Abstract
Adipose-derived mesenchymal stem cells (ASCs) are readily available multipotent mesenchymal progenitor cells and have become an attractive therapeutic tool for regenerative medicine. We herein investigated the mechanistic role of how miR-27b modulated regenerative capacities of ASCs. Intravenous administration of miR-27b-transfected ASCs (ASCs-miR-27b) was conducted after 70% partial hepatectomy (PH). After PH, rats injected with ASCs-miR-27b had decreased inflammatory cytokines and increased hepatocyte growth factor and other related growth factors. We showed that the nature of ASCs-miR-27b to inhibit hepatic stellate cell activation was dependent upon peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) in vitro. Moreover, expression of miR-27b in ASCs induced heme oxygenase-1 (HO-1), resulting in increased production of ATP, protective cytokines/growth factors, and genes involved in mitochondrial biogenesis in a PGC-1α-dependent manner. RNA sequencing (RNA-Seq) analysis revealed drastic transcriptional changes in livers treated with ASCs-miR-27b after PH. The differentially expressed genes classified into “regeneration,” “fibrosis,” and “mitochondrial biogenesis” clusters were mainly mitochondrial. The potential biological context reflecting the effects of PGC-1α by ASCs-miR-27b treatment was also observed by the subnetwork analysis with HO-1 and PGC-1α being the top-ranked regulatory genes. We demonstrate autologous ASCs-miR-27b enhances liver regeneration and, importantly, preserves hepatic function through paracrine actions which offers a viable therapeutic option to facilitate rapid recovery after liver injury.
Keywords: adipose-derived mesenchymal stem cells, heme oxygenase-1, interleukin-1 receptor antagonist, miRNA, mitochondrial biogenesis, peroxisome proliferator-activated receptor γ coactivator-1 alpha
Introduction
Mesenchymal stem cells (MSCs) are nonhematopoietic multipotent stem cells, elaborated from various tissues such as bone marrow, cord blood, adipose tissue, amniotic fluid, etc. and are defined as adherent, fibroblastoid-like cells that can differentiate into osteoblasts, adipocytes, and chondrocytes in vitro.1,2 They typically do not express hematopoietic stem cell markers, but they do express a quite specific pattern of molecules, such as SH2 (CD105), SH3, SH4 (CD73), CD106 (vascular cell adhesion molecule 1 (VCAM1)), CD54 (intracellular adhesion molecule 1 (ICAM1)), CD44, CD90, CD29, and STRO-1.3,4 In recent years, researchers have found that MSCs exert immunomodulatory effects and suppress allogeneic antigen-induced T lymphocyte proliferation.5,6 The multipotent capacity of MSCs has made them an attractive tool for cell therapy and tissue engineering. However, such therapies can be restricted by the limited ex vivo expansion rate. In the bone marrow, there is only a small fraction of MSC population and, therefore, it is necessary to expand them in vitro to obtain a sufficient number of cells for therapeutic purposes. Due to the abundance and ease of harvest, adipose-derived MSCs (ASCs) are readily available adult stem cells that have become increasingly popular for use in regenerative cell therapy. A recent study demonstrated long-term engraftment of human ASCs-derived hepatocyte-like cells in a xenogeneic transplantation model of liver regeneration.7 The special ability of ASCs for hepatocyte differentiation in vitro and liver regeneration in vivo has been highlighted by Banas et al.8 Their work suggests that ASCs may be a superior choice for the establishment of therapeutic transplantation for liver injury.
There are several studies regarding the immunological aspects of MSCs in transplantation. ASCs have also been reported to inhibit activation, proliferation, and function of immune cells, including T cells, B cells, natural killer cells, and antigen-presenting cells.9,10 Ryan et al. reported that MSCs could avoid allogeneic rejection and suggested potential uses in regenerative medicine.11 MSCs have been shown to prolong graft survival in allogeneic skin and heart transplantation models.12 Furthermore, Wan et al. applied MSCs to a rat liver transplantation model and demonstrated that MSCs clearly inhibit recipient-derived T lymphocyte proliferation and alleviate acute rejection following orthotopic liver transplantation.6 However, others have revealed that MSC infusion resulted in faster rejection as compared to controls.13,14 Recently, it has been shown that MSCs exhibit an opposite stimulating effect only on Th17 cells while T-cell activation has occurred.15 Baertschiger et al. also demonstrated that MSCs in certain circumstances might be harmful due to their fibrogenic potential and this should be seriously considered before use of MSCs for stem cell therapy.16 Thus, further investigations are required to determine the molecular mechanisms of ASCs in their immunosuppressive and regenerative properties in detail.
We have primarily found an important role of miR-27b in immunosuppressive activity in ASCs in our rat orthotopic liver transplantation models and reported that the regenerative capacities of ASCs with overexpressed miR-27b were significantly higher compared with control ASCs.17,18 However, the enhanced regeneration, hepatic differentiation, and suppressed liver inflammation were significantly reverted by coadministration of heme oxygenase-1 (HO-1) inhibitor indicating an important role of HO-1 in the regenerative and cytoprotective activities of miR-27b-transfected ASCs. In the present study, we found that miR-27b-increased HO-1 expression in ASCs was mediated by a peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α)-dependent pathway. The greater expression of PGC-1α in ASCs with miR-27b overexpression, a consequence from suppression of Fbxw7, also resulted in induction of several anti-inflammatory cytokines and protective factors, augmenting liver regeneration of hepatectomized rats that underwent ASC therapy. We attempted to determine the interplay between miR-27b, PGC-1α, and HO-1 in ASCs and investigate their importance in stem cell therapy using sequencing technology for transcriptome analysis in our model of liver regeneration. Our data demonstrated that the enhancement of regenerative capacities in miR-27b-transfected ASCs might result from elevated levels of genes involved in mitochondrial biogenesis as well as anti-inflammatory and cytoprotective factors that act in a paracrine manner.
Results
Intravenously introduced ASCs with miR-27b overexpression inhibit liver enzyme release and systemic inflammation after partial resection
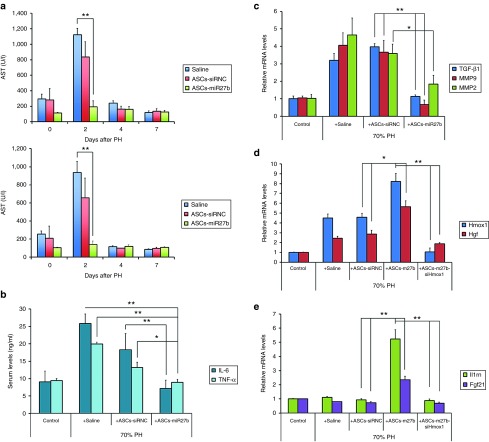
To evaluate the effects of miR-27b on the anti-inflammatory properties of ASCs in an animal model, cells (2 × 106) were transfected with control siRNA (siRNC) or miR-27b-mimic and i.v. injected into the LEW rats after a 1-day recovery period following 70% partial hepatectomy (PH). Levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured as specific indicators of liver inflammation and liver function to assess hepatocyte damage. As shown in Figure 1a, elevated levels of serum ALT and AST at day 2 after 70% PH were slightly reduced by control ASC (ASCs-siRNC) administration, whereas the elevation was drastically abolished by treatment with ASCs transfected with miR-27b (ASCs-miR-27b). Furthermore, elevated protein levels of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) in the serum and mRNA levels of transforming growth factor-beta 1 (TGF-β1), matrix metalloproteinase 2 (MMP2), and MMP9 in rat liver, responsible for the induction of inflammatory/fibrogenic response after injury, were significantly reduced by administration of ASCs-miR-27b rather than ASCs-siRNC at day 2 after partial resection (Figure 1b,c). These results indicated a critical role of miR-27b overexpression in ASCs on the anti-inflammatory activities.
Figure 1.
MicroRNA-27b-transfected ASCs led to abolished liver enzyme release and decreased systemic inflammation and enhanced expression of HGF, IL-1Ra, and FGF-21 mRNAs in an heme oxygenase-1 (HO-1)-dependent manner in vivo. ASCs (2 × 106) were transfected with control siRNA (siRNC), miR-27b-mimic, or miR-27b + Hmox1 siRNA (ASCs-miR-27b-siHmox1) and injected into the 5-week-old LEW rats (five rats per group) after a 1-day recovery period following 70% PH. Blood samples were harvested at several time points for ALT and AST measurements. Serum ALT and AST were significantly higher in the saline and siRNC-transfected ASCs (ASCs-siRNC) groups but was drastically reduced to almost normal values in the miR-27b-transfected ASCs (ASCs-miR-27b) at day 2 after partial liver resection (a). ELISA demonstrated that increased serum levels of TNF-α and IL-6, responsible for the induction of inflammatory responses, were significantly reduced by i.v. injection of ASCs-miR-27b rather than ASCs-siRNC at day 2 after 70% PH (b). The induced mRNA levels of fibrogenic genes TGF-β1, MMP9, and MMP2 in resected livers were also significantly reduced by administration of ASCs-miR-27b but not ASCs-siRNC (c). qRT-PCR analysis demonstrated that enhanced mRNA level of HGF in resected livers by administration of ASCs-miR-27b, were significantly inhibited by injection of ASCs transfected with both miR-27b and Hmox1 siRNA (d). The induced mRNA levels of Il1rn (encoding IL-1Ra) and Fgf-21 (encoding FGF-21) in resected livers were observed only in rats injected with ASCs-miR-27b and were also HO-1 dependent (e). The data are representative of three independent experiments. All experiments were conducted three times and similar results were observed. The statistics were analyzed with Student's t-test. *P < 0.05; **P < 0.01. ALT, alanine aminotransferase; ASCs, adipose-derived mesenchymal stem cells; AST, aspartate aminotransferase; Fgf, fibroblast growth factor; Hgf, hepatocyte growth factor; IL, interleukin; MMP, matrix metalloproteinase; PH, partial hepatectomy; TGF, transforming growth factor; TNF, tumor necrosis factor.
