Abstract
Human pluripotent stem cells (hPSCs) hold great promise for cell therapy. However, a major concern is the risk of tumor formation by residual undifferentiated cells contaminating the hPSC-derived cell product. Suicide genes could safeguard against such adverse events by enabling elimination of cells gone astray, but the efficacy of this approach has not yet been thoroughly tested. Here, we engineered a lentivirally encoded herpes simplex virus thymidine kinase (HSVtk) with expression restricted to undifferentiated hPSCs through regulation by the let7 family of miRNAs. We show that induced pluripotent stem cells (iPSCs) expressing a let7-regulated HSVtk transgene are selectively killed by ganciclovir (GCV), whereas differentiated cells are fully protected. However, in contrast to previous studies, we find that in vivo GCV administration results in longer latency but does not prevent teratoma formation by iPSCs expressing either a constitutive or a let7-regulated HSVtk, without evidence of silencing of the HSVtk. Clonal analyses of iPSCs expressing HSVtk revealed frequent emergence of GCV resistance which, at least in some cases, could be attributed to preexisting inactivating mutations in the HSVtk coding sequence, selected for upon GCV treatment. Our findings have important consequences for the future use of suicide genes in hPSC-based cell therapies.
Keywords: HSVtk, induced pluripotent stem cells (iPSCs), stem cell therapy, suicide gene, teratoma formation
Introduction
Induced pluripotent stem cells (iPSCs) have opened exciting new prospects for regenerative medicine. However, the same properties of unlimited self-renewal and pluripotency which are unique to human pluripotent stem cells (hPSCs) and central to their therapeutic promise are also intrinsically linked to their potential for extensive growth and tumorigenicity.1 Several preclinical studies in rodent and nonhuman primate models have established the risk of tumorigenicity associated with administration of hPSC-derived cell grafts.2,3,4,5,6,7 Tumor formation may follow dedifferentiation and transformation of terminally differentiated cells, aberrant proliferation of incompletely differentiated progenitors, or teratoma development by residual pluripotent cells that may persist after differentiation and contaminate the graft.8,9,10 Although the latter are benign tumors, their growth can have catastrophic consequences in particular anatomic sites such as joints, brain, spinal cord, or the eye.11,12,13 The immune-privileged status of undifferentiated, as opposed to differentiated cells—at least in part due to low major histocompatibility complex antigen expression by the former—may further increase this risk, due to evasion of immune surveillance even in a nonautologous cell therapy setting.14
The use of suicide genes has been proposed as a promising strategy to safeguard against teratoma formation of hPSC-derived cell products. Herpes simplex virus thymidine kinase (HSVtk) is the most common suicide gene with a long track record in the clinic.15 It encodes an enzyme that converts a prodrug, ganciclovir (GCV), into a cytotoxic metabolite. Several recent studies have suggested that HSVtk can prevent and/or ablate teratomas derived from human or murine PSCs.16,17,18,19 Ideally a suicide approach should be selective to pluripotent undifferentiated cells, while sparing their differentiated progeny. Attempts to engineer such an expression pattern in suicide genes have included the use of pluripotent cell-specific promoter/enhancers, such as the Oct4 (knockin at the endogenous Oct4 locus),20 NANOG (knockin at the endogenous NANOG locus19 or on a lentiviral vector18), or the synthetic promoter/enhancer element Early Transposon promoter and Oct4 and Sox2 enhancers, EOS-C(3+),21 previously developed by Hotta et al.22
We and others have previously shown that regulation by endogenous miRNAs is a very effective and robust strategy to endow transgenes with tissue- and developmental stage-specific expression.23,24,25 Tagging of a transgene with sequences complementary to a mature miRNA subjects the former to posttranscriptional regulation by the specific miRNA, so that its expression is suppressed only in the presence of the miRNA but not in its absence. Because expression of most miRNAs is transcriptionally regulated in a cell type- and differentiation stage-specific manner, this mechanism of transgene regulation has found several applications in genetic engineering.24
The let7 family of miRNAs are ubiquitously expressed in differentiated tissues, but absent from pluripotent cells. Here, in order to sequester expression of HSVtk strictly in undifferentiated hPSCs and render the latter selectively sensitive to GCV-induced cell death, we engineered HSVtk to be regulated by the let7 family of miRNAs. While we observed no teratoma formation for up to 5 weeks in mice that received HSVtk-expressing iPSCs with administration of GCV, no protection from teratomas was observed in the longer term, likely due to inactivating mutations of the HSVtk transgene. Our study has fundamental implications for the use of HSVtk as a suicide gene in hPSC-based cell and gene therapy. It also highlights the need for longer observation periods in preclinical testing for teratoma formation.
