0.01 |
| Mc Connell’s Sign, n (%) | 52 (26) | 38 (22) | 14 (41) | 0.03 |
| Geneva score | 7.5 ± 3.0 | 7.7 ± 3.1 | 6.4 ± 2.4 | 0.01 |
| Wells score | 5.1 ± 2.1 | 5.2 ± 2.1 | 4.4 ± 2.0 | 0.06 |
| PESI score | 94 ± 39 | 83 ± 24 | 149 ± 52 | 0.01 |
| Thrombolytic therapy, n (%) | 43 (21) | 33 (20) | 10 (30) | 0.2 |
| Clinical outcome | ||||
| Follow-up, months | 20 [17.0] | 21.0 [18.0] | 16.5 [16.2] | 0.21 |
| Mortality at 30 days, n (%) | 14 (7) | - | 14 (42) | |
| Long term mortality, n (%) | 20 (10) | - | 20 (58) |
BP, blood pressure; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; PE, pulmonary embolism; PESI, pulmonary embolism severity index; RV, right ventricle; sPAP, systolic pulmonary arterial pressure.
* p value is calculated by comparison of survivors to non-survivors.
Table 2. Comparison of laboratory parameters in the study population.
| Laboratory parameters | Study population (n = 203) | Survivors (n = 169) | Non-survivors (n = 34) | p value* |
| Hemoglobin (g/dL) | 12.41 ± 2.12 | 12.31 ± 2.01 | 12.12 ± 2.23 | 0.62 |
| WBC count (103/L) | 9.23 ± 4.73 | 9.77 ± 4.72 | 8.55 ± 4.77 | 0.49 |
| Neutrophils (103/L) | 8.75 ± 4.17 | 8.22 ± 3.83 | 11.3 ± 4.83 | 0.01 |
| Lymphocytes (103/L) | 1.68 ± 0.77 | 1.82 ± 0.80 | 1.26 ± 0.50 | 0.01 |
| Platelets (103/L) | 234 ± 83 | 225 ± 103 | 251 ± 160 | 0.25 |
| RDW (%) | 14.91 ± 2.13 | 14.90 ± 2.21 | 15.20 ± 2.02 | 0.46 |
| PDW (%) | 16.78 ± 1.21 | 16.70 ± 1.10 | 16.80 ± 0.81 | 0.71 |
| MPV (fL) | 8.50 ± 1.45 | 8.46 ± 1.44 | 8.61 ± 1.96 | 0.63 |
| NLR | 5.01 [4.91] | 4.17 [4.64] | 9.02 [4.05] | 0.01 |
| PLR | 145 [92] | 139 [83] | 215 [154] | 0.01 |
| Fasting glucose (mg/dL) | 124 [53] | 110[43] | 105[41] | 0.81 |
| Urea (mg/dL) | 25 ± 13 | 25 ± 12 | 26 ± 14 | 0.77 |
| Creatinine (mg/dL) | 1.12 ± 0.40 | 1.11 ± 0.41 | 1.20 ± 0.40 | 0.18 |
| Sodium, mmol/L | 136.10 ± 5.00 | 136.01 ± 6.11 | 135.23 ± 5.01 | 0.8 |
| Potassium, mmol/L | 4.30 ± 0.71 | 4.30 ± 0.81 | 4.20 ± 1.01 | 0.53 |
| Troponin-I (ng/mL) | 0.09 [0.42] | 0.11 [0.31] | 0.51 [0.88] | 0.01 |
| BNP (pg/mL) | 352 [650] | 344 [552] | 618 [803] | 0.01 |
| D-Dimer (ng/ml) | 1540 [2045] | 1460 [1870] | 1870 [4500] | 0.73 |
| Median INR during follow-up | 2.73 ± 0.60 | 2.77 ± 0.62 | 2.69 ± 0.59 | 0.49 |
BNP, brain natriuretic peptide; INR, international normalized ratio; MPV, mean platelet volume; NLR, neutrophil-to-lymphocyte ratio; PDW, platelet distribution width; PLR, platelet-to-lymphocyte ratio; RDW, red cell distribution width; WBC, white blood cell.
* p value is calculated by comparison of survivors to non-survivors.
