Abstract
Kaempferol is a flavonoid that has been reported to exhibit antitumor activity in various malignant tumors. However, the role of kaempferol on cholangiocarcinoma (CCA) is largely unknown. In this article, we found that kaempferol inhibited proliferation, reduced colony formation ability, and induced apoptosis in HCCC9810 and QBC939 cells in vitro. Results from transwell assay and wound-healing assay demonstrated that kaempferol significantly suppressed the migration and invasion abilities of HCCC9810 and QBC939 cells in vitro. Kaempferol was found to decrease the expression of Bcl-2 and increase the expressions of Bax, Fas, cleaved-caspase 3, cleaved-caspase 8, cleaved-caspase 9, and cleaved-PARP. In addition, kaempferol also downregulated the levels of phosphorylated AKT, TIMP2, and MMP2. In vivo, it was found that the volume of subcutaneous xenograft (0.15 cm3) in the kaempferol-treated group was smaller than that (0.6 cm3) in the control group. Kaempferol also suppressed the number and volume of metastasis foci in the lung metastasis model, with no marked effects on body weight of mice. Immunohistochemistry assay showed that the number of Ki-67-positive cells was lower in the kaempferol-treated group than that in the control group. We further confirmed that the changes of apoptosis- and invasion-related proteins after kaempferol treatment in vivo were similar to the results in vitro. These data suggest that kaempferol may be a promising candidate agent for the treatment of CCA.
Keywords: cholangiocarcinoma, kaempferol, proliferation, invasion
Introduction
Cholangiocarcinoma, abbreviated as CCA, is the most common biliary malignancy and the second common hepatic malignancy after hepatocellular carcinoma [1]. CCA is officially recognized as a major health issue in Southeast Asia, where the incidence of CCA is the highest in the world [2,3]. Although CCA is currently not a common tumor in western countries, the incidence of CCA has increased in the past decades partly as a result of the immigration from Asia [4]. Furthermore, oil sand pollution has been reported to be one of the reasons for the increased incidence of CCA in Northern Alberta, Canada [5]. In the past years, a lot of efforts have been made to treat this disease, but outcomes are not satisfactory. More unfortunately, CCA is always diagnosed at an advanced stage and patients always lose the chance to have an operation at this stage [6–8]. So, more effective therapeutic strategies for the treatment of CCA are urgently needed.
Kaempferol is a flavonoid that is often found in plant-derived food and Chinese herbal plants [9]. It is widely accepted that kaempferol possesses antioxidant and anti-inflammatory activities [10,11]. Recently, its antitumor ability has been gradually discovered. Epidemiological studies have confirmed that kaempferol reduces the risk of developing cancer in smokers [12–14]. It also exhibits antitumor activity in various cancers, including renal cell carcinoma, breast cancer, gastric cancer, hepatocellular carcinoma, colon cancer, and CCA [15–20]. Lee et al. [21] have reported that kaempferol competes with ATP in binding to PI3K in mouse epidermal JB6 P+ cells and subsequently inhibits the downstream activity of AKT and its transcription factor targets [21]. In addition, kaempferol has been reported to disrupt breast cancer metastasis by suppressing matrix metalloproteinase-3 (MMP3) activity [22]. However, the exact role of kaempferol in CCA still needs to be investigated.
The purpose of the present study was to investigate the role of kaempferol in CCA and its possible underlying mechanism.
Materials and Methods
Cell lines and reagents
The human CCA cell lines HCCC9810 and QBC939 were obtained from Shanghai Bioleaf Biotech Co., Ltd (Shanghai, China). HCCC9810 and QBC939 cell lines were maintained in RPMI 1640 (Hyclone, Los Angeles, USA) and Dulbecco's modified Eagle's medium (Hyclone), respectively, supplemented with 10% fetal bovine serum (FBS; Hyclone) in a humidified incubator at 37°C and 5% CO2. Kaempferol (purity of ≥97.0%) and LY294002 were purchased from Shanghai Winherb Medical S & T Development (Shanghai, China). Kaempferol was dissolved in dimethyl sulfoxide (DMSO) for further experiment. Primary monoclonal antibodies against Bcl-2 (1 : 1000), Bax (1 : 1000), survivin (1 : 500), TIMP1 (1 : 1000), TIMP2 (1 : 1000), MMP2 (1 : 500), MMP9 (1 : 500), and β-actin (1 : 2000); polyclonal antibody against GAPDH (1 : 4000); and horseradish peroxidase (HRP)-conjugated secondary antibodies against mouse IgG or rabbit IgG were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, USA). Monoclonal antibodies against Fas (1 : 1000), Bcl-xL (1 : 1000), cleaved-caspase 3 (1 : 1000), cleaved-caspase 8 (1 : 1000), cleaved-caspase 9 (1 : 1000), cleaved-PARP (1 : 2000), PI3K (1 : 1000), p-STAT3 (1 : 1000), STAT3 (1 : 1000), p-extracellular signal-regulated kinase (p-ERK, 1 : 2000), ERK (1 : 2000), p-AKT (1 : 2000), AKT (1 : 2000), and Ki-67 (1 : 200) were purchased from Cell Signaling Technology, Inc. (Danvers, USA).