Results
Let7 regulation suppresses expression of HSVtk in differentiated but not pluripotent cells
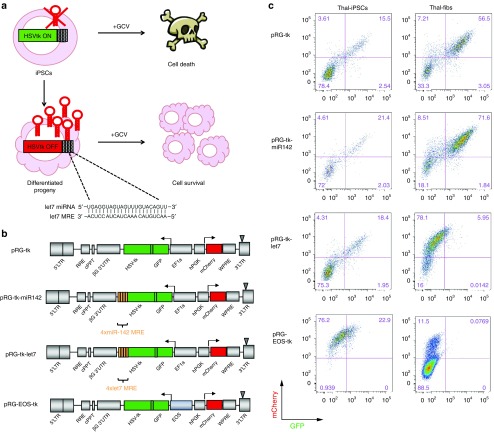
We set to engineer a lentivirally encoded HSVtk to be specifically expressed in undifferentiated hPSCs, rendering them sensitive to cell death by GCV, but not in their differentiated progeny, which would thus be resistant to GCV using miRNA regulation (Figure 1a). We selected the let7 family of miRNAs on the basis of its well-documented widespread and high-level expression in practically all differentiated cells, but not in undifferentiated pluripotent stem cells.26,27,28 We constructed bidirectional lentiviral vectors expressing HSVtk linked by a P2A peptide to GFP driven by the EF1α promoter or the pluripotent-specific EOS-C(3+) promoter and constitutively expressed hPGK-driven mCherry (vectors pRG-tk and pRG-EOS-tk, respectively) (Figure 1b). EF1α-driven HSVtk was tagged to four tandem miRNA recognition elements (MRE) complementary to mature miRNAs let7a, let7d, let7f, and let7g (vector pRG-tk-let7) or to four MREs complementary to mature miR-142. miR-142 is a miRNA predominantly expressed in hematopoietic cells, but not in pluripotent stem cells or fibroblasts. The four vectors were transduced into an iPSC line (thal-iPS thal-5.10-Cre8) derived from a beta-thalassemia patient,29 as well as in bone marrow fibroblasts derived from the same individual (thal-fibs). As expected, both iPSCs and fibroblasts coexpressed mCherry with GFP-HSVtk expressed by the nonregulated (pRG-tk) and miR-142-regulated (pRG-tk-miR142) vectors (Figure 1c). In contrast, let7 regulation resulted in suppression of GFP-HSVtk expression in fibroblasts but not in undifferentiated iPSCs (Figure 1c and Supplementary Figure S1). GFP-HSVtk expression levels relative to mCherry were practically identical in iPSCs with or without let7 regulation, showing that let7 regulation has no detectable effect in HSVtk expression in pluripotent cells (Supplementary Figure S2a). EOS-driven HSVtk expression was also undetectable in fibroblasts but maintained in iPSCs at low levels (Figure 1c). This vector was thus not further used.
Figure 1.
Let7-regulated HSVtk is expressed in undifferentiated but not differentiated cells. (a) Scheme of the approach to engineering an HSVtk transgene specifically expressed in undifferentiated iPSCs by incorporation of MREs complementary to the let7 family of miRNAs in its 3′ end. HSVtk is expressed in iPSCs, which lack endogenous let7 and can thus be eliminated by GCV administration. In contrast, HSVtk is posttranscriptionally silenced in differentiated cell derivatives which thus remain insensitive to GCV. (b) Lentiviral vectors used in this study. The vectors bidirectionally drive expression of a constitutively expressed mCherry reporter gene driven by the EF1α promoter and HSVtk together with GFP (linked through a P2A peptide) driven by hPGK or the EOS-C(3+) promoter/enhancer with or without four MREs of miRNAs let7 or miR-142 attached in the 3′ of the GFP-2A-HSVtk transcription unit. (c) Expression of mCherry (constitutive reporter) and GFP-HSVtk (constitutive or regulated) in iPSCs (left panels) and fibroblasts (right panels) transduced with the respective vectors as indicated. GCV, ganciclovir; HSVtk, herpes simplex virus thymidine kinase; iPSCs, induced pluripotent stem cells; MRE, miRNA recognition element.
Let7-regulated HSVtk enables selective killing of pluripotent but not differentiated cells
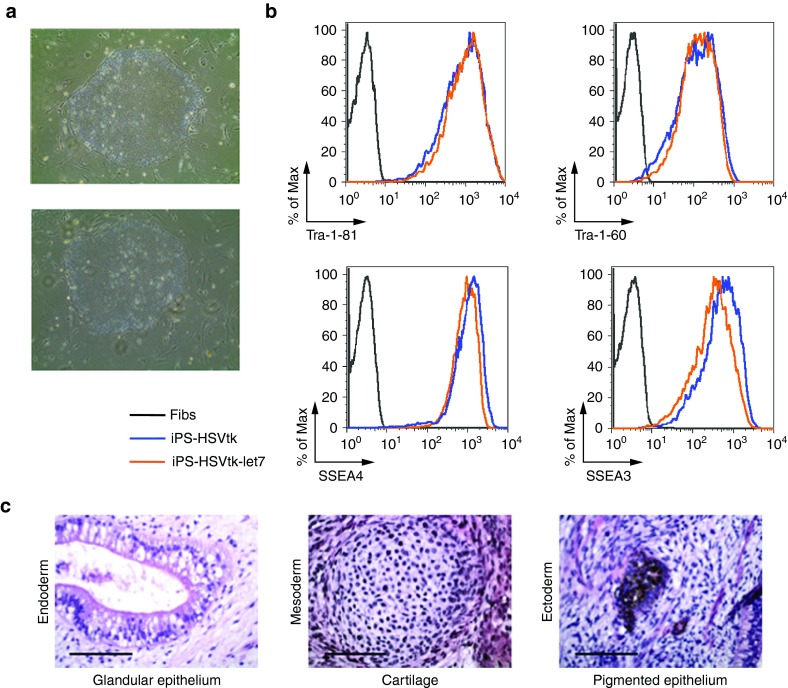
To next test if let7-regulated HSVtk could operate as a “death switch,” iPSC colonies homogeneously expressing GFP-HSVtk and GFP-HSVtk-let7 were individually picked under a microscope and expanded (Supplementary Figure S2b). iPSCs expressing GFP-HSVtk and GFP-HSVtk-let7 showed unchanged morphology (Figure 2a) and expression of pluripotency markers (Figure 2b) and could form trilineage teratomas upon injection into immunodeficient mice (Figure 2c).
Figure 2.
iPSCs expressing HSVtk are pluripotent and form trilineage teratomas. (a) Brightfield images of representative colonies of the thal-5.10-Cre8 iPSC line transduced with pRG-tk (upper panel) and pRG-tk-let7 (lower panel). (b) Flow cytometric evaluation of expression of pluripotent stem cell markers Tra-1–81, Tra-1–60, SSEA3, and SSEA4 in iPSCs expressing HSVtk. (c) Hematoxylin–eosin staining of a representative teratoma derived from the thal-iPSC line thal-5.10-Cre8 transduced with pRG-tk-let7 showing differentiation into tissues of all three embryonic germ layers, mesoderm, endoderm, and ectoderm. Bars = 100 µm. Fibs, fibroblasts; HSVtk, herpes simplex virus thymidine kinase; iPSCs, induced pluripotent stem cells.
