Abstract
Formation of the contractile myofibril of the skeletal muscle is a complex process which when perturbed leads to muscular dystrophy. Herein, we provide a mRNAseq dataset on three different zebrafish mutants affecting muscle organization during embryogenesis. These comprise the myosin folding chaperone unc45b (unc45b−/−), heat shock protein 90aa1.1 (hsp90aa1.1−/−) and the acetylcholine esterase (ache−/−) gene. The transcriptome analysis was performed in duplicate experiments at 72 h post-fertilization (hpf) for all three mutants, with two additional times of development (24 hpf and 48 hpf) for unc45b−/−. A total of 20 samples were analyzed by hierarchical clustering for differential gene expression. The data from this study support the observation made in Etard et al. (2015) [1] (http://dx.doi.org/10.1186/s13059-015-0825-8) that a failure to fold myosin activates a unique transcriptional program in the skeletal muscles that is different from that induced in stressed muscle cells.
Keywords: Genetics, mRNASeq, Zebrafish, Muscle mutant, Development
Specifications Table
| Subject area | Biology |
| More specific subject area | Developmental/Stem cell biology |
| Type of data | Transcriptome, figures, tables |
| How data was acquired | High-throughput RNA sequencing using Illumina HiSeq 1000 |
| Data format | Processed |
| Experimental factors | Wild type siblings versus mutants |
| Experimental features | Comparison of mRNA from unc45b−/−, hsp90aa1.1−/−, ache−/− to their respective wild type siblings at 3 stages of development. Duplicates are used for each condition. |
| Data source location | Institute of Toxicology and Genetics, Karlsruhe Institute of Technology, Campus Nord, Eggenstein-Leopoldshafen, Germany |
| Data accessibility | Data are available with this article, and via NCBI׳s GEO accession number GEO: GSE74202 (through the direct linkhttp://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE74202) |
Value of the data
-
•
This dataset comprises the transcriptome analysis of the zebrafish mutants unc45b−/−, hsp90aa1.1−/− and ache−/− during embryonic development.
-
•
It provides the list of regulated genes and associated Gene Ontology analysis of skeletal muscle cells under cellular stress and defective chaperoning activity.
-
•
It is anticipated that this dataset can serve as a reference point for other analysis on myopathies.
1. Data
This data consists of 20 high-throughput sequencing samples of unc45b−/−at 24 hpf, 48 hpf and 72 hpf, as well as ache−/− and hsp90aa1.1−/− and their respective siblings at 72 hpf (n=2 for each genotype and stage) generated on an Illumina HiSeq 1000 [1]. The data are deposited under the Gene Expression Omnibus (GEO) number GEO: GSE74202 at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE74202.
2. Experimental design, materials and methods
2.1. Production of mutants and experimental design
Homozygous mutant zebrafish embryos were produced from incrosses of identified heterozygote mutant carriers in the AB genetic background for the lines unc45b+/− [2], hsp90aa1.1+/− (referred here after as hsp90a) [3] and ache+/− [4]. Wild type and mutant siblings were identified at 72 hpf for hsp90a−/− and ache−/−, and at 24 hpf, 48 hpf and 72 hpf for unc45b−/− by their morphology under the binocular. Embryos were collected from several incrosses in two independent collections. About 20–50 manually dechorionated embryos of each genotype were collected in fish water and homogenized in 200 µL Trizol (Thermo Fisher) after removing fish water with a pipette. The extraction of total RNA was performed as described in the manufacturer׳s protocol, with the modification that an additional extraction with chloroform was performed before precipitation with isopropanol. Total RNA pellets were resuspended in 50 µl RNase-free water (Ambion). RNA integrity was checked by loading about 100 ng total RNA on a RNA6000 Nanochip using an Agilent 2100 Bioanalyser (Agilent Technologies). Samples showed no sign of degradation (RNA index number>9). The list of genotypes and stages of samples collected in the study are provided in Table 1.
Table 1.
