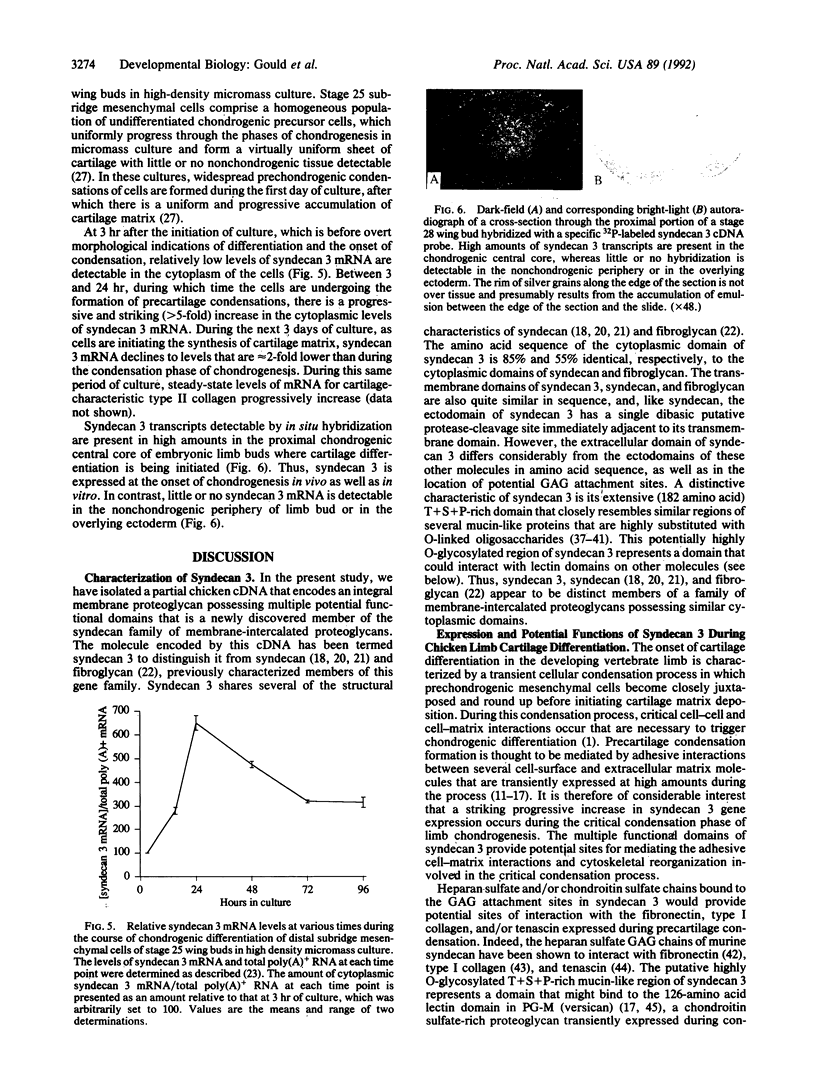
Abstract
A partial cDNA that encodes a newly discovered member of the syndecan family of integral membrane proteoglycans, which we have termed syndecan 3, has been isolated from an embryonic chicken limb bud cDNA library. Syndecan 3 is distinct from but structurally related to syndecan and fibroglycan, two previously characterized members of this family of membrane-intercalated proteoglycans. Syndecan 3 contains a cytoplasmic domain potentially associated with the cytoskeleton that is 85% identical in amino acid sequence to the cytoplasmic domain of syndecan. Syndecan 3 also possesses a hydrophobic transmembrane domain and an extracellular domain containing several clustered potential glycosaminoglycan attachment sites. Like syndecan, the ectodomain of syndecan 3 has a single dibasic protease-susceptible site adjacent to the transmembrane domain, which might be involved in shedding the ectodomain from the cell surface. A striking feature of syndecan 3 is an extensive (182 amino acid) threonine, serine, and proline (T+S+P)-rich domain that closely resembles T+S+P-rich regions in several mucin-like proteins in which O-linked oligosaccharides are bound to the threonine and serine residues. Syndecan 3 is expressed in high amounts during a critical phase of chicken limb chondrogenesis in which limb mesenchymal cells condense, round up, and interact with one another before depositing a cartilage matrix. The multiple functional domains of syndecan 3 provide potential sites for mediating the adhesive cell-matrix interactions and cytoskeletal reorganization involved in this critical condensation process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):2241–2245. doi: 10.1093/nar/19.suppl.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M., Sanderson R. D. Syndecan, a developmentally regulated cell surface proteoglycan that binds extracellular matrix and growth factors. Philos Trans R Soc Lond B Biol Sci. 1990 Mar 12;327(1239):171–186. doi: 10.1098/rstb.1990.0052. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coelho C. N., Kosher R. A. Gap junctional communication during limb cartilage differentiation. Dev Biol. 1991 Mar;144(1):47–53. doi: 10.1016/0012-1606(91)90477-k. [DOI] [PubMed] [Google Scholar]
- Coelho C. N., Sumoy L., Rodgers B. J., Davidson D. R., Hill R. E., Upholt W. B., Kosher R. A. Expression of the chicken homeobox-containing gene GHox-8 during embryonic chick limb development. Mech Dev. 1991 Jun;34(2-3):143–154. doi: 10.1016/0925-4773(91)90051-7. [DOI] [PubMed] [Google Scholar]
- David G., Lories V., Decock B., Marynen P., Cassiman J. J., Van den Berghe H. Molecular cloning of a phosphatidylinositol-anchored membrane heparan sulfate proteoglycan from human lung fibroblasts. J Cell Biol. 1990 Dec;111(6 Pt 2):3165–3176. doi: 10.1083/jcb.111.6.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessau W., von der Mark H., von der Mark K., Fischer S. Changes in the patterns of collagens and fibronectin during limb-bud chondrogenesis. J Embryol Exp Morphol. 1980 Jun;57:51–60. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt A. E., Timpte C. S., Abernethy J. L., Zhao Y., Hill R. L. Porcine submaxillary mucin contains a cystine-rich, carboxyl-terminal domain in addition to a highly repetitive, glycosylated domain. J Biol Chem. 1991 May 25;266(15):9678–9686. [PubMed] [Google Scholar]
