Abstract
Dicentric chromosome analysis (DCA) is the gold standard for individual radiation dose estimation. Two limiting factors of DCA are the time-consuming lymphocyte stimulation and proliferation using the lectin PHA-M and the upper dose limit of individual dose assessment of ∼4 Gy. By measuring the mitotic index (MI), the authors investigated systematically whether the stimulation of lymphocytes can be improved after administration of alternative (and combined) mitogens. The authors compared the lymphocyte stimulation effectiveness of the traditionally used PHA-M (from Phaseolus vulgaris) with seven cited mitogens by determination of MIs: five lectins namely CNA (concanavalin A), PW (pokeweed), LMA (Maackia amurensis), LTV (T. vulgaris), PHA-L (P. vulgaris) as well as LPS (lipopolysaccharide, Escherichia coli) and SLO (streptolysine O, Streptococcus pyogenes) were applied. The conventional protocol using PHA-M for lymphocyte stimulation proved to be superior over lower/higher PHA-M concentrations as well as seven other mitogens administered either alone or combined with SLO or LPS.
INTRODUCTION
In case of large-scale accidental or deliberate radiation exposure, rapid biodosimetry triage tools are needed to help identify the severely exposed individuals who may require clinical monitoring or treatment and to identify and reassure the many ‘worried wells’ to prevent them overwhelming the emergency response and health care infrastructure(1, 2). Two cytogenetic methods, dicentric chromosome and cytokinesis-block micronucleus analysis (DCA/CBMN), have been established as the main biodosimetry techniques to assess low-LET ionising radiation exposure and to estimate absorbed doses(3). Both methods show high (DCA) or reasonable (CBMN) specificity, sensitivity of ∼100 mGy and persistence of the biomarker for several months. However, the upper dose limit of both methods is ∼4 Gy, because at higher radiation doses, the formation of the respective aberration in vitro does not follow any more a linear-quadratic function but reaches a plateau (saturation). That may be due to the checkpoint arrests during the cell cycle, ensuring that DNA damage and/or incomplete replication are detected and repaired(3). Because of the time expenditure and labour intensity of these methods, several strategies are pursued to increase the biodosimetry capacity such as progressive automation(3), validation of new fast scoring protocols(4–6) and network formation of cooperating biodosimetry laboratories(7–9).
The authors have already implemented several approaches to increase their lab's DCA capacity: (1) they automated single working steps such as fully automatic lymphocyte fixation using an automatic cell harvester (Hanabi PII, Transgenomic, UK) and automatic metaphase (MP) acquisition followed by semi-automatic dicentric (dic) scoring with the metafer4 platform (Metasystems, Germany)(10). (2) A basis for mutual assistance during a large-scale radiation incident was established by an inter-comparison with another German biodosimetry lab aimed at the validation of the DCA as a cytogenetic triage tool(11). (3) In the frame of two EU projects, ‘Multibiodose’(4) and ‘RENEB’(7), the authors verified their capabilities in the application of different dic scoring strategies (triage and web-based scoring) as well as in the assessment of different exposure scenarios, such as homogeneous, heterogeneous and protracted radiation exposures by DCA in triage mode(5, 8–10, 12).
However, in the frame of the NATO biodosimetry study organised by the authors’ institute, it was shown that biological dose assessment using new molecular methods (γ-H2AX-foci-, gene expression analysis) is much faster than the application of the well-established cytogenetic techniques(6). The molecular methods delivered biodosimetric estimates only 1 d after sample receipt in the lab whereas the cytogenetic methods provided results not until 2.4 (DCA) and 4 d (CBMN). This is due to the essential lymphocyte stimulation of at least 48 (DCA) or 70 h (CBMN) prior to cell fixation and quantification of the biomarker. The NATO study further confirmed that the upper limit of applicability of the four methods (DCA, CBMN, γ-H2AX and gene expression) ranges below 6.4 Gy (highest blind dose in the study), which was systematically underestimated by all techniques(6). Some attempts to overcome this limitation have already been made. Some authors have extended the culture time of the conventional DCA to allow more cells to reach metaphase(13–15). Recently, Pujol et al.(16) have proposed a new integrated model for low- and high-radiation doses combining the DCA with caffeine treatment of cells during an extended culture time and including a Gompertz-type function as the dose–response curve for dic formation(16). Another approach was the development of another cytogenetic assay, premature chromosome condensation, showing an upper limit of 20 Gy for dose estimation, which however is not yet widely used(3).