Cell viability assay
Cell viability was determined using CCK-8 assay (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer's instruction. CCA cells (3 × 103) were seeded in a 96-well plate and cultured in 150 µl of medium supplemented with 10% FBS. After 24 h, kaempferol (0, 30, 60, 90, 120, or 150 µM) was added into the culture medium. Then, after the cells were incubated at 37°C for indicated time (24, 48, or 72 h), the medium was replaced by 100 µl of medium and 10 µl of CCK-8 reagent. The cells were incubated for another 2 h at 37°C. Finally, the optical density was measured using an EnSpire™ 2300 Multilabel Reader (PerkinElmer, Waltham, USA) at 450 nm.
Bromodeoxyuridine proliferation assay
CCA cells (3 × 103 cells/well) were seeded in a 96-well plate and cultured in 150 µl of medium supplemented with 10% FBS. After 24 h, kaempferol (0, 30, 60, 90, 120, or 150 µM) was added into the culture medium and cultured for two more days. Then, cell proliferation was determined using a BrdU kit (Roche, Mannheim, Germany) according to the manufacturer's instruction.
Colony formation assay
CCA cells were trypsinized and collected by centrifugation. Then, resuspended cells were seeded in a 6-well plate at a density of 1000 cells/well in 2 ml of medium. After 24 h of incubation, culture medium was replaced by fresh medium containing kaempferol (0, 50, or 100 µM). Two weeks later, CCA cell clones were stained with a solution containing 1% crystal violet and counted.
Apoptosis assay
CCA cells were seeded in a 6-well plate and treated with kaempferol (0, 50, or 100 µM). After incubation for 48 h, cells were collected, washed with phosphate buffered saline (PBS), resuspended in binding buffer, stained with an annexin-V/PI solution (BD Biosciences, California, USA) at room temperature, and then analyzed using a FACScan system (BD Biosciences).
Wound-healing assay
CCA cells were seeded in a 6-well plate and cultured until confluence, and then scratched with a 10-μl pipette tip. The wounded cell monolayer was then incubated in fresh complete medium with or without kaempferol. Then images were captured at 0 and 24 h after scratching.
Transwell chamber migration and invasion assay
Cell migration assay was conducted using Transwell/Boyden chambers (BD Biosciences). CCA cells were pretreated with kaempferol for 8 h, and then 3 × 104 cells were seeded into transwell chambers. Invasion assay was performed using Transwell/Boyden chambers coated with Matrigel. CCA cells were pretreated with kaempferol for 8 h, and then 3 × 104 cells were seeded into transwell chambers. After 24 h, CCA cells that had migrated or invaded through the membrane were stained with 0.5% crystal violet and counted under an optical microscope. Each experiment was repeated at least three times.
Western blot analysis
Briefly, cells were treated with kaempferol (0, 50, or 100 µM) followed by lysing in lysis buffer [20 mM Tris (pH 7.4), 250 mM NaCl, 2 mM ethylenediaminetetraacetic acid (pH 8.0), 0.1% Triton X-100, 0.01 mg/ml aprotinin, 0.005 mg/ml leupeptin, 0.4 mM phenylmethylsulfonylfluoride, and 4 mM NaVO4]. Protein concentration was measured by bicinchoninic acid assay. Samples of the total protein lysate (15 µg protein) were resolved on 10% or 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and then electrophoretically transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% skimmed milk in PBST buffer (PBS plus 0.1% Tween-20). The membranes were incubated overnight with primary antibodies at 4°C, and then incubated with the corresponding HRP-conjugated secondary antibody for 1 h at room temperature. The membranes were then washed, and the protein bands were visualized with an enhanced chemiluminescence detection kit (Pierce, Rockford, USA) followed by exposure to X-ray film. GAPDH and β-actin were used as a loading control.