- Frenz D. A., Jaikaria N. S., Newman S. A. The mechanism of precartilage mesenchymal condensation: a major role for interaction of the cell surface with the amino-terminal heparin-binding domain of fibronectin. Dev Biol. 1989 Nov;136(1):97–103. doi: 10.1016/0012-1606(89)90133-4. [DOI] [PubMed] [Google Scholar]
- Gay S. W., Kosher R. A. Uniform cartilage differentiation in micromass cultures prepared from a relatively homogeneous population of chondrogenic progenitor cells of the chick limb bud: effect of prostaglandins. J Exp Zool. 1984 Nov;232(2):317–326. doi: 10.1002/jez.1402320219. [DOI] [PubMed] [Google Scholar]
- Gendler S. J., Lancaster C. A., Taylor-Papadimitriou J., Duhig T., Peat N., Burchell J., Pemberton L., Lalani E. N., Wilson D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990 Sep 5;265(25):15286–15293. [PubMed] [Google Scholar]
- Gum J. R., Byrd J. C., Hicks J. W., Toribara N. W., Lamport D. T., Kim Y. S. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989 Apr 15;264(11):6480–6487. [PubMed] [Google Scholar]
- Harley C. B. Hybridization of oligo(dT) to RNA on nitrocellulose. Gene Anal Tech. 1987 Mar-Apr;4(2):17–22. doi: 10.1016/0735-0651(87)90013-6. [DOI] [PubMed] [Google Scholar]
- Jalkanen M., Rapraeger A., Saunders S., Bernfield M. Cell surface proteoglycan of mouse mammary epithelial cells is shed by cleavage of its matrix-binding ectodomain from its membrane-associated domain. J Cell Biol. 1987 Dec;105(6 Pt 2):3087–3096. doi: 10.1083/jcb.105.6.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M. C., Stephans J. C., Crawford K., Okino K., Barr P. J. Ligand-affinity cloning and structure of a cell surface heparan sulfate proteoglycan that binds basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6985–6989. doi: 10.1073/pnas.87.18.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata K., Oike Y., Tani K., Shinomura T., Yamagata M., Uritani M., Suzuki S. A large chondroitin sulfate proteoglycan (PG-M) synthesized before chondrogenesis in the limb bud of chick embryo. J Biol Chem. 1986 Oct 15;261(29):13517–13525. [PubMed] [Google Scholar]
- Koda J. E., Rapraeger A., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. Cell surface proteoglycan as a receptor for interstitial collagens. J Biol Chem. 1985 Jul 5;260(13):8157–8162. [PubMed] [Google Scholar]
- Kosher R. A., Gay S. W., Kamanitz J. R., Kulyk W. M., Rodgers B. J., Sai S., Tanaka T., Tanzer M. L. Cartilage proteoglycan core protein gene expression during limb cartilage differentiation. Dev Biol. 1986 Nov;118(1):112–117. doi: 10.1016/0012-1606(86)90078-3. [DOI] [PubMed] [Google Scholar]