In this study, the authors examined systematically the application of different mitogens for lymphocyte stimulation. For biodosimetry, the usage of the mitogen phytohemagglutinin (PHA-M), which stimulates human T cells in vitro (mainly CD4+ and CD8+), is recommended(3). Several other mitogens are commercially available, but less is known about the subsets of lymphocytes which they stimulate and about their actual suitability for the assessment of chromosomal damage in human cells. The authors searched the literature to find out studies comparing those different mitogens for human lymphocyte stimulation specifically for biodosimetry but did not find any systematic study on this subject. Due to that lack of knowledge, they investigated whether lymphocyte stimulation can be improved by administration of other mitogens or a combination and examined the mitotic index (MI) as a surrogate for lymphocyte stimulation.
MATERIAL AND METHODS
Blood sampling and lymphocyte culture
Peripheral blood was taken with informed consent from healthy donors using heparinised vials. Replicate lymphocyte cultures were set up adding 0.5 ml of whole blood to 4.5 ml of RPMI-1640 supplemented with 200 mm l-glutamine (Gibco-BRL, Germany), antibiotics (100 IU ml−1 penicillin, 100 µg ml−1 streptomycin; Sigma–Aldrich, Germany) and 20 % foetal calf serum (FCS; PAA, Austria). Additionally, 10 µm bromodeoxyuridine (BrdU, Sigma–Aldrich) was added to permit the analysis of exclusively first-division MP. Finally, 100 µl PHA-M (10576-015, Gibco-BRL) and other mitogens (see below) were added in three different concentrations to stimulate lymphocytes. Culture flasks were wrapped in aluminium foil and incubated for 48 h or 72 h at 37°C in a 5 % CO2 atmosphere. Colcemid (0.15 µg ml−1; Gibco-BRL) was added 45 h (48 h cultures) or 48 h (72 h cultures) after culture set up to block lymphocytes at MP.
Dilution and administration of mitogens
In addition to the routinely used PHA-M, five lectins, concanavalin A (CNA), pokeweed (PW), LMA, LTV as well as PHA-L were selected due to citations regarding their proliferative ability on lymphocytes (Table 1). Further cited mitogens comprised lipopolysaccharide from E. coli (LPS) and streptolysin O (SLO). All drugs except PHA-M (Gibco-BRL) were purchased from Sigma–Aldrich (Schnelldorf, Germany). Three concentrations were chosen for each drug based on product specifications and literature search (Table 1). All drugs were diluted and stored as recommended (Table 2).
Table 1.
Based on literature search and supplied product specifications, the authors compared seven mitogens with PHA-M, the traditional mitogen used for lymphocyte stimulation in DCA.