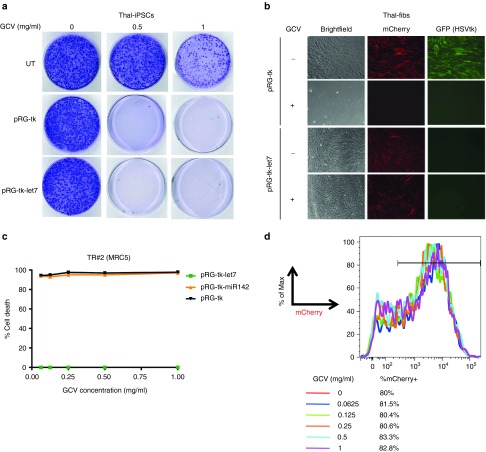
Once clonal iPSC cultures homogeneously expressing GFP-HSVtk were established, GCV sensitivity was assessed. iPSCs expressing either HSVtk or HSVtk-let7 were completely eliminated over 14 days of GCV treatment (Figure 3a). No live cells could be detected after treatment of 1–2 million iPSCs with GCV at 0.5 mg/ml or 1 mg/ml. In contrast, fibroblasts expressing let7-regulated, but not constitutive, HSVtk were completely protected over a range of GCV concentrations up to 1 mg/ml (Figure 3b). No cell death was observed by assaying cell counts even at the highest GCV dose (Figure 3c,d). In contrast, fibroblasts expressing constitutive or miR-142-regulated HSVtk were sensitive even at very low GCV concentrations (Figure 3c). Assessed further by a more sensitive flow cytometry-based assay, fibroblasts expressing HSVtk-let7 (Figure 3d, mCherry+) remained resistant to GCV, similarly to untransduced cells (mCherry−), as the % of mCherry+ cells remained stable over a range of GCV doses and similar to that of untreated cells (Figure 3d). These results collectively demonstrate that let7-regulated HSVtk is robustly expressed in undifferentiated iPSCs, rendering them sensitive to killing by GCV, while it is posttranscriptionally suppressed in differentiated cells, preserving their resistance to GCV.
Figure 3.
Selective killing of pluripotent stem cells but not of their differentiated progeny. (a) Crystal violet staining of thal-iPSCs harboring the pRG-tk or pRG-tk-let7 vectors or UT after treatment with GCV at the indicated concentration for 14 days. (b) Fibroblasts transduced with pRG-tk or pRG-tk-let7 with or without treatment with 1 mg/ml GCV for 14 days. (c) Percent cell death estimated from the % viable cells by manual cell counts and Trypan blue staining of fibroblasts transduced with the indicated vectors and treated with GCV at the indicated concentrations, compared to untreated cultures that were plated simultaneously and grown in parallel. (d) Percent Cherry+ cells by flow cytometry in fibroblast cultures transduced with pRG-tk-let7 and treated with a range of GCV concentrations, as indicated, for 14 days. GCV, ganciclovir; HSVtk, herpes simplex virus thymidine kinase; iPSCs, induced pluripotent stem cells; UT, un transduced.
HSVtk delays but does not abolish the emergence of teratomas
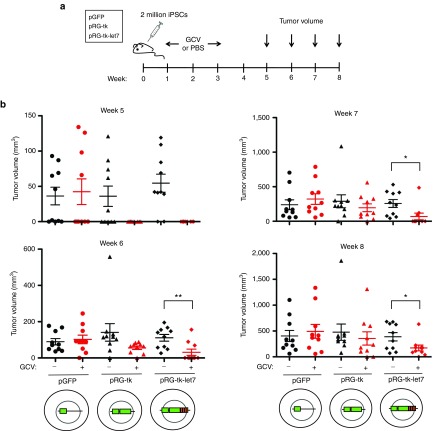
To next test if HSVtk with or without let7 regulation can prevent teratoma formation, we injected the iPSC clones transduced with pRG-tk, pRG-tk-let7, or a control GFP vector subcutaneously into NSG mice. One week later, administration of GCV or phosphate-buffered saline through a minipump was initiated and maintained for 2 weeks. Tumor size was monitored over 8 weeks (Figure 4a). By week 5 after cell injection, over half of the mice that received iPSCs not expressing HSVtk or were not treated with GCV developed palpable teratomas (Figure 4b). In contrast, none of the mice that received iPSCs harboring HSVtk or HSVtk-let7 and GCV developed teratomas. However, by week 6 after injection, tumors appeared in several GCV-treated mice from both groups injected with iPSCs harboring pRG-tk or pRG-tk-let7. These had initially overall smaller size than the tumors in untreated mice but continued growing over the next 2 weeks until they reached a size comparable to the tumors in the untreated mice and in the mice that received iPSCs expressing GFP only (Figure 4b). By week 8, teratomas had developed in 8 out of 10 injection sites of iPSCs expressing HSVtk and in 9 out of 10 injection sites of iPSCs expressing HSVtk-let7 with GCV treatment. All (10 out of 10) sites of injection of iPSCs not expressing HSVtk or not treated with GCV showed tumor growth.
Figure 4.
In vivo protection from teratoma formation. (a) Scheme of in vivo teratoma formation experiments. (b) Tumor volume in weeks 5–8 in mice which received iPSCs transduced with vectors expressing HSVtk-GFP with or without let7 regulation or GFP alone, as indicated, with or without GCV treatment. Five mice were included in each group and teratomas were injected in two sites per mouse (total 10 injection sites for each test and control group). GCV, ganciclovir; HSVtk, herpes simplex virus thymidine kinase; iPSCs, induced pluripotent stem cells; PBS, phosphate-buffered saline. Statistical significance was assessed using an unpaired unequal variance t-test.*P < 0.05. **P < 0.01.