ASCs-miR-27b affects hepatocyte growth factor and cytokine expression after partial resection
After massive hepatectomy, many growth factors are immediately induced and play important roles in liver regeneration, most notably of which is hepatocyte growth factor (HGF), known to be secreted primarily by hepatic stellate cells (HSCs).19 We have previously demonstrated that decreased protein expression of alpha-smooth muscle actin (α-SMA), increased expression of HO-1, and an accelerated liver-to-body ratio as the indicators of liver regeneration were observed in rats after 70% PH with i.v. injection of ASCs-miR-27b compared with control ASCs-siRNC.18 Furthermore, ASCs-miR-27b-mediated liver-to-body ratio increase was dependent on HO-1. The expression of HGF was measured by real-time quantitative reverse transcription–PCR (qRT–PCR) and western blot analysis in liver tissue. As shown in Figure 1d, mRNA expression of HGF as well as HO-1 was increased above basal level in all groups after PH. However, ASCs-miR-27b-injected rat livers showed significantly higher HGF expression at day 2 after PH, whereas livers from rats injected with ASCs-siRNC showed no further increase compared with those from saline controls. Moreover, knockdown of Hmox1 (the gene that encodes for HO-1) in ASCs abolished liver HGF increase by ASCs-miR-27b administration, indicating that the promoting effect on HGF production is mediated by HO-1 activity. Interestingly, IL-1 receptor antagonist (IL-1Ra, IL1RN) and fibroblast growth factor-21 (FGF-21), two factors important for hepatic regeneration and protection after liver injury, were significantly elevated at day 2 after resection with injection of ASCs-miR-27b and we observed no difference between saline and ASCs-siRNC treated rats (Figure 1e). The increases of IL-1Ra and FGF-21 by introducing ASCs-miR-27b were also HO-1 dependent, as the elevations of both genes by ASCs-miR-27b were blocked when HO-1 was simultaneously knocked down. These findings indicate that miR-27b-transfected ASCs enhance regeneration, protection, and anti-inflammatory capacities not only by increasing HGF expression but also by elevating the levels of IL-1Ra and FGF-21. These elevations were mediated through HO-1 activity.
Coculture with ASCs-miR-27b abrogates activation of HSCs
Due to the effect on HGF and other factors produced by HSCs in the animal experiment, we further established an in vitro coculture model system to analyze the outcome of ASCs-miR-27b on TGF-β1-induced HSC activation. This indirect coculture system was assembled where HSC-T6 cells were plated in a six-well plate and ASCs were in intercup chambers. Cocultures were maintained in HSC medium for 2–4 days.
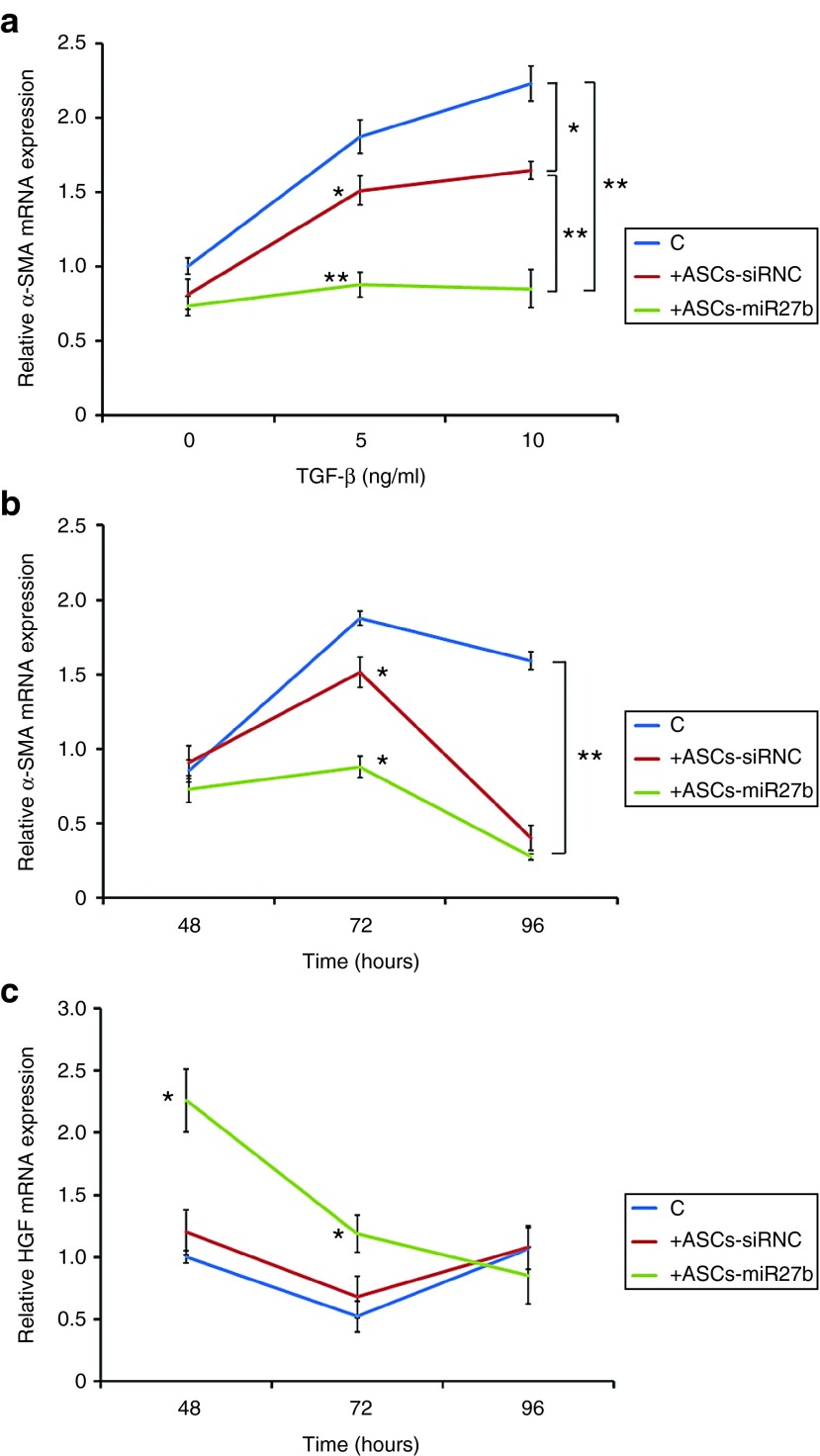
As shown in Figure 2a, a dose-dependent increase of α-SMA expression by TGF-β1 in HSCs was partially suppressed in cocultures with control ASCs, whereas the increase was almost abolished when HSC-T6 cells were cocultured with ASCs-miR-27b. This effect was best observed at 72 hours (Figure 2b). Furthermore, greater induction of HGF was also observed only in cocultures with ASCs-miR-27b at 48 and 72 hours (Figure 2c).
Figure 2.
ASCs-miR-27b led to decreased activation of cocultured hepatic stellate cells (HSCs) in vitro. The effects of ASCs-siRNC and ASCs-miR-27b on TGF-β1-activated HSCs were measured in Transwell coculture system. qRT-PCR demonstrated that dose-dependent (a) and time-dependent (b) TGF-β1-induced α-SMA mRNA expression in HSCs was significantly reduced in the presence of ASCs-siRNC, however, was drastically abolished by ASCs-miR-27b. The significant increase of HGF mRNA was observed only in activated HSCs cocultured with ASCs-miR-27b (c). However, no significant difference was found between HSCs cocultured with ASCs-siRNC and medium control. The cells were cultured in six-well culture plates. The data are representative of three independent experiments. All experiments were conducted three times and similar results were observed. The statistics were analyzed with Student's t-test. *P < 0.05; **P < 0.01. ASCs, adipose-derived mesenchymal stem cells; HGF, hepatocyte growth factor; SMA, smooth muscle actin; TGF, transforming growth factor.
miR-27b induces PGC-1α-dependent expression of anti-inflammatory and antioxidant genes in ASCs
Recently, Lu et al. discovered that PGC-1α could enhance the therapeutic engraftment of MSCs and improve the perfusion recovery in diabetic hindlimb ischemia.20 However, little is known about the underlying mechanisms of MSCs' beneficial effects on the regenerative and protective properties.