| Order # (Sigma) | Source | Name | Abbreviation | Mitogene activity |
Notes | ||
|---|---|---|---|---|---|---|---|
| Assay | Concentration (µg ml−1) | Reference | |||||
| L9379 | Phytolacca americana | Pokeweed | PW | BrdU, human PBMN 3H-thymidine |
0.03–0.3 2.5 10 0.2/2.0/10.0 |
Sigma product specific(17, 18) | Performed in canine lymphocytes Performed in humans |
| L4391 | Lipopolysaccharide from E. coli | Lipopolysaccharide | LPS | BrdU, human PBMN 3H-thymidine |
5, maximum 62 330 0.2–20 |
Sigma(18, 19) | No mitogenic effect in rhesus monkeys No mitogenic effect in human lymphocytes |
| C0412 |
Canavalia ensiformis, Jack bean |
Concanavalin A | CNA | BrdU, human PBMN 3H-thymidine 3H-thymidine 3H-thymidine |
≤125 7 0.83 5 0.2/2/10 |
Sigma product specific(17–20) | Examined in rhesus monkeys Examined in canine lymphocytes |
| S0149 | Haemolytic streptococci | Strepolysin-O | SLO |
3H-thymidine (maximum incorporation 4–5 d) |
100 50 |
(17, 21) | Permeabilises animal membranes, T lymphocytes targeted, additive effect in combination with other mitogens(17) |
| L8025 | M. amurensis | Haemagglutinating and mitogenic haemagglutinin (MAH & MAM) | LMA |
3H-thymidine (maximum incorporation 3 d) |
MAM = 2 MAH = 20 |
(20) | Agglutinates human blood group 0 erys |
| L9640 | T. vulgaris | Lectin from T. vulgaris | LTV | ≤5 | Sigma | Agglutination activity | |
| L4144 | P. vulgaris, red kidney bean | Phytohemagglutinin | PHA-L | BrdU, human PBMN 3H-thymidine |
≤5 3 |
Sigma product specific(20) | Leukocyte agglutination at ≤1 µg ml−1 |
| Gibco-BRL, 10576-015 | P. vulgaris, red kidney bean | Phytohemagglutinin | PHA-M | 100 µl ml−1 | (3) | Recommended for biodosimetry purposes | |
Additional information from the literature: 15 % homologous serum significantly enhanced (∼2-fold) the response of rhesus lymphocytes to PHA, CNA and PW(19). One study used even 20 % human AB serum(18). Under optimal conditions in rhesus monkeys, CNA (∼2-fold) > PHA ∼ PW(19). With higher concentration mitogenic effect of phytolectins decreasing(19). LPS in rhesus monkey appeared not stimulatory(19) and in human T&B lymphocytes as well(18). SLO in canine lymphocytes has an additive effect in combination with phytolectins (∼2–3-fold), but phytolectins combined do not(17). SLO is a B-cell mitogen requiring T-cell help and phytolectins stimulate T cells(17). PHA stimulates human T & B cells (ratio ∼ 2:1), and PHA ∼ CNA > PW(18). Max. 3H-thymidine incorporation at 4 d of human lymphocyte culture(18).
Table 2.
Drugs used in the authors’ experiments had to be dissolved to prepare stock solutions using certain solvents and considering specific storage conditions.
| Drugs name | Abbreviation | Delivered quantity | Solvent | Stock solution | Storage conditions | Experimental lectin concentrations (µg ml−1) |
|---|---|---|---|---|---|---|
| Pokeweed | PW | 10 mg | NaCl (0.9 %) | 1 mg ml−1 | −20°C | 1/5/10 |
| E. coli lipopolysaccharide | LPS | 1 mg | NaCl (0.9 %) | 1 mg ml−1 | −20°C | 2/10/50 |
| Concanavalin A | CNA | 5 mg | PBS | 1 mg ml−1 | 2/10/100 | |
| Strepolysine O | SLO | ∼1 mg | Aqua dest (cold) | 1 mg ml−1 | 2–8°C | 10/50/100 |
| Lectin (from M. amurensis) | LMA | 5 mg | PBS | 1 mg ml−1 | 2–8°C | 2/5/10 |
| Lectin (from T. vulgaris) | LTV | 10 mg | PBS | 1 mg ml−1 | 2–8°C | 2/5/10 |
| Phytohemagglutinin L | PHA-L | 5 mg | PBS | 1 mg ml−1 | −20°C | 2/5/10 |
| Phytohemagglutinin M | PHA-M | 10 ml | Medium | — | −20°C | 13/26/52 µl ml−1 |
Final drug concentrations (µg ml−1 medium) differed depending on cited literature and companies' product specifications.