These results show that HSVtk (with or without let7 regulation) does not ultimately prevent teratoma formation. Teratomas derived from HSVtk-expressing iPSCs developed with a longer latency than teratomas derived from iPSCs not expressing HSVtk in the presence of GCV. These kinetics are consistent with initial sensitivity to GCV and emergence of resistance over the course of treatment.
GCV resistance is mediated by HSVtk mutation
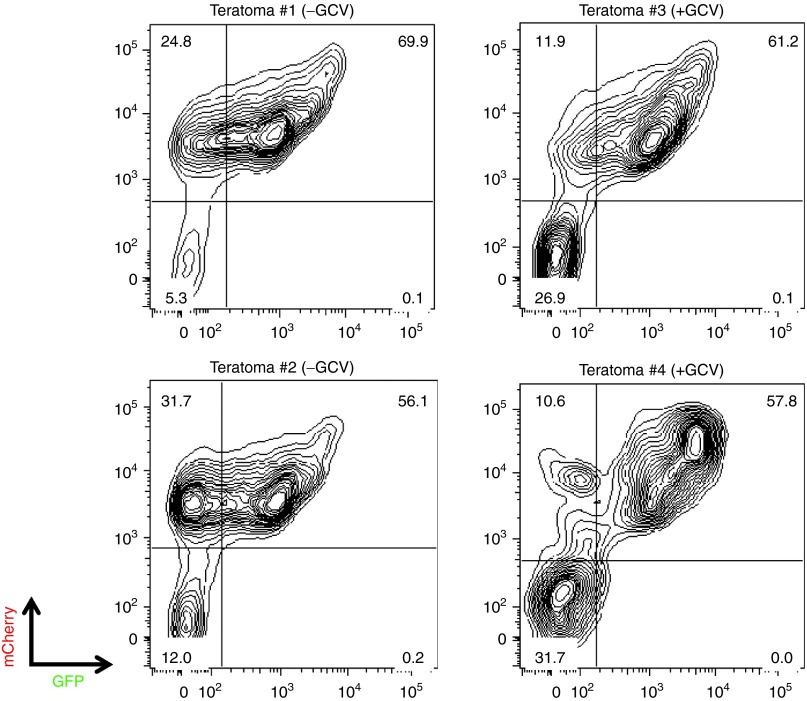
Resistance to GCV-mediated killing in cells expressing HSVtk has previously been reported to occur through various genetic and epigenetic mechanisms.30,31,32 To first test if GCV resistance was due to HSVtk silencing, teratomas grown in mice injected with iPSCs transduced with pRG-tk with or without GCV treatment were collected and their cellular components were analyzed by flow cytometry for GFP-HSVtk expression. All four teratomas tested showed robust expression of GFP-HSVtk in the majority of the cells (Figure 5). This result precludes transgene silencing as the cause of GCV resistance.
Figure 5.
GCV resistance of HSVtk-expressing iPSCs is not due to silencing. Analysis of GFP-HSVtk and mCherry expression in cells from four teratomas grown in mice injected with iPSCs clonally transduced with the pRG-tk vector in the absence (left panels) or presence (right panels) of in vivo GCV administration. GCV, ganciclovir; HSVtk, herpes simplex virus thymidine kinase; iPSCs, induced pluripotent stem cells.
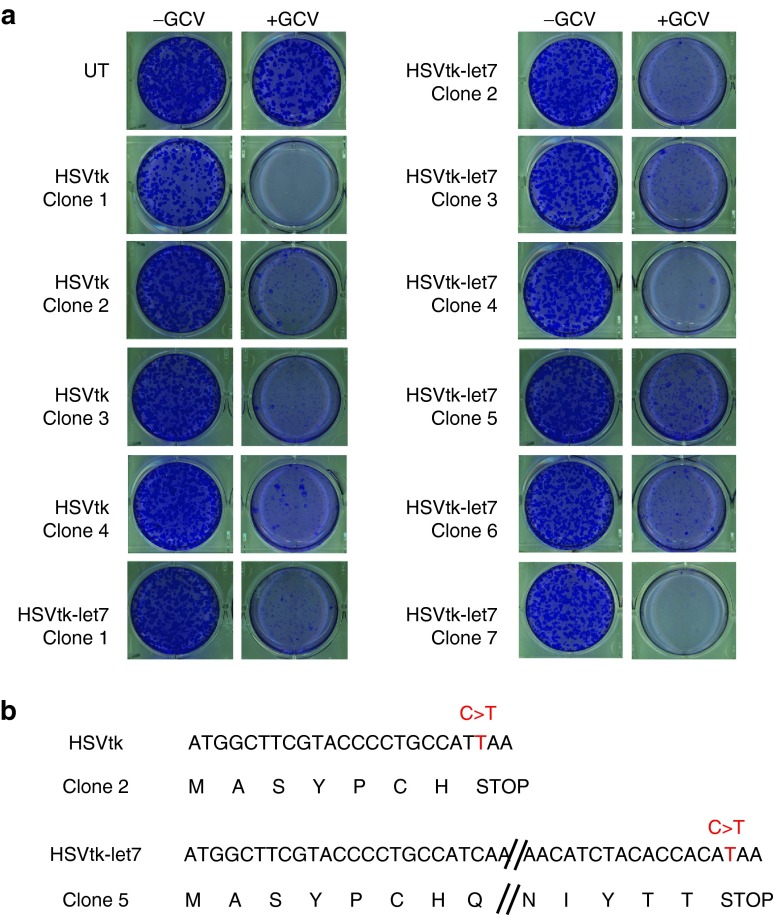
To further investigate the mechanism of GCV resistance, we derived single-cell iPSC clones expressing either pRG-tk or pRG-tk-let7. Eleven clones were expanded for ~3 weeks and their sensitivity to GCV was tested. The clones showed variable sensitivity to GCV with few but detectable resistant colonies remaining in 6 of the 11 clones after exposure to GCV (1 mg/ml) for 14 days (Figure 6a). Sequencing of the HSVtk in two of the GCV-resistant clones revealed nonsense mutations in the HSVtk coding region (Figure 6b). These results show that HSVtk mutation is a frequent mechanism of resistance of human iPSCs to GCV.