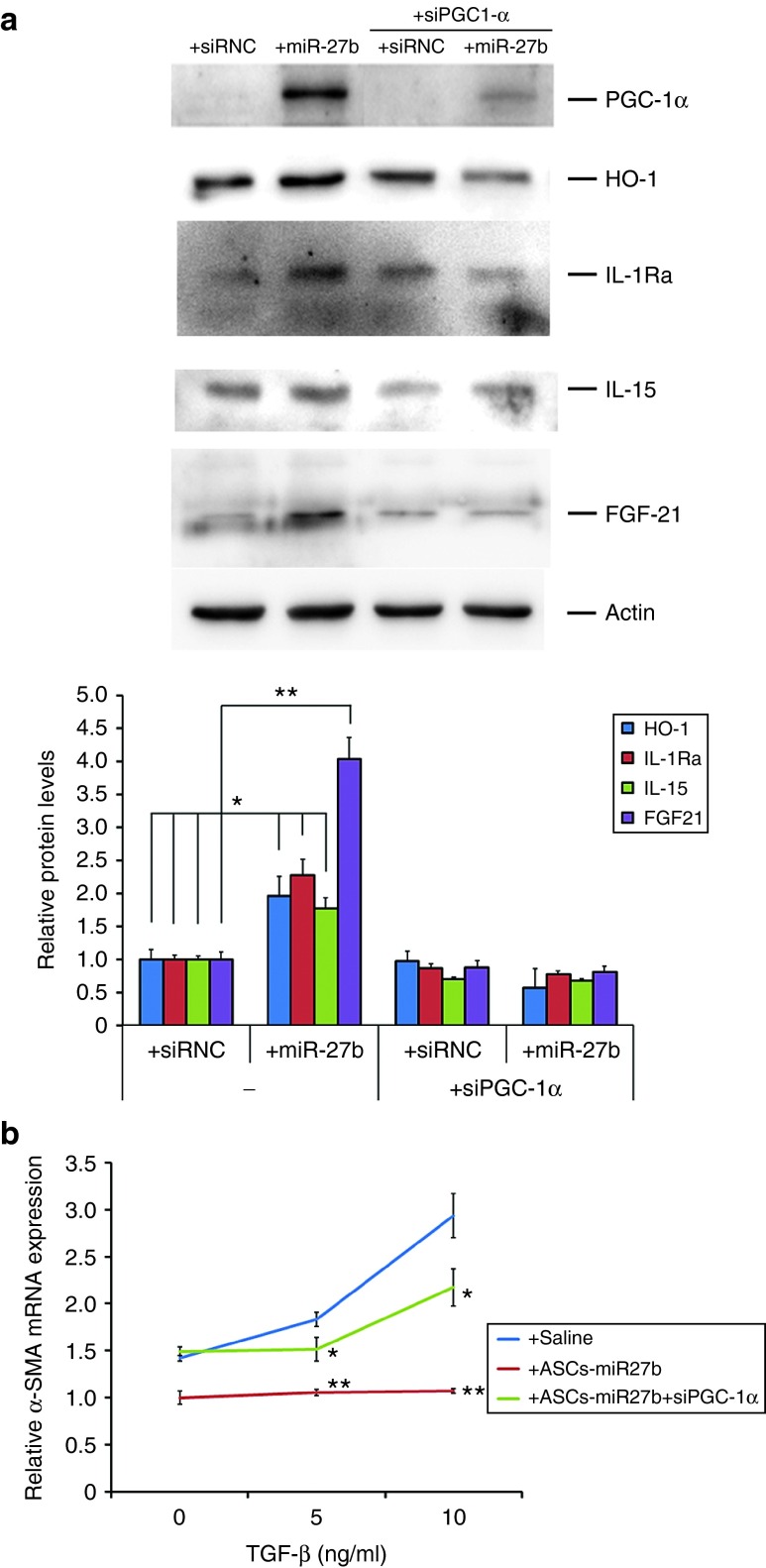
To determine whether PGC-1α mediates the induction of HO-1 expression in ASCs transfected with miR-27b, we cotransfected ASCs with siRNA for PGC-1α and miR-27b or siRNC for 24 hours and then examined the protein level of HO-1 after another 12-hour incubation. As shown in Figure 3a, the protein level of PGC-1α was significantly increased by transfection of miR-27b in ASCs (4.27-folds, P < 0.001). Small interfering RNA against PGC-1α significantly reduced the induction of HO-1 observed in ASCs-miR-27b. Furthermore, knockdown of PGC-1α also abrogated miR-27b-induced cytokines IL-15, IL-1Ra, and growth factor FGF-21 expression, indicating that PGC-1α may orchestrate HO-1 levels in mediating miR-27b-induced anti-inflammatory, antioxidant, and regenerative properties of ASCs.
Figure 3.
PGC-1α mediated the increased protein levels of anti-inflammatory and antioxidant genes by miR-27b transfection in ASCs. To evaluate potential effects of miR-27b transfection which contribute to anti-inflammatory and antioxidant gene expression in ASCs, four genes currently known to be involved in counteracting damaging effects were examined compared to effects of control siRNA. Western blot results demonstrated that HO-1, IL-1Ra, IL-15, and FGF-21 were significantly increased by miR-27b transfection for 24 hours with additional 12-hour incubation and were PGC-1α dependent (a). Knockdown of PGC-1α in ASCs-miR-27b also significantly reverted hepatic stellate cell activation (b). The cells were cultured in six-well culture plates. The data are representative of three independent experiments. All experiments were conducted three times and similar results were observed. The statistics were analyzed with Student's t-test. *P < 0.05; **P < 0.01. ASCs, adipose-derived mesenchymal stem cells; FGF, fibroblast growth factor; HO, heme oxygenase; IL, interleukin; PGC, peroxisome proliferator-activated receptor gamma coactivator; SMA, smooth muscle actin; TGF, transforming growth factor.
To further investigate the effect of PGC-1α in ASCs-miR-27b for the suppression of HSC activation, ASCs cotransfected with PGC-1α siRNA and miR-27b for 24 hours were cocultured with TGF-β1-treated HSCs for another 72 hours. As shown in Figure 3b, the abolished α-SMA expression by ASCs-miR-27b was significantly reverted by PGC-1α silencing. These results suggest that the abolishment of HSC activation by ASCs-miR-27b is mediated through the PGC-1α activity.
Fbxw7 is involved in increased PGC-1α levels mediated by miR-27b in ASCs
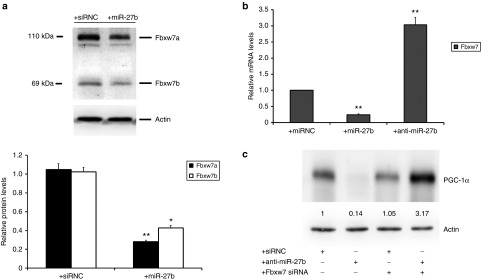
It has been demonstrated that miR-27 overexpression leads to a decrease in the expression of the F-box protein Fbxw7/Cdc4, the substrate recognition component of an Skp1-Cul1-F-box-protein (SCF) ubiquitin ligase which mediates the ubiquitin transfer to target proteins for proteasomal degradation.21 Olson et al. previously found that reduction of Fbxw7 in neurons resulted in an increased level of PGC-1α protein, which was mediated by Fbxw7-dependent proteasomal degradation and lead to increased PGC-1α-dependent transcription.22 In miR-27b-overexpressed ASCs, we observed a significant decrease in the expression of two major isoforms of Fbxw7 compared with transfection controls (Figure 4a). To investigate whether miR-27b could affect the expression of Fbxw7 through translational repression or degradation of mRNA, we examined the levels of its mRNA in ASCs transfected with miR-27b mimic or the anti-miR inhibitor anti-miR-27b. qRT–PCR results showed that the expression of Fbxw7 in ASCs transfected with 20 nmol/l miR-27b mimic resulted in a 75% decrease in mRNA levels, whereas in ASCs transfected with anti-miR-27b inhibitor, Fbxw7 expression was drastically increased by more than threefold compared with transfection controls (Figure 4b). These data indicate that miR-27b negatively regulates Fbxw7 at the mRNA level.
Figure 4.