- Kosher R. A., Kulyk W. M., Gay S. W. Collagen gene expression during limb cartilage differentiation. J Cell Biol. 1986 Apr;102(4):1151–1156. doi: 10.1083/jcb.102.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger R. C., Jr, Fields T. A., Hildreth J., 4th, Schwartz N. B. Chick cartilage chondroitin sulfate proteoglycan core protein. I. Generation and characterization of peptides and specificity for glycosaminoglycan attachment. J Biol Chem. 1990 Jul 15;265(20):12075–12087. [PubMed] [Google Scholar]
- Kulyk W. M., Coelho C. N., Kosher R. A. Type IX collagen gene expression during limb cartilage differentiation. Matrix. 1991 Aug;11(4):282–288. doi: 10.1016/s0934-8832(11)80236-2. [DOI] [PubMed] [Google Scholar]
- Kulyk W. M., Rodgers B. J., Greer K., Kosher R. A. Promotion of embryonic chick limb cartilage differentiation by transforming growth factor-beta. Dev Biol. 1989 Oct;135(2):424–430. doi: 10.1016/0012-1606(89)90191-7. [DOI] [PubMed] [Google Scholar]
- Kulyk W. M., Upholt W. B., Kosher R. A. Fibronectin gene expression during limb cartilage differentiation. Development. 1989 Jul;106(3):449–455. doi: 10.1242/dev.106.3.449. [DOI] [PubMed] [Google Scholar]
- Lan M. S., Batra S. K., Qi W. N., Metzgar R. S., Hollingsworth M. A. Cloning and sequencing of a human pancreatic tumor mucin cDNA. J Biol Chem. 1990 Sep 5;265(25):15294–15299. [PubMed] [Google Scholar]
- Leonard C. M., Fuld H. M., Frenz D. A., Downie S. A., Massagué J., Newman S. A. Role of transforming growth factor-beta in chondrogenic pattern formation in the embryonic limb: stimulation of mesenchymal condensation and fibronectin gene expression by exogenenous TGF-beta and evidence for endogenous TGF-beta-like activity. Dev Biol. 1991 May;145(1):99–109. doi: 10.1016/0012-1606(91)90216-p. [DOI] [PubMed] [Google Scholar]
- Mackie E. J., Thesleff I., Chiquet-Ehrismann R. Tenascin is associated with chondrogenic and osteogenic differentiation in vivo and promotes chondrogenesis in vitro. J Cell Biol. 1987 Dec;105(6 Pt 1):2569–2579. doi: 10.1083/jcb.105.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali M., Jaakkola P., Arvilommi A. M., Jalkanen M. Sequence of human syndecan indicates a novel gene family of integral membrane proteoglycans. J Biol Chem. 1990 Apr 25;265(12):6884–6889. [PubMed] [Google Scholar]
- Mallein-Gerin F., Kosher R. A., Upholt W. B., Tanzer M. L. Temporal and spatial analysis of cartilage proteoglycan core protein gene expression during limb development by in situ hybridization. Dev Biol. 1988 Apr;126(2):337–345. doi: 10.1016/0012-1606(88)90144-3. [DOI] [PubMed] [Google Scholar]
- Marynen P., Zhang J., Cassiman J. J., Van den Berghe H., David G. Partial primary structure of the 48- and 90-kilodalton core proteins of cell surface-associated heparan sulfate proteoglycans of lung fibroblasts. Prediction of an integral membrane domain and evidence for multiple distinct core proteins at the cell surface of human lung fibroblasts. J Biol Chem. 1989 Apr 25;264(12):7017–7024. [PubMed] [Google Scholar]