Description of the genotype and stage of development of the zebrafish embryos collected in the study. The mutants ache−/−, unc45b−/− and hsp90a−/− are caused by recessive mutations. The genotype of each sample is indicated. The mutant samples symbolized by (−/−) are homozygous mutant. The wild type siblings are annotated by WT and are constituted of embryos without any overt phenotype with a mixed genotype (+/+ or +/−). Biological duplicates are indicated by the number 1 and 2.
| Sample name | Genotype | Stage | Description |
|---|---|---|---|
| ache−/− 1 | ache−/− | 72 hpf | Mutant ache−/− at 72 hpf, replicate 1 |
| ache−/− 2 | ache−/− | 72 hpf | Mutant ache−/− at 72 hpf, replicate 2 |
| hsp90a−/− 1 | hsp90a−/− | 72 hpf | Mutant hsp90a−/− at 72 hpf , replicate 1 |
| hsp90a−/− 2 | hsp90a−/− | 72 hpf | Mutant hsp90a−/− at 72 hpf , replicate 2 |
| unc45b−/− 1_24 hpf | unc45b−/− | 24 hpf | Mutant unc45b−/− at 24 hpf, replicate 1 |
| unc45b−/− 1_48 hpf | unc45b−/− | 48 hpf | Mutant unc45b−/− at 48 hpf, replicate 1 |
| unc45b−/− 1_72 hpf | unc45b−/− | 72 hpf | Mutant unc45b−/− at 72 hpf, replicate 1 |
| unc45b−/− 2_24 hpf | unc45b−/− | 24 hpf | Mutant unc45b−/− at 24 hpf, replicate 2 |
| unc45b−/− 2_48 hpf | unc45b−/− | 48 hpf | Mutant unc45b−/−at 48 hpf, replicate 2 |
| unc45b−/− 2_72 hpf | unc45b−/− | 72 hpf | Mutant unc45b−/− at 72 hpf, replicate 2 |
| ache WT 1 | ache+/− ; ache+/+ | 72 hpf | Wild type siblings of ache−/− at 72 hpf, replicate 1 |
| ache WT 2 | ache+/− ; ache+/+ | 72 hpf | Wild type siblings of ache−/− at 72 hpf, replicate 2 |
| hsp90a WT 1 | hsp90a+/+ ; hsp90a+/− | 72 hpf | Wild type siblings of hsp90a−/− at 72 hpf, replicate 1 |
| hsp90a WT 2 | hsp90a+/+ ; hsp90a+/− | 72 hpf | Wild type siblings of hsp90a−/− at 72 hpf, replicate 2 |
| unc45b WT 1_24 hpf | unc45b+/− ; unc45b+/+ | 24 hpf | Wild type siblings of unc45b−/− at 24 hpf, replicate 1 |
| unc45b WT 1_48 hpf | unc45b+/− ; unc45b+/+ | 48 hpf | Wild type siblings of unc45b−/− at 48 hpf, replicate 1 |
| unc45b WT 1_72 hpf | unc45b+/− ; unc45b+/+ | 72 hpf | Wild type siblings of unc45b−/− at 72 hpf, replicate 1 |
| unc45b WT 2_24 hpf | unc45b+/− ; unc45b+/+ | 24 hpf | Wild type siblings of unc45b−/− at 24 hpf, replicate 2 |
| unc45b WT 2_48 hpf | unc45b+/− ; unc45b+/+ | 48 hpf | Wild type siblings of unc45b−/− at 48 hpf, replicate 2 |
| unc45b WT 2_72 hpf | unc45b+/− ; unc45b+/+ | 72 hpf | Wild type siblings of unc45b−/− at 72 hpf, replicate 2 |
2.2. Library preparation, quality control and data analysis
Sequencing libraries were prepared with the TruSeq RNA Library Prep kit v2 (Illumina), following manufacturer׳s protocol. Briefly, total RNA (1 µg) for each sample was used for poly(A) RNA selection using poly-dT coated magnetic beads followed by fragmentation. First strand cDNA synthesis was performed with the Superscript II (Thermo Fisher) using random hexamer primers. The cDNA fragments were subjected to end-repair and dA-tailing, and finally ligated to specific double stranded bar-coded adapters. Libraries were amplified by 12 cycles of PCR. The quality and concentration of the resulting sequencing libraries were determined on a DNA1000 chip using an Agilent 2100 Bioanalyser (Agilent Technologies). The mRNASeq libraries were sequenced at 7 pM on a HiSeq1000 device (Illumina) to generate 50 bp paired-end reads. Cluster detection and base calling were performed using RTA v.1.13 and quality of reads assessed with CASAVA v.1.8.1 (Illumina). The mapping was performed with TopHat version 1.4.1 [5], setting the distance between mates to 180 bp and a standard deviation of 80 bp. Other TopHat options were -butterfly-search -coverage-search -microexon-search -a 5 -p 5 -library-type fr-unstranded and using the known exon-exon junctions from Ensembl release 75. Quantification of the mapped reads was determined with HTSeq version 0.5.3p3 [6] using the options --stranded=no --mode=union and using the gtf file from Ensembl release 75.