Fbxw7 is responsible for the regulation of PGC-1α levels in adipose-derived mesenchymal stem cells (ASCs) transfected with miR-27b. ASCs transfected with siRNC or miR-27b (20 nmol/l) for 24 hours and the protein levels of Fbxw7 were measured by Western blot analysis. The results showed that miR-27b overexpression significantly decreased two major isoforms Fbxw7a (110 kDa) and Fbxw7b (69 kDa) in ASCs compared with transfection control (a). Furthermore, the mRNA level of Fbxw7 was significantly suppressed by miR-27b whereas was highly increased by the anti-miR-27b inhibitor in ASCs (b). Knockdown of Fbxw7 significantly increased protein level of PGC-1α compared to both siRNC and inhibitor controls (c). These results indicate a Fbxw7-dependent regulation of PGC-1α in response to miR-27b levels in ASCs. The cells were cultured in six-well culture plates. The data are representative of three independent experiments. All experiments were conducted three times and similar results were observed. The statistics were analyzed with Student's t-test. *P < 0.05; **P < 0.01. PGC, peroxisome proliferator-activated receptor gamma coactivator.
We next asked whether the miR-27b-modulated Fbxw7 regulates PGC-1α expression in ASCs. To test this, we examined the expression level of PGC-1α after transiently cotransfecting ASCs with anti-miR-27b inhibitor and Fbxw7 siRNA. As shown in Figure 4c, the protein expression of PGC-1α was drastically increased when both Fbxw7 siRNA and anti-miR-27b were transfected. These results demonstrate that miR-27b directs PGC-1α expression via Fbxw7-dependent regulation.
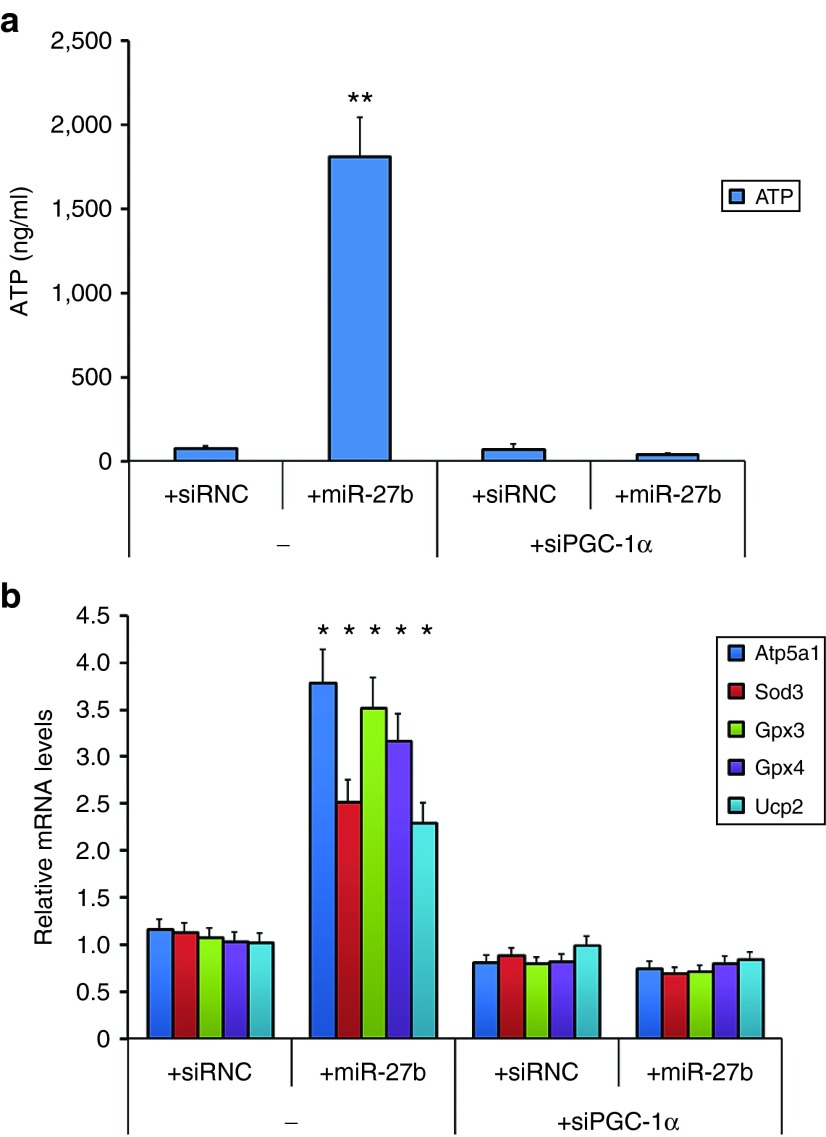
miR-27b markedly increases ATP production and mitochondrial biogenesis in ASCs in a PGC-1α-dependent manner
PGC-1α has been reported to activate mitochondrial-related genes which are involved in biogenesis and metabolism in suppressing reactive oxygen species production and preventing stress-induced apoptosis in different cell types.23,24,25,26 In the liver, PGC-1α is induced in response to fasting27,28,29 and resection30 and coordinates the activation of targets necessary for increasing ATP generation. To determine the contribution of miR-27b and PGC-1α in ATP content in cultured ASCs, we measured the ATP levels in ASCs transfected with control siRNC, miR-27b mimic in the absence or presence of cotransfected PGC-1α siRNA. As shown in Figure 5a, ATP levels were drastically increased in miR-27b-transfected ASCs in comparison with transfection control (over 20-folds, P value = 0.003), but the increase was significantly inhibited by siRNA for PGC-1α (P value = 0.006). ATP levels were also significantly reduced by a miR-27b inhibitor in comparison with transfection control (data not shown). Our observations also reflected on the mRNA levels of PGC-1α target genes which are involved in mitochondrial biogenesis and reactive oxygen species metabolism. As shown in Figure 5b, miR-27b activated a number of mitochondrial genes, including ATP synthase ATP5A1 and reactive oxygen species-detoxifying enzymes superoxide dismutase 3 (SOD3), glutathione peroxidase 3 (GPx3), glutathione peroxidase 4 (GPx4), and uncoupling protein 2 (UCP2). In contrast, miR-27b-induced mitochondrial gene expression was reversed by knockdown of PGC-1α in ASCs. Transfection of siRNC did not affect the activation of PGC-1α target genes.
Figure 5.
PGC-1α mediated the increase of ATP production and mitochondrial genes by transfected miR-27b in adipose-derived mesenchymal stem cells (ASCs). ASCs transfected with control siRNA or miR-27b with or without PGC-1α siRNA for 24 hours, and the levels of ATP production were measured. ATP determination assay showed that miR-27b transfection drastically increases ATP levels in ASCs in comparison with transfection control, but the increase was inhibited by knockdown of PGC-1α (a). The mRNA levels of five mitochondrial genes were induced by miR-27b transfection in ASCs, and their induction was also PGC-1α dependent (b). The cells were cultured in six-well culture plates. The data are representative of three independent experiments. All experiments were conducted three times and similar results were observed. The statistics were analyzed with Student's t-test. *P < 0.05; **P < 0.01. PGC, peroxisome proliferator-activated receptor gamma coactivator.
RNA-Seq analysis predicts ASCs-miR-27b-regulated genes
RNA-Seq analysis serves as an effective approach to identify genes in hepatic tissue of partially hepatectomized rats treated by i.v. administration of ASCs-miR-27b, ASCs-siRNC, or saline controls in comparison with naive liver tissue. We sequenced cDNA libraries from three pooled samples of liver tissues for each group using Illumina Miseq platform as described in Materials and Methods section. The sequencing runs yielded a total of 22.67 M reads with an average length of 100 bp. The Strand NGS software (version 2.1) was performed using default parameters for prealignment and postalignment quality control analysis and 100% of the raw reads remained in the dataset. Of these, 19.02 M reads (84%) were aligned into contigs of the rat genome (rn5), and 31,457 transcripts were identified. The unaligned reads were excluded from further analytical steps. The high-throughput sequencing performed for liver samples with different treatments showed similar numbers of yielded reads ranged from 5.57 to 5.74 M and the same average length. The number of reads for each condition of liver sample and the number of reads mapped to reference sequences are shown in Supplementary Table S1.