Figure 6.
GCV resistance is mediated by nonsense mutations in the HSVtk coding region. (a) Crystal violet staining of iPSC clones derived from single cells transduced with pRG-tk or pRG-tk-let7 and expanded after treatment with 1 mg/ml GCV for 14 days. (b) Nonsense mutations in the HSVtk coding sequence in two of the clones containing GCV-resistant cells shown in (a). The nucleotide sequence is shown above with the translation shown below. In both clones, a C > T substitution introduces a premature stop codon (TAA) in the HSVtk reading frame. GCV, ganciclovir; HSVtk, herpes simplex virus thymidine kinase; iPSCs, induced pluripotent stem cells; UT, untransduced.
Discussion
Given the very high stakes for the stem cell field, translation of stem cell therapies needs to proceed with extreme caution to avoid the occurrence of serious adverse events in early-stage trials. Suicide genes, among other strategies, will be valuable tools to increase safety in stem cell therapies. There is thus an urgent need to evaluate the efficacy of such an approach.
Here, we showed that miRNA regulation by let7 can be used to engineer a robust genetic “switch” in a suicide gene, so that the latter is “on” in undifferentiated hPSCs but gets turned “off” once the cells are differentiated to the desired cell type. To the best of our knowledge, this constitutes the most effective pluripotent cell-specific regulation of a vector-encoded transgene reported to date and can find many applications given the general scarcity of tools for engineering pluripotent-specific gene expression. Alternative approaches involve either the much more cumbersome targeting of a suicide gene in an endogenous pluripotent cell-specific locus, such as OCT4 or NANOG.19,20 This approach requires multiple engineering steps and it may interfere with expression of the endogenous gene and the pluripotent state and the properties of the target cell. On the other hand, incorporation of promoter/enhancer elements from genes such as NANOG and OCT4 in vectors has not so far resulted in robust transgene expression and/or regulation.18,22 Synthetic composite promoters/enhancers, like EOS-C(3+)22, may be more promising. In our study, expression of HSVtk by the EOS-C(3+) promoter was relatively low (Figure 1c) but might have been sufficient for effective suicide gene therapy. Since our miRNA-based strategy is robust and very simple to implement, as it only requires the incorporation of short sequences in the vector, it can have broader application in transgene regulation for basic stem cell research, in addition to suicide gene therapy. While expressing exogenous MREs can potentially exert some inhibitory effects on endogenous miRNAs, this would require very high levels of MRE expression that are unlikely to be reached with our strategy.24
Previous studies proposed the prevention of teratomas by an HSVtk suicide gene.16,17,18,19
Differences in various experimental parameters, such as the onset and the duration of GCV treatment following cell injection, the vector, the dose of GCV, and the HSVtk variant, can be noted between studies. A major difference that can account for the difference in the observed outcome is that, in contrast to the previous studies, we used a larger cohort of mice and a longer observation period. Indeed if we had evaluated our mice at week 5 with no further follow-up, our conclusion would also have been that HSVtk completely abolishes teratoma formation (Figure 4b). This illustrates that short-term studies can miss heterochronic teratoma formation between test and control groups as a result of resistance acquired later in the course of an experiment and highlights the need for long observation periods in future teratoma formation studies.
Our findings document acquisition of resistance to GCV by iPSCs expressing HSVtk. The injected cells were clonal and confirmed to be sensitive to GCV before injection. Furthermore, the kinetics of teratoma growth were consistent with initial sensitivity and subsequent emergence of resistance to GCV. GCV resistance has been observed in various murine and human cell lines, including human iPSCs30,31,32 and relapse after GCV suicide treatment has been reported even in clonal tumors.30 Epigenetic silencing as well as a variety of genetic events including point mutations, small frameshifting insertions/deletions, deletions of the entire gene, loss of part or of an entire chromosome, and postinsertional recombination of retrovirally encoded HSVtk have all been documented as mechanisms mediating GCV resistance.30,31,32 Of all the different mechanisms, we were able to document inactivation of the HSVtk gene by point mutations. Since our cells were transduced at high multiplicity of infection and likely harbored more than one HSVtk copies and we did not test for large-scale rearrangements or chromosomal deletions, we cannot exclude the possibility that additional mechanisms of resistance—genetic and epigenetic—might also have contributed to escape and teratoma formation in our study.
Our clonal analyses suggest that escape mutants arise frequently (Figure 6a). In a translational setting, many factors can influence the frequency of mutational HSVtk inactivation, such as the vector system used and the degree of expansion of the iPSCs before and after differentiation and before and after administration to the patient. Both mutations in our clones were G-to-A substitutions (Figure 6b). Wild-type HIV-1 shows a high rate of G-to-A substitutions, due to the error-prone retroviral reverse transcriptase and/or RNA editing by mammalian cytidine deaminases of the APOBEC family.33 Therefore, the genetic stability and mutation rate of the viral vector may need to be considered in suicide gene transfer into hPSCs. Since malignant transformation often results in genetic and epigenetic instability, the emergence of GCV-resistant clones in the event of malignancy arising from iPSC derivatives may be even more likely. The specific type of tumor and the driver genetic alterations that underlie transformation and tumor development can also influence the genomic stability and frequency of GCV resistance.
Acquired GCV resistance and teratoma growth through escape mutational events is akin to drug resistance in malignant tumors. The cancer field has extensively documented and studied the emergence of tumor resistance to cytotoxic agents through genetic events that are subject to selection and clonal evolution in a Darwinian process.