Immunohistochemistry
Immunohistochemistry analysis was performed using the anti-Ki-67 antibody. Sections of a paraffin-embedded tissue block were deparaffinized three times in xylene for 5 min and rehydrated through graded ethanol solutions. Then, endogenous peroxidases were inactivated by immersing the sections in 3% hydrogen peroxide for 10 min. Sections were brought to a boil in 10 mM sodium citrate buffer (pH 6.0) and subsequently heated for 10 min in a microwave oven for antigen retrieval. The slides were blocked with 10% bovine serum albumin for 30 min and then stained with the anti-Ki-67 antibody overnight. The sections were further incubated with the corresponding secondary antibody for 1 h at room temperature. Finally, the sections were developed with DAB color solution (50 µl/section) for 5 min at room temperature. The positive cells were counted under a light microscope.
Animal experiments
Male BALB/c athymic nude mice (4 weeks old) were obtained from the Experimental Animal Center of Shanghai Institute for Biological Sciences (Shanghai, China) and housed under standard conditions and cared for according to the institutional guidelines for animal care. All of the animal experiments were approved by the Institutional Animal Care and Use Committee of Harbin Medical University. Eight mice were used in each group to establish subcutaneous xenograft model. QBC939 cells (2.5 × 106) in 150 μl of PBS were subcutaneously injected into the flanks of nude mice. After the tumor volume reached 100 mm3, kaempferol (20 mg/kg/day) was administered i.p. daily for 3 weeks. At the end of treatment, tumors were excised and tumor volume was calculated by the following equation.
For the lung metastasis model, CCA cells (5 × 106) in 200 μl of PBS were injected into the nude mice through the tail vein (n = 8/group). Animals were randomized to receive either kaempferol (20 mg/kg/day) or DMSO at 1 week after injection. After 8 weeks, the mice were sacrificed, and their lungs were collected for further experiment.
Statistical analysis
Results were expressed as the mean ± SD from at least three independent experiments. The difference between groups was analyzed using Student's t-test when only two groups were compared or by a one-way analysis of variance when more than two groups were compared. Significant difference was taken as P < 0.05.
Results
Kaempferol inhibits proliferation, reduces colony formation ability, and induces apoptosis in CCA cells
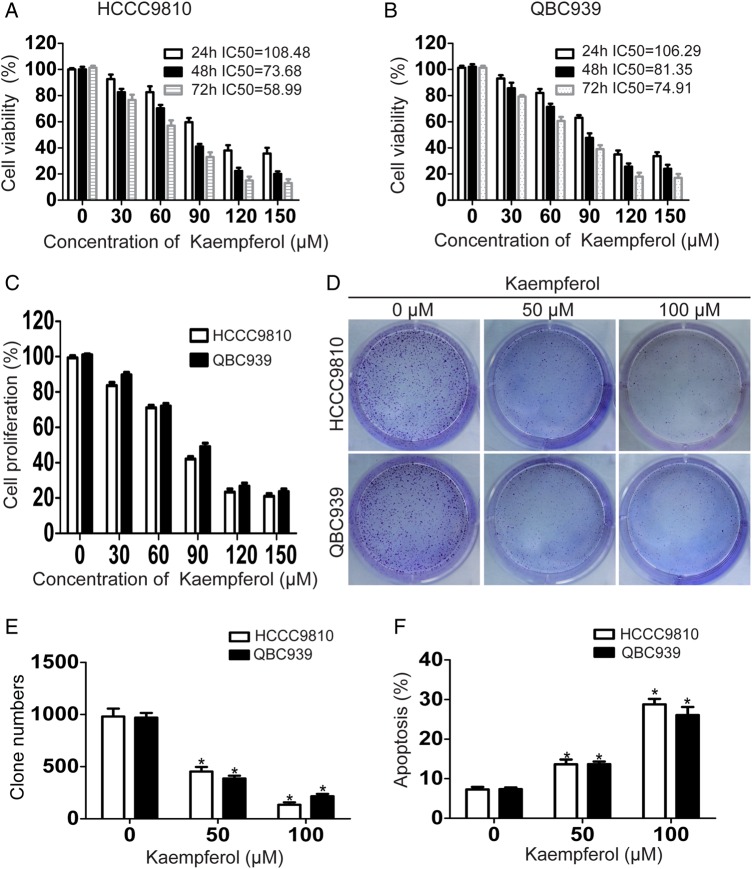
In order to explore the effect of kaempferol on the viability of CCA cells in vitro, CCK-8 assay was performed at 24, 48, or 72 h after drug administration. Results showed that kaempferol significantly suppressed the viability of CCA cells in a time- and dose-dependent manner (Fig. 1A,B). According to the results of bromodeoxyuridine proliferation assay, kaempferol also inhibited the proliferation of CCA cells in a dose-dependent manner (Fig. 1C). We then investigated whether kaempferol could affect the capacity of CCA cells to form foci. Data from colony formation assay showed that kaempferol reduced the number of colonies in a dose-dependent manner, compared with the controls (Fig. 1D,E). To find out whether the inhibition of cell viability was induced by stimulating cell apoptosis, flow cytometric analysis with annexin-V/PI staining was carried out. Results showed that kaempferol induced apoptosis in a dose-dependent manner in CCA cells (Fig. 1F).