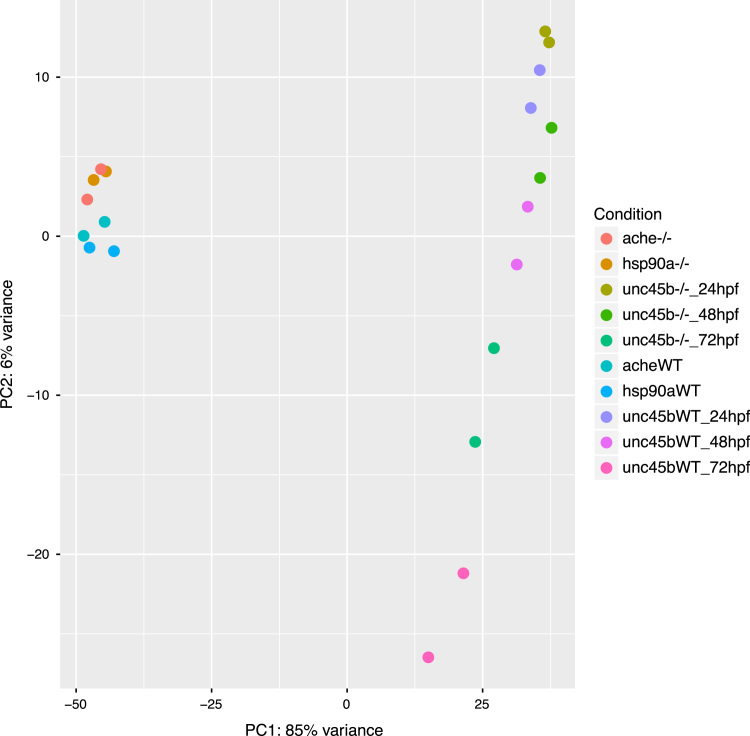
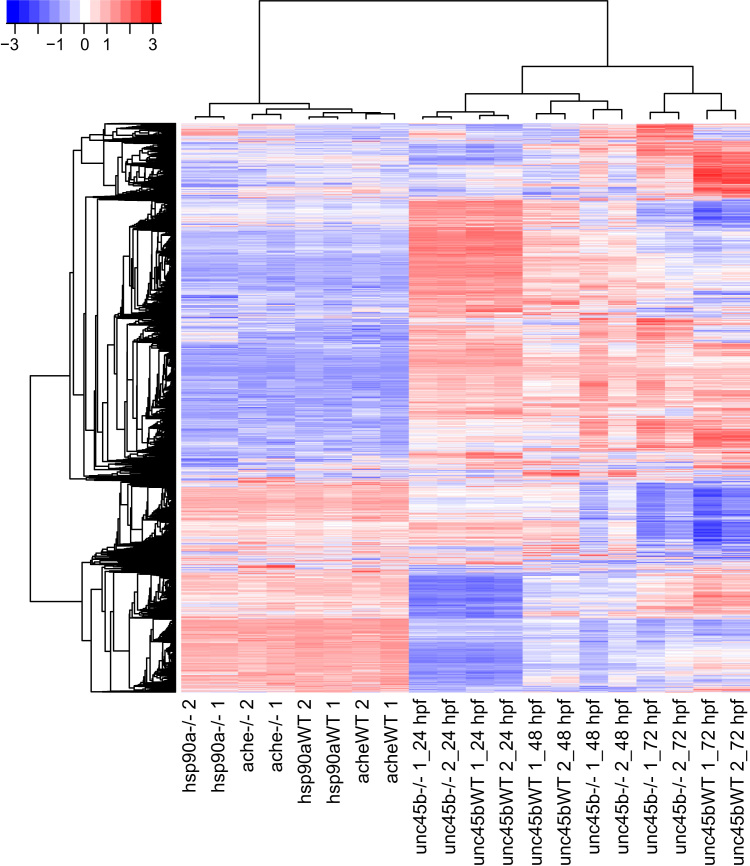
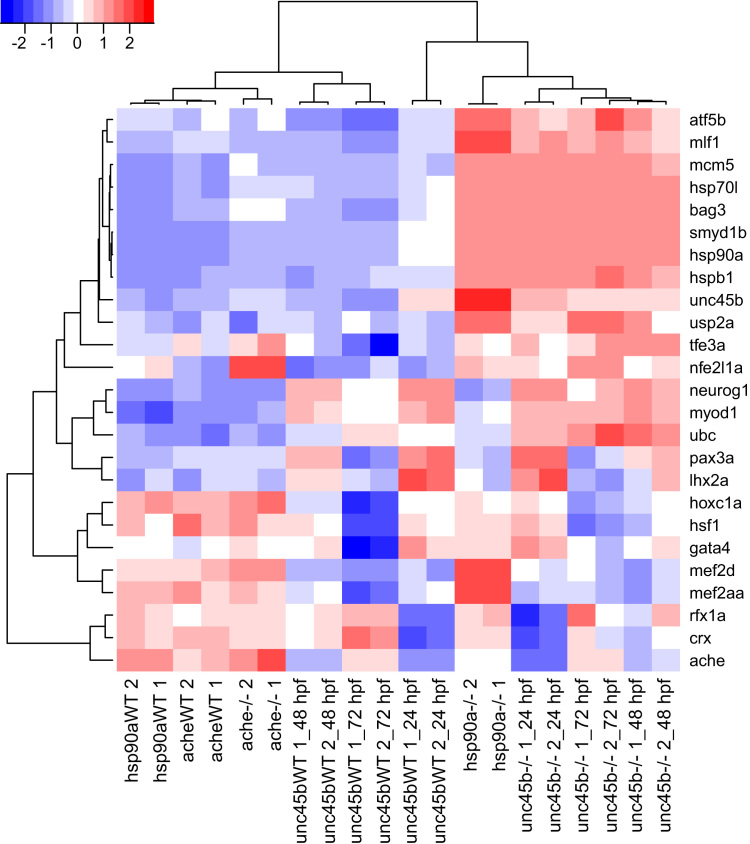
The principal component analysis of the regularized log transformed (rlog) data from DESeq2 [7] shows that the biological duplicates are consistent and that the variance is mainly a factor of the stage and genotype (Fig. 1). The 9453 genes with rlog expression consistently>9 in at least one set of duplicate were subjected to hierarchical clustering with Pearson׳s correlation and the complete-linkage methods using the R packages hclust and gplots (Fig. 2). The hierarchical clustering of 24 selected genes involved in various biological processes such muscle development and neurogenesis shows specific patterns of expression depending on the genotype (Fig. 3).
Fig. 1.
PCA plot. Visualization of the effects of experimental covariates and batch effect of the 20 samples analyzed by mRNASeq by their first and second principal components. Expression data were normalized using the regularized log transformation method from DESeq2.