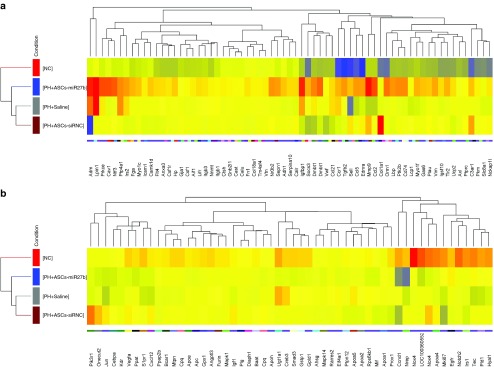
Differentially expressed gene (DEGs) from resected livers 3 days after i.v. treatments of saline, ASCs-siRNC, and ASCs-miR-27b compared with naive liver samples were calculated from the raw reads using the DESeq method. The significance level applied to the RNA-Seq data was corrected for multiple testing using Benjamini–Hochberg correction allowing rigorous identification of DEGs in partially hepatectomized liver tissues treated with ASCs-miR-27b, ASCs-siRNC, or saline control. We identified 2,013 DEGs in hepatectomized rats injected with saline (PH-Saline), more than 60% (1,235 genes) of which were also observed in the set of 1,992 DEGs in hepatectomized rats injected with ASCs-siRNC (PH-ASCs-siRNC) (Supplementary Figure S1a). Furthermore, 2,843 DEGs were identified in hepatectomized rats injected with ASCs-miR-27b (PH-ASCs-miR-27b), less than 50% (1,398 genes) of which overlapped with the set of DEGs in PH-ASCs-siRNC (Supplementary Figure S1b). This reflects greater accumulation of transcriptomic changes in PH-ASCs-miR-27b compared with those in PH-ASCs-siRNC. Among the selected DEGs, we further investigated gene functions and categorized according to specific biological processes by using the GO enrichment analysis. In the liver samples, the enriched GO “regeneration” and “positive regulation of cell migration” terms were increasingly assigned additive 98, 108, and 121 DEGs in PH-Saline, PH-ASCs-siRNC, and PH-ASCs-miR-27b, respectively. The genes classified in each functional group are listed in Supplementary Table S2. Unsupervised hierarchical clustering of all the 121 DEGs to these three groups compared to naive control demonstrated two clearly distinct up- and downregulated subsets shared between naive control and PH-ASCs-miR-27b groups (Figure 6a,b). Interestingly, several abundantly expressed genes in this cluster of PH-ASCs-miR-27b group (e.g., adm, lpin1, prkce, cav1, ntf3, nr0b2, and ucp2), involved in regeneration or wound healing, were relatively low or even downregulated when compared with the PH-ASCs-siRNC group (Table 1). This result indicates a beneficial effect of miR-27b overexpression in regenerative capacities of ASCs. However, we found that four fibrotic genes (Col1a1, C3ar1, Pik3r1, and Nox4) were highly expressed in the PH-ASCs-siRNC group and this might reflect on the fibrogenic potential of using unmodified ASCs for therapeutic use. Notably, half of these genes listed in Table 1 affected by miR-27b were present in mitochondria. We further investigated several gene signatures involved in mitochondrial biogenesis in the datasets and found that not only PGC-1α but Sirt3, Cluh, and Prkaa1 were significantly upregulated in PH-ASCs-miR-27b but not in PH-ASCs-siRNC, indicating that the pivotal role of miR-27b expression in comprehending the regenerative ability of ASCs could be mediated through mitochondrial bioenergetic response of PGC-1α.
Figure 6.
Hierarchical clustering and dendrograms showing differentially expressed genes (DEGs) in liver samples. The red blocks represent overexpressed genes, and the blue blocks represent underexpressed genes. Totally, 67 DEGs upregulated in PH + ASCs-miR-27b group compared to naive control (NC) group (a) and 54 DEGs downregulated in PH + ASCs-miR-27b group compared to NC group (b). PH: liver samples from 70% PH with saline injection; PH + ASC: liver samples from 70% PH with ASCs-siRNC injection; PH + ASCs-miR-27b: liver samples from 70% PH with ASCs-miR-27b injection. ASCs, adipose-derived mesenchymal stem cells; NC, native control; PH, partial hepatectomy.
Table 1. Differential expression in selected DEGs of liver samples from control and hepatectomized rats with saline or ASCs delivered via i.v. injection.

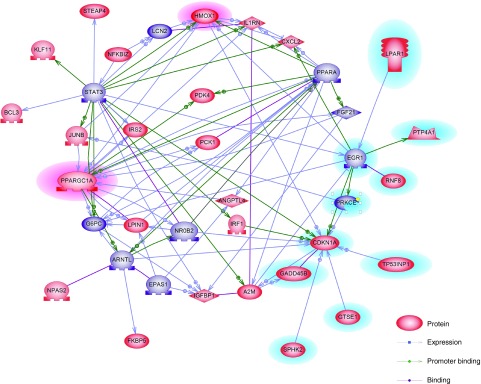
To better understand the biological context of the changes in resected livers affected by highly induced PGC-1α following ASCs-miR-27b treatment, 200 genes selected from 2,843 DEGs which were downregulated in the PH-ASCs-siRNC group and upregulated in the PH-ASCs-miR-27b group were analyzed using Ariadne's pathway buildup for direct interaction and enriched by the subnetwork enrichment analysis to identify putative expression networks and their corresponding regulators. The enriched subnetworks consisting of a set of single seed genes with target genes associated to the seed among these DEGs are listed in Supplementary Table S3 and presented in a circular pathway layout (Figure 7). As shown in Figure 7, 10 subnetworks altered between PH-ASCs-miR-27b and PH-ASCs-siRNC groups (assigned to purple) were built around the seed of PGC-1α (Ppargc1a), and Hmox1 as well as Ppargc1a are the top-ranked genes among the 11 subnetworks (highlighted on pink). Overall, our data reveal an important physiological role for miR-27b in comprehending the anti-inflammatory, antioxidant, antifibrotic as well as regenerative properties of ASCs which are orchestrated by PGC-1α with the involvement of HO-1 expression and mitochondrial biogenesis (summarized in Supplementary Figure S2).
Figure 7.
Direct interaction network of the significantly regulated genes between PH-ASCs-miR-27b and PH-ASCs-siRNC groups using Ariadne's Pathway Studio Software. The seed genes enriched by the subnetwork analysis among these differentially expressed genes were assigned to purple and also listed in Supplementary Table S3. The genes involved in machinery of cell proliferation were highlighted on light blue. ASCs, adipose-derived mesenchymal stem cells; PH, partial hepatectomy.
Discussion
Recent studies have reported that MSCs including ASCs do not express costimulatory molecules and exert relevant immune modulatory effects in suppressing many functions of immune cells,31,32 as these properties have been experimentally demonstrated in cell-based therapy for liver diseases.33 The rationale of using MSCs for cell therapies is based on the intrinsic properties that MSCs can be recruited to peripheral tissue in times of stress or injury. Several lines of evidence in animal and clinical studies support this idea, as reviewed by Meier et al.34 In the current study, we evaluated the therapeutic capacities of miR-27b-expressing ASCs in liver regeneration. We first ensured that the injected ASCs could localize to the site of liver resection by visualizing the fluorescent labeled ASCs (using XenoLight DiR) in rats that underwent PH and monitoring their localization using an IVIS Spectrum System. By day 3, most of the labeled ASCs were highly concentrated at the site of liver injury (Supplementary Figure S3). However, despite the benefits, MSCs have been shown to have increased fibrotic potential and to be unable to differentiate into hepatocytes but into myofibroblasts under certain conditions.16,35 Therefore, using MSCs in cell-based therapy should be very careful and the potentially deleterious outcome should be seriously taken into consideration. In the current study, we investigated the impact of administration of ASCs, modified by miR-27b overexpression, on liver regeneration after PH. Although regeneration of the residual lobes following PH is mostly mediated by processes relevant to growth (proliferation of hepatocytes), accompanying inflammatory responses as well as activation of resident stellate cells should not be disregarded. It has been illustrated that massive production of initial inflammatory cytokines after extensive hepatectomy is associated with harmful effects on liver regeneration. Suppression of these cytokines improved liver regeneration and function.36 We previously investigated the effects of miR-27b overexpression in ASCs on engraftment during liver regeneration and observed significant inhibition of α-SMA induction, a drastic increase of HO-1 expression as well as HO-1-dependent liver-to-body ratio value of injured liver after i.v. injection of ASCs-m27b.18 These results suggested that specific factors secreted by ASCs-m27b might be responsible for the reduction of myofibrotic response and improvement of liver function in the resected liver, which could also participate in promoting liver regeneration via suppression of systemic inflammation. Therefore in this study, we further investigated the mechanism of ASCs-m27b facilitation in liver regeneration and demonstrated that administration of ASCs-m27b highly reduced AST, ALT, TNF-α, and IL-6 serum levels and suppressed TGF-β1, MMP2, and MMP9 levels in liver tissue comparing with little to moderate effects by ASCs-siRNC. The results indicate that these effects are related to systemic inhibition of both TNF-α and TGF-β1 signaling pathways which could be mediated by the secreted factors from ASCs with miR-27b overexpression. Besides our results, more evidence demonstrates that downregulation of inflammatory as well as profibrotic factors, which are systemically activated and responsible for the severity of fibrosis, is significantly associated with upregulation of HO-1.37,38 However, several other studies also demonstrate that overexpression of HO-1 is not exclusively protective or antifibrotic but increases oxidative injury.39,40,41 Thus, the dual role of HO-1 in either cytoprotection or increased fibrogenesis may depend upon the contextual differences of injured tissue, such as participation of other antifibrotic/anti-inflammatory factors (e.g., IL-1Ra) and/or coupling with PGC-1α-mediated mitochondrial biogenesis.42 Increased expression of HO-1 in damaged tissue is necessary but not sufficient for cytoprotection, anti-inflammation, and regeneration in the liver.18
In the present study, we found that HO-1 induction in ASCs by transfected miR-27b depends on the expression of PGC-1α. The protein level of PGC-1α was significantly increased by miR-27b transfection; however, PGC-1α knockdown abolished miR-27b-induced HO-1 expression. Moreover, it has been reported that miR-27 negatively controls the expression of the F-box protein Fbxw7/Cdc4, which targets the cell cycle regulator cyclin E43,44,45,46 and several well-known oncoproteins47,48 as well as this master regulator of mitochondrial biogenesis PGC-1α,22,49 for ubiquitination-mediated turnover. Since we first observed that miR-27b was highly expressed in stromal cells of the immune tolerant rat model and found that it may play an important role in the immunosuppressive and regenerative properties of ASCs, here we further confirmed that the anti-inflammatory, antioxidant as well as tissue protective actions via increased levels of secreted soluble molecules by miR-27b overexpression were mediated by PGC-1α-dependent signaling pathways. We demonstrated that miR-27b significantly suppressed Fbxw7 level in ASCs, indicating that promotion of PGC-1α expression from the suppression of Fbxw7 by miR-27b is responsible for the cytoprotective and anti-inflammatory abilities as well as regenerative activity of administrated ASCs. Indeed, we found that miR-27b drastically increased ATP production, various reactive oxygen species-detoxifying enzymes, and expression of cytokines IL-1Ra, IL-15, and the hepatocyte-secreted protective hormone FGF-21 in ASCs as well as in resected liver (data not shown) in a PGC-1α-dependent manner. The expression of vascular endothelial growth factor (VEGF) and angiopoietin-1 (ANGPT1), which were highly expressed in activated HSCs, were significantly reduced in liver samples of PH-ASCs-miR-27b group than in those of PH-ASCs-siRNC group. Moreover, blockade of PGC-1α in ASCs-miR-27b also reverted TGF-β1-mediated HSC activation and decreased HGF production in 70% PH rats indicating that a positive feedback loop may exist between PGC-1α and these secreted factors of ASCs-miR-27b and remnant liver tissue, which is mediated by the induction of HO-1 expression.