Automated lymphocyte fixation
The Hanabi PII automated metaphase harvester (Transgenomic, Glasgow, UK) was used to harvest MP from lymphocyte cultures fully automatic including the following steps: all centrifugations at 230×g, aspiration/disposal of supernatants, agitation, suspension and incubation (12 min) of cells in prewarmed (37°C) hypotonic solution (75 mm KCl; Gibco-BRL) and the treatment of cells with up to five changes of fixative (3:1 methanol:acetic acid; Roth, Germany). All steps were performed at room temperature (RT).
Slide preparation, staining and scoring
Slides were prepared at RT around 21°C and humidity around 45 % by manually dispensing 35 µl of a suitable concentrated and thoroughly mixed cell suspension with a pipette onto clean wet slides. Slides were coded, air-dried and stored at RT until ‘Fluorescence plus Giemsa’ (FpG) staining, which was performed as described elsewhere with some modifications(22). Slides were immersed for 12 min in Hoechst 33258 [12.5 µg in 0.25 l distilled water (A.d.); Serva, Germany]. After washing in A.d., slides were placed onto a heating plate at 60°C, covered with phosphate-buffered saline (PBS, pH 6.8) plus coverslip and illuminated with UV light for 10 min. Slides were rinsed in A.d. and immersed in Giemsa stain (9.5 % in GURR buffer, pH 6.8; Gibco-BRL) for 3–5 min. After washing three times in A.d., slides were air-dried at RT, mounted (Eukitt; Merck, Germany) and cover-slipped. Stained slides were stored at RT, whereas cell suspensions were stored at −20°C.
One or two cell cultures were set up per condition, and cytogenetic slides were evaluated using a light microscope (10× objective) by two technicians. MIs were calculated scoring at least 1000 cells dividing the number of MP by the total cells. Therefore, stimulated lymphocytes (large and weakly stained cells) were considered as recommended in the IAEA manual(3).
RESULTS
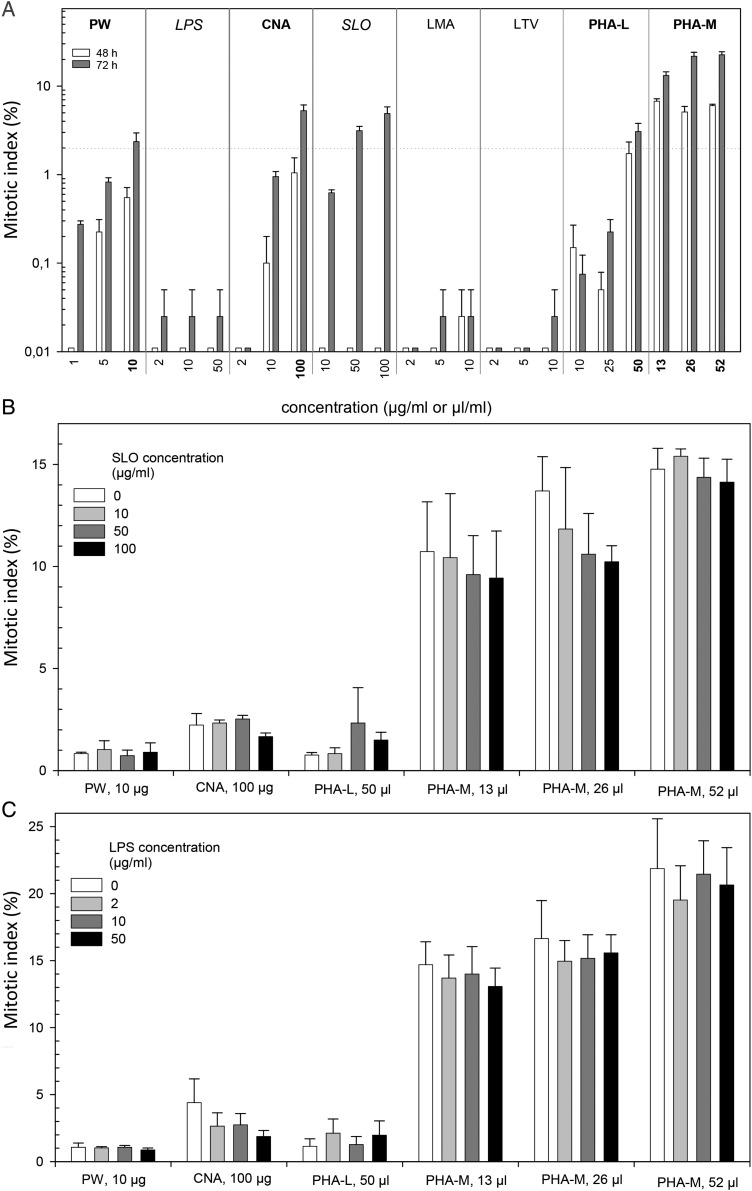
Incubation of mitogens over 48 h resulted in mitotic indices (MI) of <2 % for all mitogens except for PHA-M at all three concentrations (6.8/5.1/6.8 %, Figure 1A). Extending the incubation time to 72 h increased the MI for PW (10 µg ml−1: 2.4 %), CNA (100 µg ml−1: 5.3 %), SLO (50 and 100 µg ml−1: 3.1 and 4.9 %), PHA-L (50 µg ml−1: 3.1 %) and PHA-M (13/26/52 µl: 13.2/21.7/22.6 %) (Figure 1A).
Figure 1.