The mean PESI scores were significantly higher in the deceased patients compared to the survived patients (149 ± 52 vs. 83 ± 24; p = 0.01). Systolic pulmonary artery pressure and right ventricular diastolic diameter were significantly higher in the deceased patients (78 ± 17 vs. 49 ± 11 mmHg, 3.25 ± 0.46 vs. 3.01 ± 0.51 cm; p = 0.01 and p = 0.02, respectively). The frequency of subjects with McConnell’s sign was significantly higher compared to the survived patients (p = 0.03). Furthermore, the frequency of patients who received thrombolytic therapy was not different between the survived and non-survived group (20% vs. 30%; p = 0.20). The median INR measurements during the follow-up were comparable between the non-survived and the survived patients (2.69 ± 0.59 vs. 2.77 ± 0.62; p = 0.49).
When the mortality rate within the 30-day period is considered, NLR and PLR levels were significantly higher in the non-survived group compared to the rest of the study population [9.9 (5.5) vs. 4.5 (4.1), p = 0.01 and 280 (74) vs. 135 (75), p = 0.01, respectively]. During long-term follow-up (excluding patients who died within 30 days), admission NLR and PLR levels were significantly different in non-survived cases (n = 20) compared to the survived study subjects (n = 169) [8.4 (2.9) vs. 4.1 (3.8), p = 0.01 and 153 (117) vs. 133 (73), p = 0.03, respectively).
In ROC analysis, NLR > 5.93 predicted total mortality with a sensitivity of 87.8% and a specificity of 74.5% (AUC 0.84, p = 0.01). It was noted that PLR > 191 predicted total mortality with a sensitivity of 60.6% and a specificity of 83.2% (AUC 0.73, p = 0.01).
In univariate regression analysis, gender, systolic BP, PESI score, right ventricular diastolic diameter, systolic pulmonary arterial pressure (sPAP), troponin-I, BNP, NLR, and PLR were found to be correlated with short-term mortality in the study population. In multivariate regression analysis, using a model adjusted for the aforementioned parameters, sPAP (OR 1.09 95% CI 1.03-1.15, p = 0.01), PESI scores (OR 1.04 95%CI 1.02-1.07, p = 0.01), elevated levels of NLR and PLR were found to be independently correlated with short-term mortality (OR 1.29 95% CI 1.03-1.63, p = 0.02; OR 1.004 95% CI 1.001-1.007, p = 0.01, respectively).
Predictors of long-term mortality were investigated using Cox regression analysis. In univariate Cox regression analysis, age, gender, systolic BP, PESI score, right ventricular diastolic diameter, sPAP, troponin-I, BNP, NLR, and PLR were found to be correlated with total mortality in the study population (Table 3). In multivariate regression analysis, using a model adjusted for aforementioned parameters, age, systolic blood pressure, sPAP, PESI scores (HR 1.02 95% CI 1.01-1.04, p = 0.01), and elevated levels of NLR and PLR were found to be independently correlated with total mortality (HR 1.13 95% CI 1.04-1.23, p = 0.01; HR 1.002 95% CI 1.001-1.004, p = 0.01, respectively).
Table 3. Univariate and multivariate Cox regression analysis of possible predictors of total mortality in the study population.
