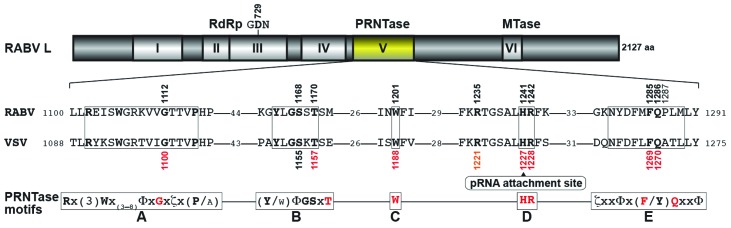
Figure 4.
The polyribonucleotidyltransferase (PRNTase) motifs in the RABV L protein: A schematic structure of the RABV L protein is shown with six conserved amino acid sequence blocks. RdRp and MTase indicate RNA-dependent RNA polymerase and methyltransferase, respectively. Local amino acid sequences of the putative PRNTase domain (block v) of the RABV L protein were aligned with those of the VSV L protein. The numbers above and below the sequences indicate the positions of amino acid residues in the L proteins of RABV and VSV, respectively. H1227 in motif D of the VSV L protein has been identified as the covalent pRNA attachment site [19]. Consensus amino acid sequences of the PRNTase motifs A–E [22] are shown on the bottom (x, Φ, and ζ indicate any, hydrophobic, and hydrophilic amino acids, respectively).