The MI was measured in lymphocytes stimulated by each of the seven mitogens and PHA-M alone using different concentrations (A) or a combination of selected mitogens with either LPS (B) or SLO (C). For PHA-L and PHA-M working solutions in microliters of rehydrated PHA/ml culture medium are given instead of weight/volume values. (A) Mitogenic activity was examined after incubation with mitogens for 48 h (white bars) and 72 h (dark gay bars) in separate experiments. Bars represent the mean of up to three independent experiments, and counts are provided from two technicians each of them counting two slides, which sums up to four to eight measurements per experimental condition. Error bars reveal the standard error of mean (n = 4–8). The 0 % MI are changed to values of 0.02 % in order to visualise them on a log scale. Drugs inducing >2 % mitosis either after 48 or 72 h of incubation time are highlighted in bold letters. The MI includes second mitosis, whose frequency was marginally for all drugs except for PHA-M with up to one-third of the MI represented by second mitosis. (B, C) In further experiments, lectin concentrations inducing >2 % MI were combined with LPS as well as SLO due to hints in the literature regarding increased mitogenic activity for lectin combinations with either LPS or SLO. The MI were determined after 72 h of incubation for four lectins comprising different concentrations (in total six conditions), which were combined with either LPS or SLO applied in three concentrations (control: without LPS/SLO). Bars represent the mean of two to three independent experiments, and counts are provided from one to two slides, which sums up to three to four measurements per experimental condition. Error bars reveal the standard error of mean (n = 3–4).
In further experiments, the lectin concentrations inducing ≥ 2 % MI were combined in separate experiments with LPS and SLO due to hints in the literature regarding increased mitogenic activity when combining lectins with either LPS or SLO. The MI was examined after 72 h of incubation time with the selected four lectins (in a total of six concentrations), which were combined with LPS or SLO added in three concentrations plus a control (Figure 1B and C). Combinations of SLO or LPS with selected lectins did not increase the MI over the application of the lectins alone (Figure 1B and C).
In the authors’ evaluation, the MI refers to all observed MP, hence including first (M1) and second mitosis MP (M2). The number of M2 was negligible for most of the lectins, except for the administration of PHA-M alone or combined with LPS or SLO. MIs were in the range of 10–20 % after 48 h and even higher after 72 h of incubation time (data not shown).