Figure 1.
Kaempferol inhibits proliferation, reduces colony formation ability, and induces apoptosis in CCAcells (A, B) CCA cells were incubated with increasing doses of kaempferol (30–150 µM) for 24–72 h and analyzed for cell viability using CCK-8 assay. (C) CCA cells were incubated with increasing doses of kaempferol (30–150 µM) for 48 h and analyzed for cell proliferation using bromodeoxyuridine proliferation assay. (D,E) CCA cells were seeded in 6-well plates, and then treated with gradually increasing concentrations of kaempferol. The number of colonies was counted on the 14th day after seeding. (F) CCA cells were incubated with 50 or 100 µM kaempferol for 48 h and analyzed for apoptosis. Assays were done in triplicate. *P < 0.05.
Kaempferol suppresses the migration and invasion of CCA cells
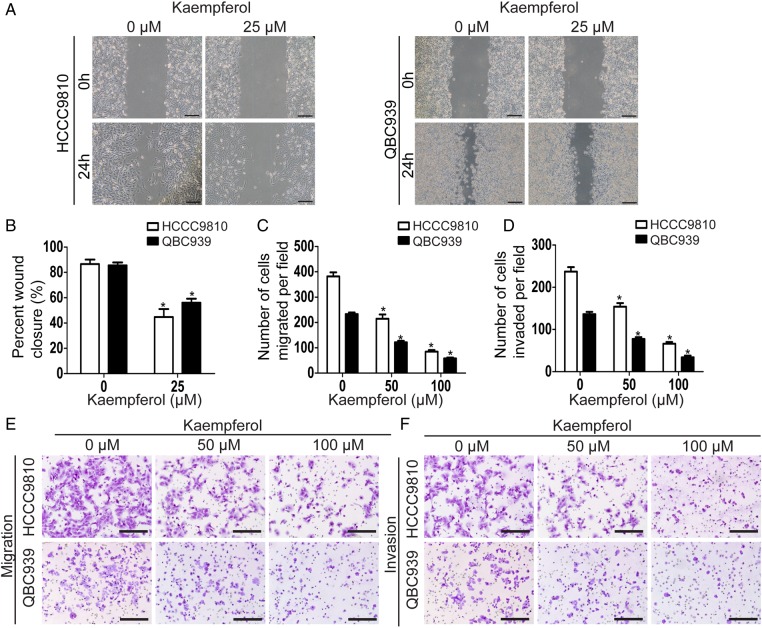
We firstly detected the effect of kaempferol on migration using wound-healing assay, which measured the rate of cell migration into the area of injury created by a sharp object. Results showed that kaempferol-treated group acquired slower closure of the scratched ‘wound’ compared with the control group (Fig. 2A,B). As wound-healing assay is semi-quantitative, we also quantitatively measured the rate of cell migration using transwell migration assay. Consistent with the above results, kaempferol significantly decreased the migration ability of CCA cells in a dose-dependent manner (Fig. 2C,E). Then, we investigated the role of kaempferol on the invasion ability of CCA cells using transwell invasion assay. Results showed that kaempferol significantly reduced the invasion ability of CCA cells in a dose-dependent manner (Fig. 2D,F).
Figure 2.