Our study reveals that PGC-1α in remnant liver of ASCs-siRNC group was decreased compared to the levels in saline control as well as ASCs-miR-27b groups in deep sequencing results. Although the mitochondrial deacetylase sirtuin 3 (Sirt3) was lower in remnant liver of saline controls compared to the ASCs-miR-27b group, a significant drop in Sirt3 expression in the ASCs-siRNC group was also observed. The mitochondrial function of the PGC-1α/Sirt3 signaling has been reported to be essential for the regulation of metabolism, biogenesis, and oxidative stress, where PGC-1α functions as an activator of Sirt3 gene transcription in hepatocytes.24,50,51 Analysis of the DEGs between ASCs-siRNC and ASCs-miR-27b groups demonstrated that various detoxifying enzymes were upregulated in ASCs-miR-27b group, whereas fibrotic genes were upregulated in ASCs-siRNC group. This result might reflect the pivotal role of PGC-1α with Sirt3 in the modulation of oxidative status with a consequent impairment of liver function (growth versus fibrosis). Moreover, FGF-21 expression, which was markedly reduced in remnant liver after PH, was rescued by ASCs-miR-27b injection, whereas an even lower level of FGF-21 was observed in ASCs-siRNC group. We also demonstrated that liver damage (as determined by serum ALT and AST levels) was not alleviated in the ASCs-siRNC group after PH (compared with saline control group), but however was drastically reduced in ASCs-miR-27b group, also suggesting a collaborative response between PGC-1α and FGF-21 against liver injury. Moreover, the expression of HGF in resected liver was significantly increased only in ASCs-miR-27b group compared with saline and ASCs-siRNC groups (data not shown). Further studies are needed to investigate the detailed underlying mechanisms involving PGC-1α in inducing secretion of anti-inflammatory, antioxidant, and protective molecules in hepatocytes interacting with miR-27b-expressing ASCs.
In conclusion, our findings provide evidence that the protective capacities of ASCs could be enhanced by “energizing” these multipotent stromal cells via miR-27b expression which derived, at least in part, from secreted cytokines and growth factors that function in proximity in the microenvironment between ASCs and target cells. Future studies will focus on cell therapies using MSCs, especially with miR-27b overexpression for liver fibrosis as well as recovery from transplantation.
Materials and methods
Animals and ethics. Male LEW (strain: RT11) rats of 4 weeks old were obtained from the National Laboratory Animal Breeding and Research Center (Taipei, Taiwan). All of the animals were maintained in specific pathogen-free animal facilities with water and commercial rat food provided ad libitum. Our experimental design was reviewed and approved by the Institutional Animal Care and Use Committee of the Kaohsiung Chang Gung Memorial Hospital (Kaohsiung, Taiwan; Approval No: 2009122102), and the Committee recognizes that the proposed animal experiment follows the Animal Protection Law by the Council of Agriculture, Executive Yuan, R.O.C. and the guideline as shown in the Guide for the Care and Use of Laboratory Animals as promulgated by the Institute of Laboratory Animal Resources, National Research Council (Washington, DC).
Isolation of ASCs from rat. Eight- to 12-week-old female LEW rats were used for the isolation of rat ASCs. Cells were isolated from rat inguinal and interscapular adipose tissues. Briefly, the adipose tissue was dissociated mechanically and digested at 37 °C in Hanks' balanced salt solution buffer (Gibco, Life Technologies, Carlsbad, CA) containing 1 mg/ml collagenase I (Worthington Biochemical, Lakewood, NJ) for 20 minutes. After digestion, the contents were filtered with a 100-μm filter prior to centrifugation at 800 × g for 10 minutes at room temperature. The cell pellet was resuspended in washing buffer (phosphate-buffered saline with 1% fetal bovine serum (FBS, Gibco)) and then centrifuged again at 800 × g for 5 minutes at room temperature. After being resuspended again in washing buffer and filtered through a 25-μm filter (Merck Millipore, Billerica, MA), mature adipocytes were separated from the stromal fraction by centrifugation (800 × g for 10 minutes) at room temperature. The pellet was resuspended in culture medium and an aliquot of the cell suspension was then seeded (10,000 cells/cm2) in Dulbecco's modified Eagle's medium (Gibco) with 10% FBS (Gibco) medium and maintained in a 5% CO2 and humidified atmosphere. Twenty-four hours after plating, all nonadherent cells were removed by changing the medium. Subconfluent ASCs were obtained after 5 days.
Reagents and transient transfection with microRNA antagomir and small interfering RNA. The small interfering RNA (siRNA) for PGC-1α, HO-1, miRNA agonist miR-27b-mimic and its negative control (siRNC) used in the present study were purchased from Sigma-Aldrich (St. Louis, MO). ASCs were cultured in basal media consisting of Dulbecco's modified Eagle's medium (Gibco) supplemented with 2 mmol/l l-glutamine (Sigma-Aldrich) and 10% FBS until the cells reached 60% confluency; cells were then transfected with the miR-27b-mimic (25 nmol/l), the miRNA negative control (siRNC, 25 nmol/l), HO-1, or PGC-1α siRNA (10 nmol/l) using GenMute siRNA Transfection Reagent (SignaGen Laboratories, Rockville, MD) according to the manufacturer's protocol. Then, the medium was changed to fresh Dulbecco's modified Eagle's medium with 10% FBS, and the cells were incubated for another 24 hours. The knockdown efficiency was assessed by measuring the mRNA expression levels. The efficiency of the PGC-1α or HO-1 knockdown was more than 95% as measured by mRNA levels.
Intravenous injection of modified ASCs into syngeneic rats after 70% PH. Four-week-old male LEW rats were obtained from the National Laboratory Animal Breeding and Research Center (Taipei, Taiwan). Under general anesthesia, a median laparotomy was performed and the left lateral, left upper, and right anterior lobes (70% PH) were ligated and removed as previously described by Greene and Puder. After 70% PH, the rats were injected with modified ASCs (siRNC, miR-27b-mimic, or HO-1 siRNA transfected, 2 × 106/rat) at day 1 after resection, and the blood and liver tissue samples were retrieved at various time points for measurement of serum factors, qRT–PCR, and next-generation sequencing (NGS) analysis.
To ensure that the injected ASCs migrated to the site of liver injury, visualization of ASCs in rats that underwent PH was performed in a parallel experiment by labeling the ASCs using Xenolight DiR and monitored their localization using an IVIS Spectrum System (PerkinElmer, Waltham, MA). Briefly, the ASCs were washed three times with phosphate-buffered saline, trypsinized, and incubated with 3.5 µg/ml DiR buffer (PerkinElmer) for 30 minutes at 37 °C according to the manufacturer's instructions. DiR-labeled ASCs (DiR(+)) were washed twice with phosphate-buffered saline and examined for viability using trypan blue. ASCs that were not labeled with DiR (DiR(−)) were only incubated in phosphate-buffered saline and treated the same way as DiR(+) cells. DiR(+) ASCs (2 × 106) were i.v. injected into LEW rats the following day after 70% PH (n = 3) as the test group. In the control group, hepatectomized rats (n = 3) were injected with DiR(−) ASCs. Migration of the injected ASCs was monitored using the Caliper IVIS Spectrum System (Caliper, Life Sciences) at days 1 (right after injection) and 3 (2 days after injection) after surgery (day 0) to obtain serial fluorescence images. The ideal filter conditions for DiR imaging were set at 710 nm for excitation and 760 nm for emission. Identical illumination settings (e.g., lamp voltage, filter, exposure time) were used in all animal imaging experiments. Gray scale photographic images and fluorescent images of each animal were analyzed and overlaid using Living Image software PerkinElmer.