DISCUSSION
Two limiting factors of DCA are the time-consuming lymphocyte stimulation and the upper limit of accurate dose estimation of ∼4 Gy where dic formation reaches a plateau (saturation) e.g. due to mitotic delay. Traditionally, PHA-M is used to shift a certain fraction of T-lymphocytes from G0 into the G1 phase of the cell cycle. The authors wondered whether improvements in lymphocyte stimulation and proliferation can be gained when using another known lymphocyte mitogen or mitogen combination. Searching the literature, the authors did not find any systematic study on the comparison of different mitogens or even combinations of drugs for improved stimulation of human peripheral blood lymphocytes. Due to that lack in knowledge, they investigated whether the stimulation of lymphocytes can be improved by administration of other mitogens and used MI as a surrogate for that. Such an effect could imply two potential benefits: (1) an increased amount of MP including a larger proportion of good quality MP especially after exposure to high radiation doses. This is beneficial for both fully automatic dic scoring requiring at least 150 high-quality MP and manual scoring being more reliable and faster evaluating high-quality MP. (2) The time for lymphocyte stimulation to pass from G0-phase into the cell cycle and possibly also to progress through mitosis could be reduced. The authors’ findings after conventional 48-h culture time clearly confirmed the traditional concentration of PHA-M to represent the best choice for lymphocyte stimulation. This PHA-M concentration was even superior over lower or higher concentrations of PHA-M and different concentrations of the seven other mitogens. The authors extended the culture time to 72 h in order to not overlook a mitogenic effect of a drug possibly being an entry point for further work with the purpose to overcome the cell cycle delay in order to increase the upper dose limit of dicentric scoring. However, after prolonged culture time as well as regarding combinations of lectins with LPS and SLO, the authors’ results confirmed the recommended use of PHA-M.
In fact, although numerous promising attempts have been made to apply the DCA for the assessment of doses considerably exceeding 4 Gy, none of those approaches got along with a culture time of <48 h. On the contrary, in each case, a prolonged culture time had been necessary(13–16). Furthermore, in order to estimate an absorbed radiation dose as accurate as possible, second mitosis MP have to be excluded from the evaluation of dic frequency and thus should occur in low quantity in the MP preparation after lymphocyte culture. Regarding the proportion of second mitosis MP in the authors’ study, the variation of PHA-M concentration or combination of PHA-M with SLO or LPS in different concentrations (data not shown) did not lead to a significantly lower frequency of second mitosis MP and thus to an advantage of a modified protocol changing the PHA-M concentration.
In conclusion, the conventional protocol using PHA-M at a concentration of 26 µl ml−1 for lymphocyte stimulation proved to be superior over lower/higher PHA-M concentrations as well as over seven other mitogens used either alone or combined.
FUNDING
This work was supported by the German Ministry of Defense.
ACKNOWLEDGEMENT
The authors thank Marcel Zegula and Miriam Kühne for excellent technical performance.
REFERENCES
- 1.Blakely W. F., Salter C. A., Prasanna P. G. S. Early-response biological dosimetry—recommended countermeasure enhancements for mass-casualty radiological incidents and terrorism. Health Phys. 89, 494–504 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Jarrett D., Sedlak R., Dickerson W., Reeves G. Medical treatment of radiation injuries—current US status. Radiat. Meas. 42, 1063–1074 (2007). [Google Scholar]
- 3.International Atomic Energy Agency. Cytogenetic dosimetry: applications in radiation emergencies. A manual. IAEA; (2011). [Google Scholar]
- 4.Multibiodose. Multi-disciplinary biodosimetric tools to manage high scale radiological casualties (MULTIBIODOSE)—Project funded within the 7th EU Framework Programme. Available on http://www.multibiodose.eu/index.htm (7 January 2015, date last accessed).
- 5.Ainsbury E. A., et al. Inter- and intra-laboratory comparison of a multibiodosimetric approach to triage in a simulated, large scale radiation emergency. Int. J. Radiat. Biol. 90(2), 193–202 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Rothkamm K., et al. Comparison of established and emerging biodosimetry assays. Radiat. Res. 180(2),111–119 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.RENEB. Realizing the European Biodosimetry Network. Project funded within the 7th EU Framework Programme. Available on http://reneb.eu/index.htm (7 January 2015, date last accessed).