- Nichols K. V., Floros J., Dynia D. W., Veletza S. V., Wilson C. M., Gross I. Regulation of surfactant protein A mRNA by hormones and butyrate in cultured fetal rat lung. Am J Physiol. 1990 Dec;259(6 Pt 1):L488–L495. doi: 10.1152/ajplung.1990.259.6.L488. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K. Oligosaccharide structures of human colonic mucin. J Biol Chem. 1985 Jul 15;260(14):8262–8271. [PubMed] [Google Scholar]
- Rodgers B. J., Kulyk W. M., Kosher R. A. Stimulation of limb cartilage differentiation by cyclic AMP is dependent on cell density. Cell Differ Dev. 1989 Dec;28(3):179–187. doi: 10.1016/0922-3371(89)90003-8. [DOI] [PubMed] [Google Scholar]
- Salmivirta M., Elenius K., Vainio S., Hofer U., Chiquet-Ehrismann R., Thesleff I., Jalkanen M. Syndecan from embryonic tooth mesenchyme binds tenascin. J Biol Chem. 1991 Apr 25;266(12):7733–7739. [PubMed] [Google Scholar]
- Sandell L. J., Yamada Y., Dorfman A., Upholt W. B. Identification of genomic DNA coding for chicken type II procollagen. J Biol Chem. 1983 Oct 10;258(19):11617–11621. [PubMed] [Google Scholar]
- Saunders S., Bernfield M. Cell surface proteoglycan binds mouse mammary epithelial cells to fibronectin and behaves as a receptor for interstitial matrix. J Cell Biol. 1988 Feb;106(2):423–430. doi: 10.1083/jcb.106.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders S., Jalkanen M., O'Farrell S., Bernfield M. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989 Apr;108(4):1547–1556. doi: 10.1083/jcb.108.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shur B. D., Vogler M., Kosher R. A. Changes in endogenous cell surface galactosyltransferase activity during in vitro limb bud chondrogenesis. Exp Cell Res. 1982 Jan;137(1):229–237. doi: 10.1016/0014-4827(82)90023-4. [DOI] [PubMed] [Google Scholar]
- Solursh M., Reiter R. S., Jensen K. L., Kato M., Bernfield M. Transient expression of a cell surface heparan sulfate proteoglycan (syndecan) during limb development. Dev Biol. 1990 Jul;140(1):83–92. doi: 10.1016/0012-1606(90)90055-n. [DOI] [PubMed] [Google Scholar]
- Solursh M., Reiter R., Ahrens P. B., Pratt R. M. Increase in levels of cyclic AMP during avian limb chondrogenesis in vitro. Differentiation. 1979;15(3):183–186. doi: 10.1111/j.1432-0436.1979.tb01049.x. [DOI] [PubMed] [Google Scholar]
- Tomasek J. J., Mazurkiewicz J. E., Newman S. A. Nonuniform distribution of fibronectin during avian limb development. Dev Biol. 1982 Mar;90(1):118–126. doi: 10.1016/0012-1606(82)90217-2. [DOI] [PubMed] [Google Scholar]
- Zanetti N. C., Solursh M. Induction of chondrogenesis in limb mesenchymal cultures by disruption of the actin cytoskeleton. J Cell Biol. 1984 Jul;99(1 Pt 1):115–123. doi: 10.1083/jcb.99.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann D. R., Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989 Oct;8(10):2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]