RNA isolation and qRT–PCR. Total RNA was isolated from the cells using the RNeasy kit from Qiagen (Valencia, CA) according to the manufacturer's protocol. Reverse transcription was performed with 1 μg RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, ABI, Waltham, MA) as described by the manufacturer. The qRT–PCR reaction was performed on an ABI 7500 Fast Real-Time PCR System with the SDS 1.4 program using the ABI TaqMan Fast Universal PCR master mix. The master mix and TaqMan MGB probes were obtained from Applied Biosystems, and the final concentration of probes was 250 nmol/l. The cycling profile for each run was 95 °C for 20 seconds and 40 cycles of 95 °C for 3 seconds followed by 60 °C for 30 seconds using the default ramp rate. The normalization was performed with rat beta-actin (ACTB) probes (P/N: 4331182, ID: Rn00667869_m1). For mRNA, the TaqMan probes (P/N: 4331182) for TGF-β1 (ID: Rn00572010_m1), MMP2 (ID: Rn01538170_m1), MMP9 (ID: Rn00579162_m1), HGF (ID: Rn00566673_m1), HO-1 (ID: Rn01536933_m1), IL-1Ra (ID: Rn02586400_m1), FGF-21 (ID: Rn04219642_g1), α-SMA (ID: Rn01759928_g1), Atp5a1 (ID: Rn01527025_m1), Sod3 (ID: Rn00563570_m1), Gpx3 (ID: Rn00574703_m1), Gpx4 (ID: Rn00820818_g1), and Ucp2 (ID: Rn01754856_m1) were obtained from Applied Biosystems. Comparative RT–PCR data including nontemplate controls was done in triplicate. The fold increase was calculated with the comparative 2−ΔΔCt method.
Relative protein expression levels and ATP production in modified ASCs. ASCs were transfected with siRNC, miR-27b-mimic, or PGC-1α siRNA for 24 hours, and the medium was replaced with growth medium for 12 hours. The total RNA and protein samples were obtained. Western blots were performed to analyze the protein expression levels by using actin (ADI-905-733-100) and HO-1 (ADI-SPA-895) antibodies from Enzo Life Sciences (Farmingdale, NY) and IL-1Ra (sc-25444), IL-15 (sc-7889), and FGF-21 (sc-292879) antibodies from Santa Cruz (Dallas, TX). Also, ATP production from modified ASCs was measured by ATP Determination Kit (A22066) from Life Technologies (Carlsbad, CA).
Indirect coculture system using HSCs and modified ASCs. HSCs (HSC-T6) were seeded in six-well plates and modified ASCs were seeded in Millicell Cell Culture Inserts (pore size: 0.4 µm; Millipore, Billerica, MA). The cells were incubated in Dulbecco's modified Eagle's medium (Gibco) with 10% FBS (Gibco) medium containing different concentration of TGF-β1 (PeproTech, Rocky Hill, CT) (0, 5, or 10 ng/ml) and maintained in a 5% CO2 and humidified atmosphere for 24, 72, and 96 hours. The total RNA samples were isolated for qRT–PCR.
NGS for RNA-Seq analysis. Total RNA was isolated from liver tissues using TRIzol reagent (Invitrogen). Total RNA (15 µg) for each sample was used for purifying the poly(A)-containing mRNA molecules, RNA amplification, and synthesis of double-stranded cDNAs that will be ligated to adapters, following the Illumina (San Diego, CA) TruSeq RNA Sample Prep guidelines. Multiplexed samples were sequenced at 100 bp length on an in-house Illumina MiSeq instrument. For data analysis, sequences called by the Illumina pipeline were mapped to the reference genome and annotated using Strand NGS 2.1 (Strand Life Sciences, Bangalore, Karnataka, India). The sequencing data were deposited in NCBI GEO database and are accessible through series accession number GSE69783.
Data analysis and statistics. The results from NGS analysis were analyzed by Mann–Whitney unpaired test or two-way analysis of variance and a Benjamini and Hochberg false discovery rate multiple gene correction was applied. To better understand the biological context of the changes, data were analyzed by Ariadne's Pathway Studio (Elsevier B.V., Amsterdam, The Netherlands) to build up for direct interaction and subnetwork enrichment analysis. Each experiment of other analyses was performed three times, and the statistical significance of the data was calculated using Student's t-test. A value of P <0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Venn diagrams showing the number of identified DEGs in liver samples using RNA-Seq analysis. Figure S2. Schematic representation showing the relationship of cells, genes, and cytokines/growth factors demonstrated in this study. Figure S3. In vivo imaging of fluorescent labeled ASCs intravenously injected into rats that underwent PH. Table S1. Basic statistics of high-throughput RNA-seq data after alignment for the 4-pooled liver samples. Table S2. Functional categories and associated genes that were differentially expressed and involved in regeneration and cell migration in resected livers of PH-ASCs-miR-27b treated rats. Table S3. Expression targets and their corresponding seeds enriched in subnetwork enrichment analysis for 200 selected DEGs upregulating in PH-ASCs-miR-27b group and downregulating in PH-ASCs-siRNC group.
Acknowledgments
This study was supported in part by grants from the Ministry of Science and Technology (MOST101-2314B-182A-031-MY3, MOST 104-2314-B-182A-018 to C.-L.C.; MOST101-2320-B-182-037-MY3 to T.N.; MOST103-2314-B-182A-054 to Y.-F.C.), the Ministry of Health and Welfare (PMRPG8E0011 to C.-L.C.), and the Chang Gung Memorial Hospital (CMRPG8B0911, CMRPG8D1021, and CMRPG8D0751 to K.-D.C.; CMRPG8D1031 and CMRPG8C1151 to K.-T.H.; CMRPG8A0433, CMRPG8B0952, and CMRPG8D1011 to C.-L.C.; CMRPG890161, CMRPG890451, and CMRPG8A1203 to C.-C.L.; CMRPD8D1381 and CMRPD8C0562 to T.N.; CMRPG8D1001 to K.-W.C.; CMRPG8B0541 to Y.-Y.M.; CMRPG881231 and CMRPG8B0861 to C.-C.W.) of Taiwan. We would like to thank Chang Gung Medical Foundation Kaohsiung Chang Gung Memorial Hospital Tissue Bank (CLRPG8B0031) for technical support. The authors declare no conflicts of interest.