Fig. 2.
Hierarchical clustering of regularized log transformed data. 9453 genes with rlog>9 in at least one condition were selected and subjected to hierarchical clustering using Pearson׳s correlation and the complete-linkage method under Euclidean metric. Each row represents the expression level of a gene. High expression is symbolized by red color, white is moderate expression and blue low expression. The genotype of each sample is indicated. Mutant samples are indicated by (−/−) and wild type siblings by WT. Biological duplicates are indicated by the numbers 1 and 2.
Fig. 3.
Heatmap of regularized log transformed data of 24 genes involved in muscle development and neurogenesis. Hierarchical clustering was performed with Pearson׳s correlation and the complete-linkage method using Euclidean metric on a selection of 24 genes involved in various biologic processes such myogenesis and neurogenesis (red: high expression, white: moderate expression, blue: low expression). The name of each gene is indicated. Mutant samples are indicated by (−/−) and wild type siblings by WT. Biological duplicates are indicated by the numbers 1 and 2.
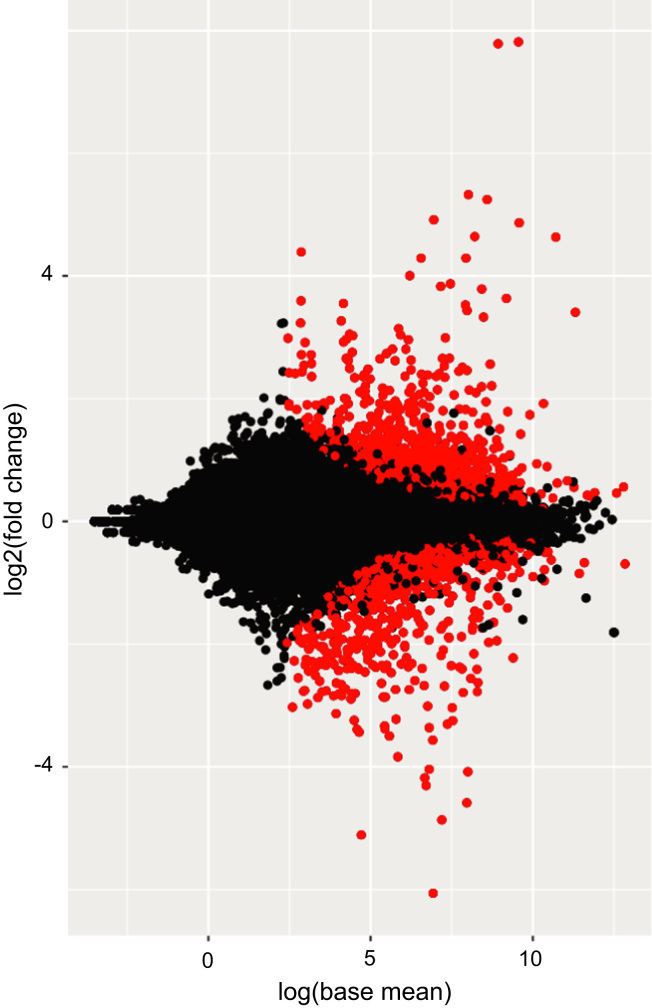
The differential gene expression of the binary comparisons between the mutant and the respective controls were made with the R package DESeq2. A MA plot for the analysis of the differential gene expression between control and unc45b−/− embryos at 72 hpf is shown (Fig. 4). For each gene, the mean expression was plotted against the logarithm of the fold change. Genes with significant deregulation are shown in red (adjusted p-value<0.01).
Fig. 4.
MA plot between the control and unc45b−/− conditions (72 hpf). For each gene the mean of the regularized log transformed expression is plotted against the log(fold change). Genes with a significant adjusted p-value (<0.01) are colored in red, and the others in black.
