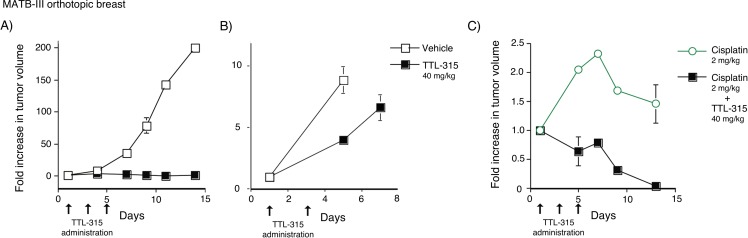
Figure 3. TTL-315 blocks the growth of MATB-III mammary carcinoma and cooperates with cisplatin to trigger regression of bulky established tumors.
Tumor volumes comparing control and experimental cohorts relative to the starting day of treatment is shown on the Y-axis in all panels. A. Prevention design. MATB-III tumor-bearing F344 rats with palpable tumors were administered 40 mg/kg TTL-315 or vehicle only (0.1 ml) by tail vein injection on the days indicated. Mean tumor volume at the start of treatment was 139±4 mm3 for mice in the control cohort (n = 5) and 127±12 mm3 for mice in the experimental cohort (n = 5). B. Treatment design. Rats with bulky tumors >2000 mm3 were treated as before on the days indicated. Mean tumor volume at the start of treatment was 2837±204 mm3 for mice in the control cohort (n = 5) and 2848±538 mm3 for mice in the experimental cohort (n = 5). C. Cooperation with cisplatin. Rats with bulky tumors were subjected to the treatment design protocol as before except for the addition of cisplatin (2 mg/kg) which was administered at the times indicated to both cohorts. Mean tumor volume at the start of treatment was 2948±180 mm3 for mice in the control cohort (cisplatin only) (n=5) and was 2402±218 mm3 for mice in the experimental cohort (cisplatin + TTL-315) (n = 5). Data was evaluated by Student's T test.