Kaempferol suppresses the migration and invasion in CCAcells (A) Wound-healing assays were used to detect the effect of kaempferol on the migration of CCA cells. Scale bar = 50 μm. (B) The widths of the gaps in wound-healing assays were measured and shown as a bar graph. (C, E) Cell migration was determined by transwell migration assays. Representative images of the migrated cells selected from three experiments were shown. Migrated cells were counted under a microscope. Scale bar = 100 μm. (D, F) Cell invasion was determined by transwell invasion assays. Representative images of the invaded cells selected from three experiments were shown. Invaded cells were counted under a microscope. Scale bar = 100 μm. Assays were done in triplicate. *P < 0.05.
Kaempferol inhibits PI3K/AKT pathway and affects the expressions of apoptosis- and invasion-related proteins in CCA cells
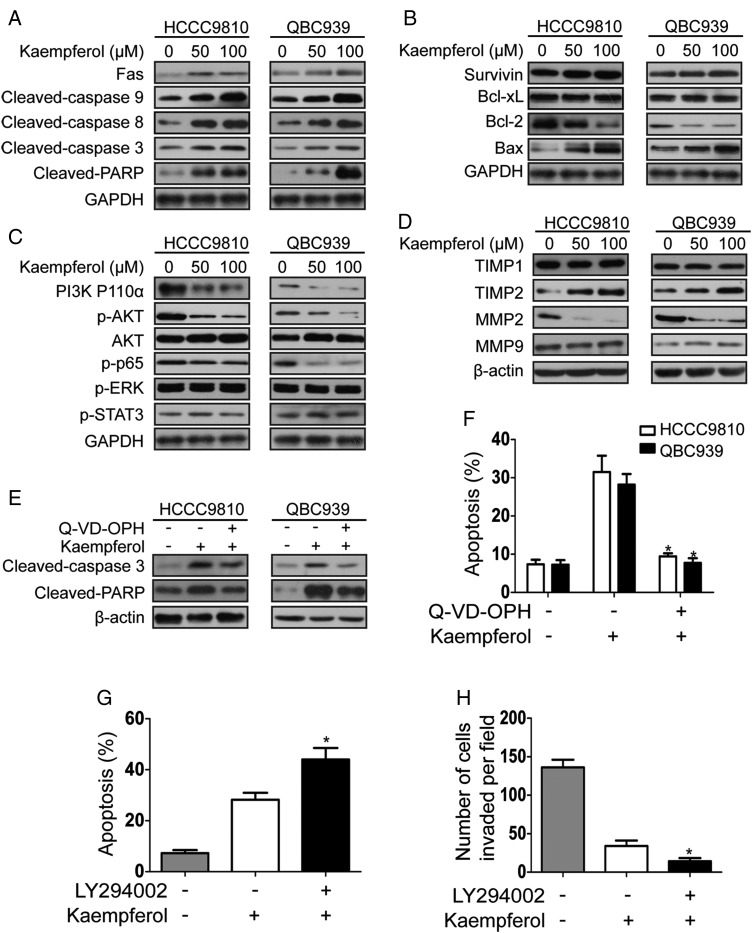
As caspase family proteins are critical enzymes to execute apoptosis, we firstly detected the effect of kaempferol on caspase-dependent apoptotic pathway (Fig. 3). Results showed that kaempferol increased the expressions of Fas, cleaved-caspase 3, cleaved-caspase 8, cleaved-caspase 9, and cleaved-PARP in a dose-dependent manner after 48 h incubation with kaempferol (Fig. 3A). We then explored the expressions of Bcl-2 family proteins which lead to the activation of caspase 3 and PARP in the execution of apoptosis [23]. Data showed that kaempferol increased the expression of Bax and reduced the expression of Bcl-2, but the expressions of Bcl-xL and survivin were not changed (Fig. 3B). To detect the dependence of kaempferol-induced apoptosis on the caspase pathway, we treated CCA cells with pan-caspase inhibitor Q-VD-OPH. The results showed that pretreatment of Q-VD-OPH reversed the expressions of cleaved-caspase 3 and cleaved-PARP, and kaempferol-induced apoptosis (Fig. 3E,F). As tumor invasion is coordinated with the increased proteolytic activity of MMPs, we assessed whether kaempferol could affect their expression. It was found that kaempferol decreased the expression level of MMP2 and increased the expression of TIMP2, but the expression of MMP9 and TIMP1 was not changed (Fig. 3D). Next, signaling pathways involved in the apoptosis and invasion of CCA were analyzed by examining the expressions of phosphorylated forms of STAT3, protein kinase B (AKT), and ERK by western blot analysis. Results showed that kaempferol only reduced AKT phosphorylation status, but the phosphorylation levels of STAT3 and ERK were not changed (Fig. 3C). We then detected the expressions of PI3K P110α (the catalytic subunit of PI3K) and p65 (a downstream transcription factor of PI3K/AKT pathway). As shown in Fig. 3C, the expressions of PI3K P110α and p-p65 were reduced. To further investigate whether kaempferol affected the apoptosis and invasion of CCA cells through the PI3K/AKT pathway, CCA cells were treated with the PI3K inhibitor LY294002 before kaempferol treatment. Results showed that the pretreatment of LY294002 enhanced the effects of kaempferol on apoptosis and invasion in QBC939 cells (Fig. 3G,H).