Zebrafish genes up-regulated or down-regulated in the comparative analysis between the unc45b−/− and control larvae at 72 hpf (adjusted p-value<0.01) were subjected to pathway enrichment analysis. First known human gene orthologues with at least 30% of identity or an orthology confidence score of 1 were obtained from Ensembl Compara [8] via Biomart (release 75) [9]. Then unique Ensembl human or zebrafish identifiers were used to obtain their associated Gene Ontology terms using the R package biomaRt [10]. Finally, enrichment of GO terms was assessed by computing p-values from a Fisher׳s exact-test (gene universe set to 18,000). A comparison between the top enriched GO terms with human and zebrafish genes up-regulated in the unc45b−/− compared to control at 72 hpf is given in Table 2, Table 3. The data show that pathways involved in muscle physiology and hypoxia are enriched in the set of genes up-regulated in unc45b−/− compared to the control at 72 hpf.
Table 2.
Human GO term enrichment. Human orthologues of zebrafish genes up-regulated in unc45b−/− at 72 hpf were used to query GO terms and assess pathway enrichment with a Fisher׳s exact-test.
| GO ID | GO Name | Fisher p-value | Genes |
|---|---|---|---|
| GO:0001666 | Response to hypoxia | 1.47E−09 | PPARA,RAF1,PLAT,SDC2,CITED2,MTHFR,CXCR4,PGF,ALKBH5,TGFB1,VEGFA,ABAT,PDLIM1,EPO,TGFBR3,ARNT,ALAS2,SMAD9,PDGFB, ANGPT2,LIMD1,MECP2,STAT5B,PLOD1 |
| GO:0032355 | Response to estradiol | 7.56E−09 | PTGS2,KCNJ11,TGFB1,RNF14,ASS1,TACR1,WNT7A,ANXA1,CASP9,NCOA1,PDGFB,IGFBP2, SLC25A36,GHR,STAT5B |
| GO:0030018 | Z disc | 2.42E−07 | MYH6,XIRP2,PDLIM5,MYPN,SLC4A1,SYNPO2,JPH2,BIN1,SLC8A1,PGM5,MYL9,SYNPO2L,OBSL1 |
| GO:0007596 | Blood coagulation | 4.44E−07 | SLC7A8,PLEK,RAF1,PSAP,PLAT,SERPINE1,ITGA4,SOS1,PLA2G4A,LYN,FLNA,ANO6,TGFB1, PDE11A,GATA1,VEGFA,PDE10A,PDE2A,PIK3R5,L1CAM,ZFPM1, PRKG1,ITGB3,LRRC16A,MERTK,SLC8A1,PDGFB,ANGPT2,DOCK1,HBE1,GATA4,SERPINB2, PRKACB,SLC16A1,SLC3A2 |
| GO:0030334 | Regulation of cell migration | 6.68E−07 | ROBO4,NOTCH1,CXCR4,TGFB1,JAG1,LAMA3,UNC5C,LAMA2,HACE1,ITGB3,PTPN23,LAMA1 |
| GO:0002040 | Sprouting angiogenesis | 7.57E−07 | NOTCH1,ESM1,PGF,NRARP,VEGFA,JMJD6,GPR124 |
| GO:0007507 | Heart development | 7.91E−07 | TBX5,PPARA,PPARG,RAF1,CITED2,RBM20,ITGA4,SALL4,ACVR2B,NOTCH1,ZFP36L1, SOX9,JMJD6,TGFBR1,PAX3,WT1,GLI2,ZFPM1,PDGFB,GATA4,RBPJ,SOX18 |
| GO:0009986 | Cell surface | 8.33E−07 | PLAT,ITGA4,NOTCH1,CXCR4,GPRC5B,LRP4,ANO6,TIMP2,TGFB1,CSF1R,VEGFA,HBEGF,EPHA2,CLMP, TACR1,WNT7A,L1CAM,ANXA1,PTGFRN,TFRC,GPR124,EPO,ITGB3,TGFBR3,KCNH2,GREM1,SDC4, CNTNAP2,LRRC8A,MST1R,PDGFB,ANXA2,SPTB,GHR,FGFR3,SLC3A2 |
| GO:0001837 | Epithelial to mesenchymal transition | 8.57E−07 | SNAI1,NOTCH1,FLNA,TGFB1,SOX9,HMGA2,TGFBR1,TGFBR3,RBPJ |
| GO:0007220 | Notch receptor processing | 9.90E−07 | NOTCH1,JAG1,NOTCH3,UBB,DLL4,DLL1 |
Table 3.