Due to their vast proliferation capacity, processes of clonal evolution are also at play in hPSCs and have fundamental implications for stem cell therapies. A mutation that confers resistance of cancer cells to chemotherapy is usually a passenger mutation before treatment, arising by chance as a result of background mutation rate. Similarly, in our study, HSVtk mutations were found to preexist in rare cells of the original population and were selected for upon treatment with GCV. Thus, in a translational setting, HSVtk mutations can be acquired by rare hPSCs at any stage of cell expansion prior to as well as following administration of the final cell product to the patient. The cells that harbor resistance mutations can lurk in the cell graft and remain inconsequential until treatment with the prodrug is initiated. Once treatment is applied, these cells immediately have a selective advantage and can readily give rise to tumor growth. In view of this, more emphasis should be placed on methods for detection by deep sequencing and purging of cells harboring potential resistance mutations to suicide gene therapy at an early stage, as well as after subsequent expansion and prior to infusion.
Suicide genes other than HSVtk have been developed and tested in human and nonhuman primate PSCs.21,34,35 These include the yeast cytosine deaminase, an enzyme that converts the nontoxic prodrug 5-fluorocytosine into a potent antimetabolite, 5-fluorouracil, and the inducible Caspase-9, a proapoptotic molecule that can be activated by a dimerizer drug (specific chemical inducer of dimerization). Whereas experience with these suicide genes is much more limited, resistance has been observed in studies utilizing inducible Caspase-9 in rhesus macaque hematopoietic stem and progenitor cells and human iPSCs, with teratoma regrowth after chemical inducer of dimerization treatment also observed in the latter case.35,36,37 Although the mechanisms of resistance to chemical inducer of dimerization treatment have not yet been thoroughly investigated, they are likely to involve the same array of genetic and epigenetic mechanisms that inactivate HSVtk and hPSCs harboring inducible Caspase-9 or other suicide genes will most likely also be subject to the same clonal dynamics. In view of this, safe stem cell therapies may require combinations of suicide genes or, more likely, the combinatorial use of more than one redundant approaches for purging and/or suicide gene therapy of a cell graft. Alternative strategies that can be combined with suicide genes are methods for purging of hPSCs in vitro prior to transplantation. These include antibodies against hPSC-specific surface antigens—either cytotoxic38 or to be used for fluorescent-activated cell sorting- or magnetic-activated cell sorting-based depletion.39 Another possibility is the development of small molecule inhibitors driven by the identification of hPSC-specific vulnerabilities arising from dependence on particular signaling or metabolic pathways.40,41
In conclusion, suicide genes can enhance the safety of stem cell therapies. For this promise to materialize, a better understanding of both the potential and the limitations of suicide gene therapy and the possibilities of combining it with other approaches will need to be the focus of future preclinical studies.
Materials and methods
Cell culture. The thal-iPSC line thal-5-10-Cre8 and the bone marrow fibroblasts (thal-fibs) that was derived from have previously been described.23 Human iPSCs were cultured on a feeder layer of mitomycin C-treated mouse embryonic fibroblasts (GlobalStem, Gaithersburg, MD) or in feeder-free conditions and passaged with dispase or accutase as previously described.29,42,43 Fibroblasts were cultured in Complete MesenCult Medium (Stem Cell Technologies, Vancouver, Canada).
Lentiviral vector construction and production. pRG-tk was derived from vector NG023 by replacing NTP with mCherry and GFP with a GFP-P2A-HSVtk cassette in which the 2A peptide is preceded by a Gly-Ser-Gly linker generated by overlapping PCR. The wild-type full-length HSVtk was used. MREs were inserted in AvrII/XmaI sites immediately downstream of HSVtk. The let7 MRE consists of four sequences complementary to each of the four mature miRNAs let7g, let7a, let7d, and let7f (in this order) with sequence 5′ to 3′ (relative to transcription of HSVtk, which is encoded in reverse orientation in the pRG-tk lentiviral vector):
AACTGTACAAACTACTACCTCAtaAACTATACAACCTACTACCTCAtaAACTATGCAACCTACTACCTCTtaAACTATACAATCTACTACCTCA
The miR-142 MRE consists of four sequences complementary to miR-142-3p and miR-142-p5 with sequence 5′ to 3′ (relative to transcription of HSVtk, which is encoded in reverse orientation in the pRG-tk lentiviral vector):
AGTAGTGCTTTCTACTTTATGtaTCCATAAAGTAGGAAACACTACAtaAGTAGTGCTTTCTACTTTATGtaTCCATAAAGTAGGAAACACTACA
The EOS-C(3+) promoter enhancer was obtained from plasmid PL-SIN-EOS-C(3+)-EiP plasmid.22 Lentiviral vector packaging and transduction of iPSCs and fibroblasts were performed as described previously.43 The pRG-tk, pRG-tk-let7, and pRG-tk-miR142 vectors yielded comparable titers.
Flow cytometry. Undifferentiated iPSCs were dissociated with accutase and stained with Alexa Fluor 647-conjugated anti-Tra-1–81 or anti-Tra-1–60 or anti-SSEA3 or anti-SSEA4 (BD Biosciences, Franklin Lakes, NJ). Data were acquired in a LSRII cytometer (BD Biosciences) and analyzed with the FlowJo software (Tree Star, Ashland, OR). iPSCs transduced with control vectors expressing mCherry only and GFP only were used for compensation setup.