| Variables | Unadjusted HR (95% CI) | p | Adjusted HR (95% CI) | p |
| Age, 1-SD increase | 1.02 (0.99-1.05) | 0.06 | 1.04 (1.02-1.07) | 0.04 |
| Female gender | 2.38 (1.11-5.12) | 0.03 | 1.42 (0.49-4.18) | 0.52 |
| Smoking | 0.89 (0.37-2.16) | 0.81 | - | - |
| Systolic BP, 1-SD decrease | 1.02 (1.01-1.04) | 0.02 | 1.05 (1.001-1.11) | 0.04 |
| PESI score | 1.02 (1.02-1.03) | 0.01 | 1.02 (1.01-1.04) | 0.01 |
| RV diameter, 1-SD increase | 2.83 (1.39-5.76) | 0.01 | 2.27 (0.82-6.39) | 0.12 |
| McConnell’s sign | 1.28 (0.61-2.70) | 0.51 | - | - |
| sPAP | 1.06 (1.04-1.08) | 0.01 | 1.06 (1.030-1.09) | 0.01 |
| Thrombolytic treatment | 1.75 (0.84-3.67) | 0.14 | - | - |
| Troponin I, 1-SD increase | 1.56 (1.11-2.21) | 0.01 | 0.81 (0.41-1.58) | 0.52 |
| D-dimer, 1-SD increase | 1.001 (0.99-1.002) | 0.25 | - | - |
| BNP, 1-SD increase | 1.002 (1.001-1.003) | 0.01 | 1.001 (0.99-1.002) | 0.15 |
| Glucose, 1-SD increase | 1.22 (0.62-2.39) | 0.56 | - | - |
| Creatinine, 1-SD increase | 1.31 (0.57-3.01) | 0.53 | - | - |
| Hemoglobin, 1-SD decrease | 0.95 (0.88-1.03) | 0.24 | - | - |
| WBC, 1-SD increase | 1.0 (0.99-1.01) | 0.55 | - | - |
| RDW, 1-SD increase | 1.07 (0.92-1.25) | 0.37 | - | - |
| Platelets, 1-SD increase | 1.01 (0.99-1.02) | 0.41 | - | - |
| MPV, 1-SD increase | 1.01 (0.82-1.25) | 0.9 | - | - |
| NLR, 1-SD increase* | 1.16 (1.10-1.22) | 0.01 | 1.13 (1.04-1.23) | 0.01 |
| PLR, 1-SD increase* | 1.003 (1.001-1.005) | 0.01 | 1.002 (1.001-1.004) | 0.01 |
BNP, brain natriuretic peptide; BP, blood pressure; HR, hazard ratio; CI, confidence interval; MPV, mean platelet volume; NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio; RDW, red cell distribution width; RV, right ventricle; sPAP, systolic pulmonary arterial pressure; WBC, white blood cell count.
* NLR and PLR are entered into the model separately in order to prevent multicollinearity.
In addition, we have calculated AUCs of the models. For that model including age, gender, systolic BP, PESI score, right ventricular diastolic diameter, sPAP, troponin-I, and BNP, AUC was 0.78 (95% CI 0.70-0.85). When PLR levels are added to the model, this resulted in an AUC of 0.79 (95% CI 0.72-0.86). Also, the addition of NLR levels to the model resulted in a larger difference of the predictive value of the model with an AUC of 0.85 (95% CI 0.78-0.90). Kaplan-Meier curve patients with NLR and PLR levels above the cut-off values had significantly higher risk for total mortality (log-rank p = 0.01 for both parameters) (Figure 1 and Figure 2).
Figure 1.
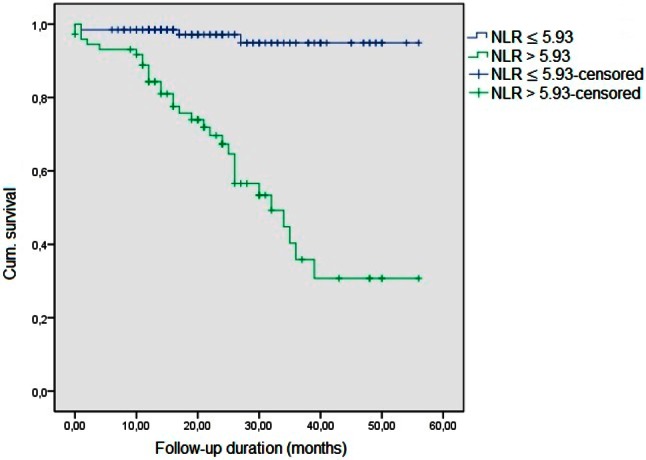
Kaplan-Meier curve for NLR. NLR, neutrophil/lymphocyte ratio.
Figure 2.
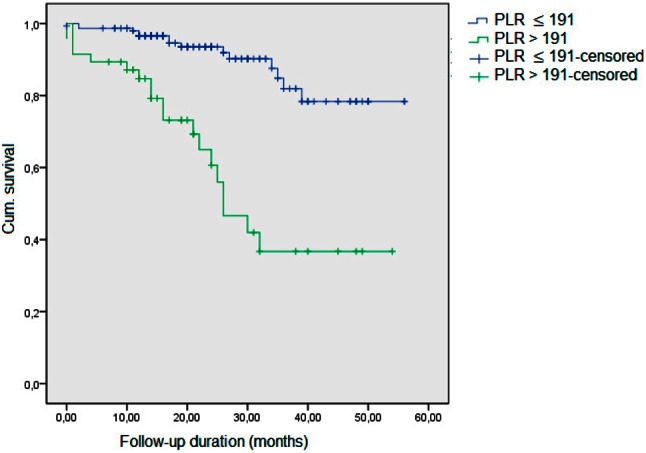
Kaplan-Meier curve for PLR. PLR, platelet to lymphocyte ratio.