Figure 3.
Kaempferol inhibits PI3K/AKT pathway and affects the expression of apoptosis- and invasion-related proteins in CCAcells (A) Lysates from CCA cells treated with kaempferol (50 and 100 µM) for 48 h were probed for Fas, cleaved-caspase 3, cleaved-caspase 8, cleaved-caspase 9, and cleaved-PARP by western blot analysis. (B) Expressions of apoptosis-related proteins (Bax, Bcl-2, Bcl-xL, and survivin) were evaluated by western blot analysis after treatment with kaempferol (50 and 100 µM) for 48 h. (C) Lysates from CCA cells treated with kaempferol (50 and 100 µM) for 48 h were probed for PI3K p110α, p-AKT, AKT, p-p65, p-ERK, and p-STAT3 by western blot analysis. (D) Lysates from CCA cells treated with kaempferol (50 and 100 µM) for 48 h were probed for MMPs and TIMPs by western blotting. (E, F) Changes in cleaved-caspase 3, cleaved-PARP, and the histograms of apoptosis rate from two cell lines treated with kaempferol alone or kaempferol in combination with Q-VD-OPH. (G) The histograms of apoptosis rate from QBC939 cells treated with kaempferol alone or kaempferol in combination with LY294002. (H) The number of invaded QBC939 cells treated with kaempferol alone or kaempferol in combination with LY294002. The western blot is representative of three independent experiments. β-Actin and GAPDH were used as the internal control. *P < 0.05.
Kaempferol inhibits tumor growth and metastasis of CCA in vivo
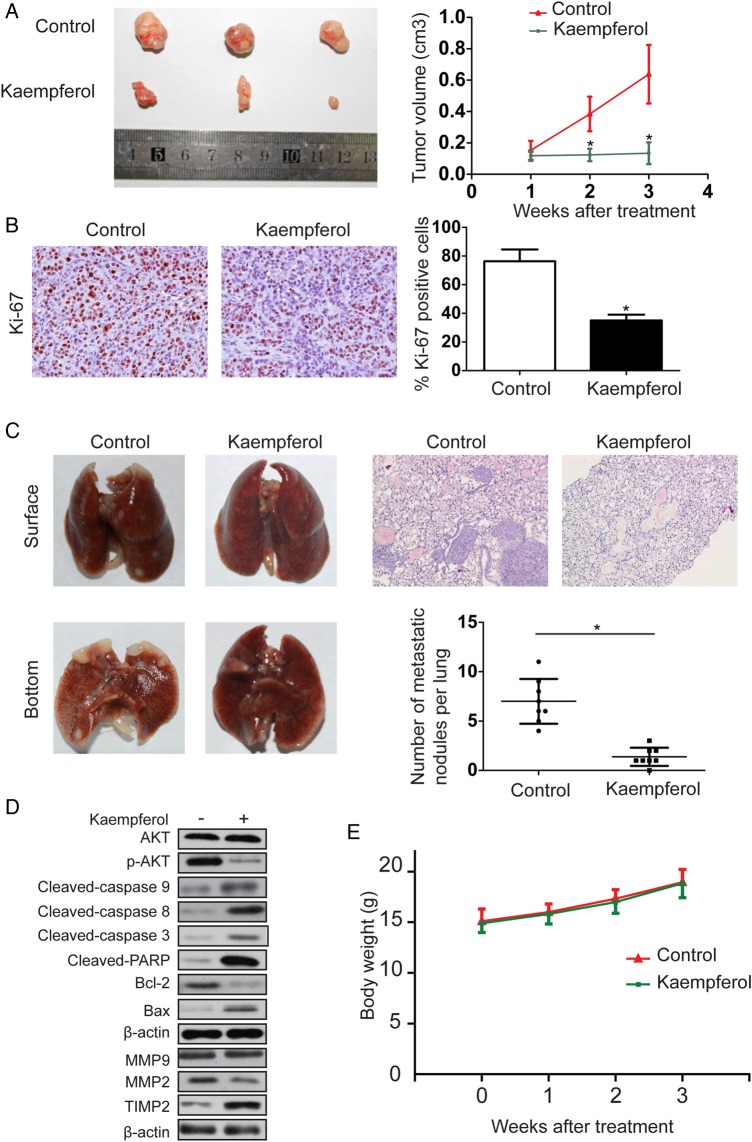
We further detected the effect of kaempferol on CCA growth by establishing a subcutaneous xenograft model in nude mice. QBC939 cell-derived xenograft tumors were allowed to grow to a size of 100 mm3, and then kaempferol (20 mg/kg/day) was administered i.p. daily for 3 weeks. As shown in Fig. 4A, kaempferol significantly inhibited the growth of the tumor, compared with the control group. Ki-67 staining was then performed to assess the role of kaempferol on the proliferative ability of tumor. Results showed that the number of Ki-67-positive tumor cells was lower in kaempferol-treated group than that in the control group (Fig. 4B). The effect of kaempferol on the metastatic phenotype of CCA was examined by injecting QBC939 cells into nude mice through the tail vein to imitate lung metastasis. Kaempferol was found to reduce the average volume and number of foci per mouse, compared with the control (Fig. 4C). The pulmonary foci were mainly located in the peripheral area of lung. The expressions of the apoptosis- and invasion-related proteins were also measured by western blot analysis (Fig. 4D). In addition, no marked change was observed in body weight in the mice, indicating that kaempferol was relatively nontoxic to mice (Fig. 4E).