Zebrafish GO term enrichment. Zebrafish genes up-regulated in unc45b−/− at 72 hpf were directly used to query GO terms and assess pathway enrichment with a Fisher׳s exact-test.
| GO ID | GO Name | Fisher p-value | Genes |
|---|---|---|---|
| GO:0007219 | Notch signaling pathway | 1.62E−06 | dll4,notch1a,notchl,jag1a,chac1,notch3,notch1b,dlc,dla,nrarpb,dld |
| GO:0002040 | Sprouting angiogenesis | 9.73E−06 | dll4,cdh5,tinagl1,rbpjb,vegfaa,sdc2,nrarpb,jmjd6,cxcr4a |
| GO:0030218 | Erythrocyte differentiation | 2.17E−05 | epb41b,tfr1a,slc4a1a,gata1a,alas2,gfi1b,stat5.1 |
| GO:0035162 | Embryonic hemopoiesis | 5.73E−05 | epb41b,tfr1a,slc4a1a,gata1a,alas2,sptb,irak3 |
| GO:0030334 | Regulation of cell migration | 4.11E−04 | robo4,LAMA3 (1 of 2),LAMA2 (2 of 2),lama1,itgb3a |
| GO:0048821 | Erythrocyte development | 1.24E−03 | spi1b,gata1a,sptb,stat5.1 |
| GO:0055002 | Striated muscle cell development | 1.60E−03 | myf5,tcf3a,vangl2,cxcr4a |
Ethic approval
All experiments were made in accordance with the German animal protection standards and were approved by the Government of Baden-Württemberg, Regierungspräsidium Karlsruhe, Germany (Aktenzeichen 35-9185.81/G-137/10).
Acknowledgments
This work was supported by the Karlsruhe Institute of Technology (KIT), the Deutsche Forschungsgemeinschaft (DFG Str439/5-1), the Association Française contre les Myopathies (AFM), EU-IP ZF-HEALTH.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dib.2016.05.007.
Appendix A. Supplementary material
Supplementary material
References
- 1.Etard C. Loss of function of myosin chaperones triggers Hsf1-mediated transcriptional response in skeletal muscle cells. Genome Biol. 2015;16:267. doi: 10.1186/s13059-015-0825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etard C. The UCS factor Steif/Unc-45b interacts with the heat shock protein Hsp90a during myofibrillogenesis. Dev. Biol. 2007;308(1):133–143. doi: 10.1016/j.ydbio.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins T.A. The ATPase-dependent chaperoning activity of Hsp90a regulates thick filament formation and integration during skeletal muscle myofibrillogenesis. Development. 2008;135(6):1147–1156. doi: 10.1242/dev.018150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behra M. The use of zebrafish mutants to identify secondary target effects of acetylcholine esterase inhibitors. Toxicol. Sci. 2004;77(2):325–333. doi: 10.1093/toxsci/kfh020. [DOI] [PubMed] [Google Scholar]
- 5.Trapnell C., Pachter L., Salzberg S.L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anders S., Pyl P.T., Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31(2):166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilella A.J. EnsemblCompara genetrees: complete, duplication-aware phylogenetic trees in vertebrates. Genome Res. 2009;19(2):327–335. doi: 10.1101/gr.073585.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinsella R.J. Ensembl bioMarts: a hub for data retrieval across taxonomic space. Database. 2011:bar030. doi: 10.1093/database/bar030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durinck S. Mapping identifiers for the integration of genomic datasets with the R/bioconductor package biomaRt. Nat. Protoc. 2009;4(8):1184–1191. doi: 10.1038/nprot.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material