DISCUSSION
Pulmonary embolism is the third most common vascular disease after acute coronary syndrome and cerebrovascular disease. Despite the new diagnostic tools and treatment modalities, the reported mortality rate remains high: between 15-25%.1,2 As in other atherothrombotic diseases, inflammation plays an important role in the pathophysiology of pulmonary embolism.2 The prognostic importance of several inflammatory parameters including BNP, NT-proBNP, IL-6, IL-8, H-FABP, troponin, and myoglobin in patients with APE has been previously demonstrated.4,5 These parameters, while useful in predicting prognosis, were not commonly used in daily practice. Therefore, the necessity of easily accessible and cheap parameters to predict the prognosis of APE has been the basis of our study.
The primary finding of our study is that, besides age, PESI scores, systolic BP and sPAP, admission NLR and PLR levels were independently associated with short and long-term mortality in patients with APE. In fact, we found that NLR may have an elevated prognostic value when compared to PLR. The total mortality rate was 16.7%, which was observed to be consistent with the literature.2 Thrombolytic therapy was used in 21% of the cases but did not result in lower mortality rates. There were no major bleeding complications observed in our study population. In general, the effect of thrombolytic therapy in long-term survival remains undetermined.1
Afzal et al. first described elevated levels of WBC in patients with pulmonary embolism and postulated that this correlation may be related to haemorrhage-infarction syndrome and comorbid conditions.16 In cancer patients, the presence of elevated WBC count was found to be associated with higher risk of venous thromboembolism and mortality.9 White blood cell subtypes, especially neutrophils, play a key role in modulating the inflammatory response in the atherosclerotic process.16 Lymphopenia is observed in inflammatory events due to increased apoptosis and is a frequent finding secondary to increased levels of corticosteroids in acute stress conditions such as acute coronary syndrome and pulmonary embolism.17,18 Also, platelets play a key role in inflammation and thrombosis.12 Hence, haematological markers, especially the ratio of subgroups (neutrophils, lymphocytes and platelets) can be used as inflammation markers in addition to biochemical markers.16-22
DVT associated with inflammatory reaction and endothelial dysfunction results in elevated levels of C-reactive protein.24-26 In APE, thrombus development is associated with pulmonary arterial and venous wall inflammation, and a leukocyte influx occurs during this process; as a result, an elevation in inflammatory mediators occurs.27-32 Studies have suggested that leukocytes contribute to venous thrombosis by damaging the endothelium.33 Elevated PLR and NLR levels indicate a higher level of inflammation, which is correlated with a more severe form of disease and increased risk for adverse events. In recent studies, prognostic use of NLR and PLR measurements has been shown in cardiovascular diseases.13,22,23
Consistent with our results, prior studies have shown that elevated NLR is associated with increased 30-day mortality rates in APE.27,28 However, neither of the studies mentioned the dose of anticoagulant therapy and INR levels. Effective anticoagulation is vital in PE treatment. In our study, the median INR measurement was not different between survived and deceased patients during the follow-up period. Thus, we may assume that they have been optimally treated. However, in patients who died during the follow-up, admission NLR and PLR measurements were higher compared to survived patients. This is the first study to indicate the long-term prognostic value of NLR and PLR levels in APE.
Limitations
A few limitations of our study deserve mention. Our investigation was a single center retrospective study which used a relatively small sample size. Despite this limitation, we have found some parameters associated with mortality, although we could not draw a causal relationship.
CONCLUSIONS
Our findings indicate that admission NLR and PLR levels may indicate short and long-term mortality risk in patients with APE. Although, we could not establish a causal relationship in this study, the results of this study may have some measure of clinical implications if found to be consistent with the results of larger scale prospective studies in the future.
Acknowledgments
None.
FUNDING
The authors received no financial support for the research, authorship, and/or publication of this article.