Supplementary Material
References
- Izadpanah, R, Trygg, C, Patel, B, Kriedt, C, Dufour, J, Gimble, JM et al. (2006). Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem 99: 1285–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, E, Guo, Z, Wang, H, Jin, J, Wang, J, Wang, H et al. (2008). High mobility group box 1 protein inhibits the proliferation of human mesenchymal stem cells and promotes their migration and differentiation along osteoblastic pathway. Stem Cells Dev 17: 805–813. [DOI] [PubMed] [Google Scholar]
- in ‘t Anker, PS, Noort, WA, Scherjon, SA, Kleijburg-van der Keur, C, Kruisselbrink, AB, van Bezooijen, RL et al. (2003). Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica 88: 845–852. [PubMed] [Google Scholar]
- Pittenger, MF, Mackay, AM, Beck, SC, Jaiswal, RK, Douglas, R, Mosca, JD et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147. [DOI] [PubMed] [Google Scholar]
- Le Blanc, K (2003). Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy 5: 485–489. [DOI] [PubMed] [Google Scholar]
- Wan, CD, Cheng, R, Wang, HB and Liu, T (2008). Immunomodulatory effects of mesenchymal stem cells derived from adipose tissues in a rat orthotopic liver transplantation model. Hepatobiliary Pancreat Dis Int 7: 29–33. [PubMed] [Google Scholar]
- Aurich, H, Sgodda, M, Kaltwasser, P, Vetter, M, Weise, A, Liehr, T et al. (2009). Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut 58: 570–581. [DOI] [PubMed] [Google Scholar]
- Banas, A, Teratani, T, Yamamoto, Y, Tokuhara, M, Takeshita, F, Osaki, M et al. (2009). Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol 24: 70–77. [DOI] [PubMed] [Google Scholar]
- McIntosh, K, Zvonic, S, Garrett, S, Mitchell, JB, Floyd, ZE, Hammill, L et al. (2006). The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells 24: 1246–1253. [DOI] [PubMed] [Google Scholar]
- Yañez, R, Lamana, ML, García-Castro, J, Colmenero, I, Ramírez, M and Bueren, JA (2006). Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells 24: 2582–2591. [DOI] [PubMed] [Google Scholar]
- Ryan, JM, Barry, FP, Murphy, JM and Mahon, BP (2005). Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew, A, Sturgeon, C, Siatskas, M, Ferrer, K, McIntosh, K, Patil, S et al. (2002). Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 30: 42–48. [DOI] [PubMed] [Google Scholar]
- Inoue, S, Popp, FC, Koehl, GE, Piso, P, Schlitt, HJ, Geissler, EK et al. (2006). Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplantation 81: 1589–1595. [DOI] [PubMed] [Google Scholar]
- Sbano, P, Cuccia, A, Mazzanti, B, Urbani, S, Giusti, B, Lapini, I et al. (2008). Use of donor bone marrow mesenchymal stem cells for treatment of skin allograft rejection in a preclinical rat model. Arch Dermatol Res 300: 115–124. [DOI] [PubMed] [Google Scholar]
- Carrión, F, Nova, E, Luz, P, Apablaza, F and Figueroa, F (2011). Opposing effect of mesenchymal stem cells on Th1 and Th17 cell polarization according to the state of CD4+ T cell activation. Immunol Lett 135: 10–16. [DOI] [PubMed] [Google Scholar]
- Baertschiger, RM, Serre-Beinier, V, Morel, P, Bosco, D, Peyrou, M, Clément, S et al. (2009). Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One 4: e6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, KD, Goto, S, Hsu, LW, Lin, TY, Nakano, T, Lai, CY et al. (2013). Identification of miR-27b as a novel signature from the mRNA profiles of adipose-derived mesenchymal stem cells involved in the tolerogenic response. PLoS One 8: e60492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, KD, Hsu, LW, Goto, S, Huang, KT, Nakano, T, Weng, WT et al. (2014). Regulation of heme oxygenase 1 expression by miR-27b with stem cell therapy for liver regeneration in rats. Transplant Proc 46: 1198–1200. [DOI] [PubMed] [Google Scholar]
- Michalopoulos, GK and DeFrances, MC (1997). Liver regeneration. Science 276: 60–66. [DOI] [PubMed] [Google Scholar]
- Lu, D, Zhang, L, Wang, H, Zhang, Y, Liu, J, Xu, J et al. (2012). Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) enhances engraftment and angiogenesis of mesenchymal stem cells in diabetic hindlimb ischemia. Diabetes 61: 1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner, M, Lundgren, J, Akhoondi, S, Jahn, A, Ng, HF, Akbari Moqadam, F et al. (2011). MiRNA-27a controls FBW7/hCDC4-dependent cyclin E degradation and cell cycle progression. Cell Cycle 10: 2172–2183. [DOI] [PubMed] [Google Scholar]
- Olson, BL, Hock, MB, Ekholm-Reed, S, Wohlschlegel, JA, Dev, KK, Kralli, A et al. (2008). SCFCdc4 acts antagonistically to the PGC-1alpha transcriptional coactivator by targeting it for ubiquitin-mediated proteolysis. Genes Dev 22: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu, S, Broxmeyer, HE and Hangoc, G (2013). Peroxisome proliferator-activated-γ coactivator-1α-mediated mitochondrial biogenesis is important for hematopoietic recovery in response to stress. Stem Cells Dev 22: 1678–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, X, Wang, R, Xue, Y, Liu, X, Zhang, H, Chen, Y et al. (2010). Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One 5: e11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver, P and Spiegelman, BM (2003). Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev 24: 78–90. [DOI] [PubMed] [Google Scholar]
- St-Pierre, J, Drori, S, Uldry, M, Silvaggi, JM, Rhee, J, Jäger, S et al. (2006). Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127: 397–408. [DOI] [PubMed] [Google Scholar]
- Herzig, S, Long, F, Jhala, US, Hedrick, S, Quinn, R, Bauer, A et al. (2001). CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413: 179–183. [DOI] [PubMed] [Google Scholar]
- Lehman, JJ, Barger, PM, Kovacs, A, Saffitz, JE, Medeiros, DM and Kelly, DP (2000). Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 106: 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, JC, Puigserver, P, Chen, G, Donovan, J, Wu, Z, Rhee, J et al. (2001). Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413: 131–138. [DOI] [PubMed] [Google Scholar]
- Wang, H, Peiris, TH, Mowery, A, Le Lay, J, Gao, Y and Greenbaum, LE (2008). CCAAT/enhancer binding protein-beta is a transcriptional regulator of peroxisome-proliferator-activated receptor-gamma coactivator-1alpha in the regenerating liver. Mol Endocrinol 22: 1596–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelli, A, Moretta, L and Pistoia, V (2008). Mesenchymal stem cells in health and disease. Nat Rev Immunol 8: 726–736. [DOI] [PubMed] [Google Scholar]
- Krampera, M, Glennie, S, Dyson, J, Scott, D, Laylor, R, Simpson, E et al. (2003). Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101: 3722–3729. [DOI] [PubMed] [Google Scholar]
- Taléns-Visconti, R, Bonora, A, Jover, R, Mirabet, V, Carbonell, F, Castell, JV et al. (2006). Hepatogenic differentiation of human mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells. World J Gastroenterol 12: 5834–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier, RP, Müller, YD, Morel, P, Gonelle-Gispert, C and Bühler, LH (2013). Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence? Stem Cell Res 11: 1348–1364. [DOI] [PubMed] [Google Scholar]
- Jeon, ES, Moon, HJ, Lee, MJ, Song, HY, Kim, YM, Cho, M et al. (2008). Cancer-derived lysophosphatidic acid stimulates differentiation of human mesenchymal stem cells to myofibroblast-like cells. Stem Cells 26: 789–797. [DOI] [PubMed] [Google Scholar]
- Tsutsumi, R, Kamohara, Y, Eguchi, S, Azuma, T, Fujioka, H, Okudaira, S et al. (2004). Selective suppression of initial cytokine response facilitates liver regeneration after extensive hepatectomy in rats. Hepatogastroenterology 51: 701–704. [PubMed] [Google Scholar]
- Wang, RQ, Nan, YM, Wu, WJ, Kong, LB, Han, F, Zhao, SX et al. (2011). Induction of heme oxygenase-1 protects against nutritional fibrosing steatohepatitis in mice. Lipids Health Dis 10: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, H, Wei, L, Sun, WL, Wang, L, Yang, ZL, Liu, Y et al. (2014). The green tea extract epigallocatechin-3-gallate inhibits irradiation-induced pulmonary fibrosis in adult rats. Int J Mol Med 34: 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froh, M, Conzelmann, L, Walbrun, P, Netter, S, Wiest, R, Wheeler, MD et al. (2007). Heme oxygenase-1 overexpression increases liver injury after bile duct ligation in rats. World J Gastroenterol 13: 3478–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaguarnera, L, Madeddu, R, Palio, E, Arena, N and Malaguarnera, M (2005). Heme oxygenase-1 levels and oxidative stress-related parameters in non-alcoholic fatty liver disease patients. J Hepatol 42: 585–591. [DOI] [PubMed] [Google Scholar]
- Suttner, DM and Dennery, PA (1999). Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J 13: 1800–1809. [DOI] [PubMed] [Google Scholar]
- Piantadosi, CA, Withers, CM, Bartz, RR, MacGarvey, NC, Fu, P, Sweeney, TE et al. (2011). Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J Biol Chem 286: 16374–16385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu, KT, Rosner, MR and Minella, AC (2012). An integrated view of cyclin E function and regulation. Cell Cycle 11: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohmaier, H, Spruck, CH, Kaiser, P, Won, KA, Sangfelt, O and Reed, SI (2001). Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 413: 316–322. [DOI] [PubMed] [Google Scholar]
- Koepp, DM, Schaefer, LK, Ye, X, Keyomarsi, K, Chu, C, Harper, JW et al. (2001). Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science 294: 173–177. [DOI] [PubMed] [Google Scholar]
- Caldon, CE, Sergio, CM, Sutherland, RL and Musgrove, EA (2013). Differences in degradation lead to asynchronous expression of cyclin E1 and cyclin E2 in cancer cells. Cell Cycle 12: 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inuzuka, H, Shaik, S, Onoyama, I, Gao, D, Tseng, A, Maser, RS et al. (2011). SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 471: 104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz, IE, Kusam, S, Lam, C, Okamoto, T, Sandoval, W, Anderson, DJ et al. (2011). Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature 471: 110–114. [DOI] [PubMed] [Google Scholar]
- Park, JH, Kang, HJ, Lee, YK, Kang, H, Kim, J, Chung, JH et al. (2015). Inactivation of EWS reduces PGC-1α protein stability and mitochondrial homeostasis. Proc Natl Acad Sci USA 112: 6074–6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, EL and Guarente, L (2011). The SirT3 divining rod points to oxidative stress. Mol Cell 42: 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rato, L, Duarte, AI, Tomás, GD, Santos, MS, Moreira, PI, Socorro, S et al. (2014). Pre-diabetes alters testicular PGC1-α/SIRT3 axis modulating mitochondrial bioenergetics and oxidative stress. Biochim Biophys Acta 1837: 335–344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.