Figure 4.
Kaempferol inhibits tumor growth and metastasis of CCA invivo (A) Representative images of mice in each group were presented. Tumor volume in kaempferol-treated mice was smaller than that in the control mice. (B) Tumors from different groups were immunostained for Ki-67. The positive cell rate of Ki-67 was reduced in kaempferol-treated group compared with the control group. (C) Images showed the representative lung tissue samples from the different experiment. The number of metastatic nodules per lung in kaempferol-treated group was lower than that in the control group. (D) Western blot analysis on the expressions of apoptosis- and invasion-related proteins as well as key proteins of signaling pathways involved in the apoptosis and invasion of CCA. (E) There was no significant change in body weight between control and kaempferol-treated mice. *P < 0.05.
Discussion
CCA is one of the most malignant tumors which have a poor response to the current chemotherapeutics, including the classic cisplatin and gemcitabine [24–29]. Kaempferol, commonly found in fruits and vegetables, has been reported to possess antitumor ability in various cancers [12]. However, in CCA, the effect of kaempferol remains unknown. In the present study, we demonstrated that kaempferol inhibited the growth and metastasis of CCA in vitro and in vivo.
We firstly demonstrated that kaempferol exhibited a dose- and time-dependent antiproliferation effect on CCA cells by CCK-8 assay and bromodeoxyuridine proliferation assay. Kaempferol also inhibited the colony formation ability and promoted apoptosis in CCA cells, which were consistent with the cell viability variation. Previous studies have reported that kaempferol induces apoptosis by regulating Bcl-2 and caspase family proteins [15–17,30]. Our results showed that kaempferol did not affect the expression of Bcl-xL or survivin, but it significantly reduced the expression of Bcl-2 and increased the expression of Bax. Our results also demonstrated that kaempferol increased the expressions of Fas, cleaved-caspase 3, cleaved-caspase 8, cleaved-caspase 9, and cleaved-PARP. More importantly, the pan-caspase inhibitor Q-VD-OPH reversed kaempferol-mediated activation of caspase 3 and PARP as well as kaempferol-induced apoptosis, indicating that kaempferol induced apoptosis partly through the Fas death receptor/caspase pathway.