DISCLOSURES
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- 1.Konstantinides SV, Torbicki A, Agnelli G, et al. ESC Committee for Practice Guidelines (CPG). Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:3033–3069. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- 2.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353:1386–1389. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 3.Donze J, Le Gal G, Fine MJ, et al. Prospective validation of the pulmonary embolism severity index. A clinical prognostic model for pulmonary embolism. Thromb Haemost. 2008;100:943–948. doi: 10.1160/th08-05-0285. [DOI] [PubMed] [Google Scholar]
- 4.Kucher N, Goldhaber SZ. Cardiac biomarkers for risk stratification of patients with acute pulmonary embolism. Circulation. 2003;108:2191–2194. doi: 10.1161/01.CIR.0000100687.99687.CE. [DOI] [PubMed] [Google Scholar]
- 5.Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041–1046. doi: 10.1164/rccm.200506-862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med. 2010;363:266–274. doi: 10.1056/NEJMra0907731. [DOI] [PubMed] [Google Scholar]
- 7.Zee RY, Glynn RJ, Cheng S, et al. An evaluation of candidate genes of inflammation and thrombosis in relation to the risk of venous thromboembolism: the women’s genome health study. Circ Cardiovasc Genet. 2009;2:57–62. doi: 10.1161/CIRCGENETICS.108.801969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox EA, Kahn SR. The relationship between inflammation and venous thrombosis. A systematic review of clinical studies. Thromb Haemost. 2005;94:362–365. doi: 10.1160/TH05-04-0266. [DOI] [PubMed] [Google Scholar]
- 9.Trujillo-Santos J, Di Micco P, Iannuzzo M, et al. Elevated white blood cell count and outcome in cancer patients with venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost. 2008;100:905–911. [PubMed] [Google Scholar]
- 10.Zhu L, Wang C, Yang YH, et al. Prognostic value of right ventricular dysfunction and derivation of a prognostic model for patients with acute pulmonary thromboembolism. Zhonghua Liu Xing Bing Xue Za Zhi. 2009;30:184–188. [PubMed] [Google Scholar]
- 11.Masotti L, Cappelli R. C-reactive protein in elderly patients with suspected and confirmed pulmonary embolism. Clin Appl Thromb Hemost. 2007;13:221–223. doi: 10.1177/1076029606299051. [DOI] [PubMed] [Google Scholar]
- 12.Balta S, Demırkol S, Kucuk U. The platelet lymphocyte ratio may be useful inflammatory indicator in clinical practice. Hemodial Int. 2013;17:668–669. doi: 10.1111/hdi.12058. [DOI] [PubMed] [Google Scholar]
- 13.Temiz A, Gazi E, Güngör Ö, et al. Platelet/lymphocyte ratio and risk of in-hospital mortality in patients with ST-elevated myocardial infarction. Med Sci Monit. 2014;20:660–665. doi: 10.12659/MSM.890152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jae KOH, James BS, A, Jamil T. Appendix 6 In: The Echo Manual. 2009;3th ed.:401–415. [Google Scholar]
- 15.McConnell MV, Solomon SD, Rayan ME, et al. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78:469–473. doi: 10.1016/s0002-9149(96)00339-6. [DOI] [PubMed] [Google Scholar]
- 16.Afzal A, Noor HA, Gill SA, et al. Leukocytosis in acute pulmonary embolism. Chest. 1999;115:1329–1332. doi: 10.1378/chest.115.5.1329. [DOI] [PubMed] [Google Scholar]
- 17.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman M, Blum A, Baruch R, et al. Leukocytes and coronary heart disease. Atherosclerosis. 2004;172:1–6. doi: 10.1016/s0021-9150(03)00164-3. [DOI] [PubMed] [Google Scholar]
- 19.Acanfora D, Gheorghiade M, Trojano L, et al. Relative lymphocyte count: a prognostic indicator of mortality in elderly patients with congestive heart failure. Am Heart J. 2001;142:167–173. doi: 10.1067/mhj.2001.115792. [DOI] [PubMed] [Google Scholar]