- 8.Romm H., Ainsbury E., Bajinskis A., Barnard S., Barquinero J. F., Barrios L., Beinke C., Puig-Casanovas R., Deperas-Kaminska M., Gregoire E., et al. Web-based scoring of the dicentric assay, a collaborative biodosimetric scoring strategy for population triage in large scale radiation accidents. Radiat. Environ. Biophys. 53(2), 241–254 (2014a). [DOI] [PubMed] [Google Scholar]
- 9.Kulka U., Ainsbury E. A., Atkinson M., Barnard S., Smith R., Barquinero J. F., Barrios L., Bassinet C., Beinke C., Cucu A., et al. Realising the European network of biodosimetry: RENEB—Status Quo. Radiat. Prot. Dosim. 164(1–2), 42–45 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romm H., et al. Validation of semi-automatic scoring of dicentric chromosomes after simulation of three different irradiation scenarios. Health Phys. 106(6), 764–771 (2014b). [DOI] [PubMed] [Google Scholar]
- 11.Beinke C., Oestreicher U., Riecke A., Kulka U., Meineke V., Romm H. Inter-laboratory comparison to validate the dicentric assay as a cytogenetic triage tool for medical management of radiation accidents. Rad. Meas. 46(9), 929–935 (2011). [Google Scholar]
- 12.Beinke C., Barnard S., Boulay-Greene H., De Amicis A., De Sanctis S., Herodin F., Jones A., Kulka U., Lista F., Lloyd D., et al. Laboratory intercomparison of the dicentric chromosome analysis assay. Radiat. Res. 180(2), 129–137 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Yao B., Jiang B. R., Ai H. S., Li Y. F., Liu G. X., Man Q. H., Qiu L. J. Biological dose estimation for two severely exposed patients in a radiation accident in Shandong Jining, China, in 2004. Int. J. Radiat. Biol. 86, 800–808 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Chen Y., Yan X. K., Du J., Wang Z. D., Zhang X. Q., Zeng F. G., Zhou P. K. Biological dose estimation for accidental supra-high dose gamma-ray exposure. Radiat. Meas. 46, 837–841 (2011). [Google Scholar]
- 15.Vinnikov V. A., Maznyk N. A. Cytogenetic dose-response in vitro for biological dosimetry after exposure to high doses of gamma-rays. Radiat. Prot. Dosim. 154:186–197 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Pujol M., Barquinero J. F., Puig P., Puig R., Caballin M. R., Barrios L. A new model of biodosimetry to integrate low and high doses. PLoS One. 9(12), e114137 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloch E. F., Schultz R. D. The canine lymphocyte: effect of streptolysin O on the proliferative response of canine lymphocytes. Vet. Immunol. Immunopathol. 8(1–2), 125–135 (1985). [DOI] [PubMed] [Google Scholar]
- 18.Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. I. Quantitative isolation of human T and B cells and response to mitogens. J. Immunol. 113(4),1113–1121 (1974). [PubMed] [Google Scholar]
- 19.Taylor D. W., Marchette N. J., Siddiqui W. A. Lymphocyte isolation, rosette formation, and mitogen stimulation in Rhesus monkeys. Dev. Comp. Immunol. 2(3), 539–546 (1978). [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi T., Matsumoto I., Osawa T. Studies on hemagglutinins from Maackia amurensis seeds. J. Biol. Chem. 249(9), 2786–2792 (1974). [PubMed] [Google Scholar]
- 21.Lea T., Michaelsen T. E., Rasmussen A. M. Characterization of the mitogenic and antigenic stimulatory properties of a purified streptolysin O preparation. Clin. Exp. Immunol. 50(1), 139–147 (1982). [PMC free article] [PubMed] [Google Scholar]
- 22.Perry P., Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature (London) 251, 156–158 (1974). [DOI] [PubMed] [Google Scholar]