Teratoma formation assays. For teratoma formation assays, iPSCs were suspended in hES medium containing 10 µmol/l of the Rho-associated kinase (Rock) inhibitor Y-27632 (Tocris Bristol, UK). Approximately 2 million cells were injected subcutaneously in the flank of female 8-week-old NOD-SCID IL2Rg-null (NSG) mice (Jackson Laboratory, Bar Harbor, ME) together with Matrigel (BD Bioscience). One week after cell implantation, the mice were randomized to receive either phosphate-buffered saline or GCV (at 5 mg/kg per day) administered via an osmotic minipump (Alzet #1007D) implanted subcutaneously. Minipumps were replaced every 7 days, for a total of 2 weeks of treatment. Mice were observed daily throughout the treatment period for signs of morbidity/mortality. Tumors were measured twice weekly using calipers, and volume was calculated using the formula: length × width2 × 0.52. Body weight was also assessed twice weekly. Some tumors were surgically dissected and analyzed by flow cytometry or histology. For the latter, teratomas were fixed in 4% formaldehyde, cryosectioned, and stained with hematoxylin and eosin. All animal experiments were conducted in accordance with guidelines and protocols approved by the University of Washington Institutional Animal Care and Use Committee (IACUC) and the Memorial Sloan-Kettering Cancer Center IACUC and Research Animal Resource Center and following National Institutes of Health guidelines for animal welfare.
SUPPLEMENTARY MATERIAL Figure S1. Let7-regulated HSVtk is expressed in undifferentiated but not differentiated cells. Figure S2. Let7 regulation does not affect HSVtk expression levels in undifferentiated pluripotent cells.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grant R00 DK087923 (E.P.P.).
Supplementary Material
Let7-regulated HSVtk is expressed in undifferentiated but not differentiated cells.
Let7 regulation does not affect HSVtk expression levels in undifferentiated pluripotent cells.
References
- Lee, AS, Tang, C, Rao, MS, Weissman, IL and Wu, JC (2013). Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med 19: 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, AS, Tang, C, Cao, F, Xie, X, van der Bogt, K, Hwang, A et al. (2009). Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle 8: 2608–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, NS, Cleren, C, Singh, SK, Yang, L, Beal, MF and Goldman, SA (2006). Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med 12: 1259–1268. [DOI] [PubMed] [Google Scholar]
- Kriks, S, Shim, JW, Piao, J, Ganat, YM, Wakeman, DR, Xie, Z et al. (2011). Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature 480: 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, L, Guan, Y, Qu, Z, Zhang, J, Liao, B, Ma, B et al. (2013). WNT signaling determines tumorigenicity and function of ESC-derived retinal progenitors. J Clin Invest 123: 1647–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi, D, Morizane, A, Kikuchi, T, Onoe, H, Hayashi, T, Kawasaki, T et al. (2012). Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson's disease. Stem Cells 30: 935–945. [DOI] [PubMed] [Google Scholar]
- Nori, S, Okada, Y, Nishimura, S, Sasaki, T, Itakura, G, Kobayashi, Y et al. (2015). Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Reports 4: 360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David, U and Benvenisty, N (2011). The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer 11: 268–277. [DOI] [PubMed] [Google Scholar]
- Fu, W, Wang, SJ, Zhou, GD, Liu, W, Cao, Y and Zhang, WJ (2012). Residual undifferentiated cells during differentiation of induced pluripotent stem cells in vitro and in vivo. Stem Cells Dev 21: 521–529. [DOI] [PubMed] [Google Scholar]
- Kiuru, M, Boyer, JL, O'Connor, TP and Crystal, RG (2009). Genetic control of wayward pluripotent stem cells and their progeny after transplantation. Cell Stem Cell 4: 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminatore, C, Polentes, J, Ellman, D, Kozubenko, N, Itier, V, Tine, S et al. (2010). The postischemic environment differentially impacts teratoma or tumor formation after transplantation of human embryonic stem cell-derived neural progenitors. Stroke 41: 153–159. [DOI] [PubMed] [Google Scholar]
- Wakitani, S, Takaoka, K, Hattori, T, Miyazawa, N, Iwanaga, T, Takeda, S et al. (2003). Embryonic stem cells injected into the mouse knee joint form teratomas and subsequently destroy the joint. Rheumatology (Oxford) 42: 162–165. [DOI] [PubMed] [Google Scholar]
- West, EL, Gonzalez-Cordero, A, Hippert, C, Osakada, F, Martinez-Barbera, JP, Pearson, RA et al. (2012). Defining the integration capacity of embryonic stem cell-derived photoreceptor precursors. Stem Cells 30: 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl, JI, Kean, LS, Davis, MM and Wu, JC (2012). Pluripotent stem cells: immune to the immune system? Sci Transl Med 4: 164ps25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini, C, Bondanza, A, Perna, SK, Kaneko, S, Traversari, C, Ciceri, F et al. (2007). The suicide gene therapy challenge: how to improve a successful gene therapy approach. Mol Ther 15: 1248–1252. [DOI] [PubMed] [Google Scholar]
- Jung, J, Hackett, NR, Pergolizzi, RG, Pierre-Destine, L, Krause, A and Crystal, RG (2007). Ablation of tumor-derived stem cells transplanted to the central nervous system by genetic modification of embryonic stem cells with a suicide gene. Hum Gene Ther 18: 1182–1192. [DOI] [PubMed] [Google Scholar]
- Schuldiner, M, Itskovitz-Eldor, J and Benvenisty, N (2003). Selective ablation of human embryonic stem cells expressing a “suicide” gene. Stem Cells 21: 257–265. [DOI] [PubMed] [Google Scholar]
- Cheng, F, Ke, Q, Chen, F, Cai, B, Gao, Y, Ye, C et al. (2012). Protecting against wayward human induced pluripotent stem cells with a suicide gene. Biomaterials 33: 3195–3204. [DOI] [PubMed] [Google Scholar]