- 20.Sunbul M, Gerin F, Durmus E, et al. Neutrophil to lymphocyte and platelet to lymphocyte ratio in patients with dipper versus non-dipper hypertension. Clin Exp Hypertens. 2014;36:217–221. doi: 10.3109/10641963.2013.804547. [DOI] [PubMed] [Google Scholar]
- 21.Öztürk S, Erdem A, Özlü MF, et al. Assessment of the neutrophil to lymphocyte ratio in young patients with acute coronary syndromes. Arch Turk Soc Cardiol. 2013;41:284–289. doi: 10.5543/tkda.2013.00344. [DOI] [PubMed] [Google Scholar]
- 22.Ghaffari S, Nadiri M, Pourafkari L, et al. The predictive value of total neutrophil count and neutrophil/lymphocyte ratio in predicting in-hospital mortality and complications after STEMI. J Cardiovasc Thorac Res. 2014;6:35–41. doi: 10.5681/jcvtr.2014.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yildiz A, Yuksel M, Oylumlu M, et al. The utility of the platelet-lymphocyte ratio for predicting no reflow in patients with ST-segment elevation myocardial infarction. Clin Appl Thromb Hemost. 2015;21(3):223–228. doi: 10.1177/1076029613519851. [DOI] [PubMed] [Google Scholar]
- 24.Jezovnik MK, Poredos P. Idiopathic venous thrombosis is related to systemic inflammatory response and to increased levels of circulating markers of endothelial dysfunction. Int Angiol. 2010;29:226–231. [PubMed] [Google Scholar]
- 25.Bucek RA, Reiter M, Quehenberger P, Minar E. C-reactive protein in the diagnosis of deep vein thrombosis. Br J Haematol. 2002;119:385–389. doi: 10.1046/j.1365-2141.2002.03886.x. [DOI] [PubMed] [Google Scholar]
- 26.Kayrak M, Erdoğan HI, Solak Y, et al. Prognostic value of neutrophil to lymphocyte ratio in patients with acute pulmonary embolism: a restrospective study. Heart Lung Circ. 2014;23:56–62. doi: 10.1016/j.hlc.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Çavuş UY, Yildirim Y, Sönmez E, et al. Prognostic value of neutrophil/lymphocyte ratio in patients with pulmonary embolism. Turk J Med Sci. 2014;44:50–55. [PubMed] [Google Scholar]
- 28.Aue G, Nelson Lozier J, Tian X, et al. Inflammation, TNF alpha and endothelial dysfunction linklenalidomide to venous thrombosis in chronic lymphocytic leukemia. Am J Hematol. 2011;86:835–840. doi: 10.1002/ajh.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Downing LJ, Strieter RM, Kadell AM, et al. Neutrophils are the initial cell type identified in deep venous thrombosis induced vein wall inflammation. Asaio J. 1996;42:677–682. doi: 10.1097/00002480-199609000-00073. [DOI] [PubMed] [Google Scholar]
- 30.Wakefield TW, Strieter RM, Downing LJ, et al. P-Selectin and TNF inhibition reduce venous thrombosis inflammation. J Surg Res. 1996;64:26–31. doi: 10.1006/jsre.1996.0301. [DOI] [PubMed] [Google Scholar]
- 31.Eagleton MJ, Henke PK, Luke CE, et al. Inflammation and intimal hyperplasia associated with experimental pulmonary embolism. J Vasc Surg. 2002;36:581–588. doi: 10.1067/mva.2002.126556. [DOI] [PubMed] [Google Scholar]
- 32.Watts JA, Zagorski J, Gellar MA, et al. Cardiac inflammation contributes to right ventricular dysfunction following experimental pulmonary embolism in rats. J Mol Cell Cardiol. 2006;41:296–307. doi: 10.1016/j.yjmcc.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Stewart GJ, Ritchie WG, Lynch PR. Venous endothelial damage produced by massive sticking and emigration of leukocytes. Am J Pathol. 1974;74:507–532. [PMC free article] [PubMed] [Google Scholar]
- 34.Dalla-Volta S, Palla A, Santolicandro A, et al. PAIMS 2: alteplase combined with heparin versus heparin in the treatment of acute pulmonary embolism. Plasminogen activator Italian multicenter study 2. J Am Coll Cardio. 1992;20:520–526. doi: 10.1016/0735-1097(92)90002-5. [DOI] [PubMed] [Google Scholar]
- 35.Schulman S. The effect of the duration of anticoagulation and other risk factors on the recurrence of venous thromboembolisms. Duration of Anticoagulation Study Group. Wien Med Wochenschr. 1999;149:66–69. [PubMed] [Google Scholar]