- Rong, Z, Fu, X, Wang, M and Xu, Y (2012). A scalable approach to prevent teratoma formation of human embryonic stem cells. J Biol Chem 287: 32338–32345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara, A, Aoki, H, Taguchi, A, Niwa, M, Yamada, Y, Kunisada, T et al. (2008). Neuron-like differentiation and selective ablation of undifferentiated embryonic stem cells containing suicide gene with Oct-4 promoter. Stem Cells Dev 17: 619–627. [DOI] [PubMed] [Google Scholar]
- Wu, C, Hong, SG, Winkler, T, Spencer, DM, Jares, A, Ichwan, B et al. (2014). Development of an inducible caspase-9 safety switch for pluripotent stem cell-based therapies. Mol Ther Methods Clin Dev 1: 14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta, A, Cheung, AY, Farra, N, Vijayaragavan, K, Séguin, CA, Draper, JS et al. (2009). Isolation of human iPS cells using EOS lentiviral vectors to select for pluripotency. Nat Methods 6: 370–376. [DOI] [PubMed] [Google Scholar]
- Papapetrou, EP, Kovalovsky, D, Beloeil, L, Sant'angelo, D and Sadelain, M (2009). Harnessing endogenous miR-181a to segregate transgenic antigen receptor expression in developing versus post-thymic T cells in murine hematopoietic chimeras. J Clin Invest 119: 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, BD and Naldini, L (2009). Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nat Rev Genet 10: 578–585. [DOI] [PubMed] [Google Scholar]
- Brown, BD, Venneri, MA, Zingale, A, Sergi Sergi, L and Naldini, L (2006). Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med 12: 585–591. [DOI] [PubMed] [Google Scholar]
- Tang, F, Hajkova, P, Barton, SC, Lao, K and Surani, MA (2006). MicroRNA expression profiling of single whole embryonic stem cells. Nucleic Acids Res 34: e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbaviy, HB, Murray, MF and Sharp, PA (2003). Embryonic stem cell-specific MicroRNAs. Dev Cell 5: 351–358. [DOI] [PubMed] [Google Scholar]
- Suh, MR, Lee, Y, Kim, JY, Kim, SK, Moon, SH, Lee, JY et al. (2004). Human embryonic stem cells express a unique set of microRNAs. Dev Biol 270: 488–498. [DOI] [PubMed] [Google Scholar]
- Papapetrou, EP, Lee, G, Malani, N, Setty, M, Riviere, I, Tirunagari, LM et al. (2011). Genomic safe harbors permit high β-globin transgene expression in thalassemia induced pluripotent stem cells. Nat Biotechnol 29: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, O, Rudolph, C, Heberlein, C, von Neuhoff, N, Schröck, E, Schambach, A et al. (2004). Tumor cells escape suicide gene therapy by genetic and epigenetic instability. Blood 104: 3543–3549. [DOI] [PubMed] [Google Scholar]
- Li, LB, Chang, KH, Wang, PR, Hirata, RK, Papayannopoulou, T and Russell, DW (2012). Trisomy correction in Down syndrome induced pluripotent stem cells. Cell Stem Cell 11: 615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal, M, Skelton, D, Pepper, KA, Jahn, T, Methangkool, E and Kohn, DB (2007). Effective suicide gene therapy for leukemia in a model of insertional oncogenesis in mice. Mol Ther 15: 183–192. [DOI] [PubMed] [Google Scholar]
- Harris, RS, Bishop, KN, Sheehy, AM, Craig, HM, Petersen-Mahrt, SK, Watt, IN et al. (2003). DNA deamination mediates innate immunity to retroviral infection. Cell 113: 803–809. [DOI] [PubMed] [Google Scholar]
- Zhong, B, Watts, KL, Gori, JL, Wohlfahrt, ME, Enssle, J, Adair, JE et al. (2011). Safeguarding nonhuman primate iPS cells with suicide genes. Mol Ther 19: 1667–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagyu, S, Hoyos, V, Del Bufalo, F and Brenner, MK (2015). An inducible caspase-9 suicide gene to improve the safety of therapy using human induced pluripotent stem cells. Mol Ther 23: 1475–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando, M, Hoyos, V, Yagyu, S, Tao, W, Ramos, CA, Dotti, G et al. (2014). Bortezomib sensitizes non-small cell lung cancer to mesenchymal stromal cell-delivered inducible caspase-9-mediated cytotoxicity. Cancer Gene Ther 21: 472–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barese, CN, Felizardo, TC, Sellers, SE, Keyvanfar, K, Di Stasi, A, Metzger, ME et al. (2015). Regulated apoptosis of genetically modified hematopoietic stem and progenitor cells via an inducible caspase-9 suicide gene in rhesus macaques. Stem Cells 33: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo, AB, Tan, HL, Ang, SN, Fong, WJ, Chin, A, Lo, J et al. (2008). Selection against undifferentiated human embryonic stem cells by a cytotoxic antibody recognizing podocalyxin-like protein-1. Stem Cells 26: 1454–1463. [DOI] [PubMed] [Google Scholar]
- Tang, C, Lee, AS, Volkmer, JP, Sahoo, D, Nag, D, Mosley, AR et al. (2011). An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol 29: 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David, U, Gan, QF, Golan-Lev, T, Arora, P, Yanuka, O, Oren, YS et al. (2013). Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell 12: 167–179. [DOI] [PubMed] [Google Scholar]
- Oricchio, E, Papapetrou, EP, Lafaille, F, Ganat, YM, Kriks, S, Ortega-Molina, A et al. (2014). A cell engineering strategy to enhance the safety of stem cell therapies. Cell Rep 8: 1677–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetrou, EP, Tomishima, MJ, Chambers, SM, Mica, Y, Reed, E, Menon, J et al. (2009). Stoichiometric and temporal requirements of Oct4, Sox2, Klf4, and c-Myc expression for efficient human iPSC induction and differentiation. Proc Natl Acad Sci USA 106: 12759–12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetrou, EP and Sadelain, M (2011). Generation of transgene-free human induced pluripotent stem cells with an excisable single polycistronic vector. Nat Protoc 6: 1251–1273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Let7-regulated HSVtk is expressed in undifferentiated but not differentiated cells.
Let7 regulation does not affect HSVtk expression levels in undifferentiated pluripotent cells.