A number of studies have revealed that kaempferol inhibits the invasion and metastasis of malignant tumor [22,31–34]. MMPs, a family of zinc-dependent endopeptidases, are involved in the invasion, metastasis, and angiogenesis of various cancers [35]. High expression of MMP9 is associated with lymph node metastasis and poor prognosis in intrahepatic CCA [36]. The upregulation of MMP2 and MMP9 is a critical factor that causes CCA cells to invade into the surrounding tissue [37,38]. In our research, kaempferol was found to suppress the migration and invasion of CCA cells in a dose-dependent manner. Moreover, kaempferol-treatment group exhibited decreased expression of MMP2 and increased expression of TIMP2, but no obvious changes were found in the expressions of MMP9 and TIMP1. Generally, all TIMPs are capable of inhibiting all known MMPs; however, the efficacy of MMP inhibition varies with each individual TIMP. For example, TIMP1 is a strong inhibitor of MMP9, while TIMP2 is capable of suppressing the expression of MMP2 [39]. Therefore, we deduced that kaempferol may suppress the migration and invasion of CCA cells partly through inhibiting the TIMP2/MMP2 pathway. It is widely accepted that inactivating the PI3K/AKT pathway is one of the mechanisms through which kaempferol exerts its biochemical effects [17,40,41]. Furthermore, previous studies have reported that the PI3K/AKT pathway is a major survival pathway involved in the apoptosis and invasion of CCA [42,43]. Our data showed that kaempferol suppressed the expression levels of PI3K P110α, p-AKT, and p-p65, with no obvious changes in the total AKT protein level. Although the effects of kaempferol on other critical proteins in CCA need to be further detected [44,45], we deduce that kaempferol may suppress the growth and metastasis of CCA partly through the PI3K/AKT pathway.
More importantly, we further confirmed the above-mentioned results in vivo. A subcutaneous xenograft model was established to investigate the effect of kaempferol on CCA growth. Kaempferol-treated group showed decreased tumor volume when compared with the control group. The expression of Ki-67, a proliferation biomarker, was also decreased after kaempferol treatment. In addition, in the lung metastasis model, kaempferol significantly decreased the number and volume of foci compared with the control group. Song et al. [17] have reported that no marked change was observed in body, liver, or spleen weight of the nude mice after the treatment of kaempferol, indicating little side effect of kaempferol at this dose of treatment. In our experiment, no apparent difference was found between the kaempferol-treated group and the control group in body weight of the nude mice.
In conclusion, we demonstrate that kaempferol has potent activity against CCA both in vitro and in vivo. These effects may be related to the inactivation of PI3K/AKT pathway and downstream proteins, which are involved in the proliferation, apoptosis, and invasion. Based on our data, kaempferol may be a potential agent for the prevention and therapy of CCA.
Funding
This work was supported by a grant from the Program for Innovative Research Team (in Science and Technology) in Higher Educational Institutions of Heilongjiang Province (No. 2009td06).
Acknowledgement
We would like to thank Dr Zhu Hong for his support in the pathobiology examination.
References
- 1.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013, 145: 1215–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang Y, Wiangnon S et al. Comparison of incidence of intrahepatic and extrahepatic cholangiocarcinoma—focus on East and South-Eastern Asia. Asian Pac J Cancer Prev 2010, 11: 1159–1166. [PubMed] [Google Scholar]
- 3.Srivatanakul P, Parkin DM, Jiang YZ, Khlat M, Kao-Ian UT, Sontipong S, Wild C. The role of infection by Opisthorchis viverrini, hepatitis B virus, and aflatoxin exposure in the etiology of liver cancer in Thailand. A correlation study. Cancer 1991, 68: 2411–2417. [DOI] [PubMed] [Google Scholar]
- 4.Hammill CW, Wong LL. Intrahepatic cholangiocarcinoma: a malignancy of increasing importance. J Am Coll Surg 2008, 207: 594–603. [DOI] [PubMed] [Google Scholar]
- 5.Al-Bahrani R, Abuetabh Y, Zeitouni N, Sergi C. Cholangiocarcinoma: risk factors, environmental influences and oncogenesis. Ann Clin Lab Sci 2013, 43: 195–210. [PubMed] [Google Scholar]
- 6.Shaib YH, Davila JA, Henderson L, McGlynn KA, El-Serag HB. Endoscopic and surgical therapy for intrahepatic cholangiocarcinoma in the United States: a population-based study. J Clin Gastroenterol 2007, 41: 911–917. [DOI] [PubMed] [Google Scholar]
- 7.Ustundag Y, Bayraktar Y. Cholangiocarcinoma: a compact review of the literature. World J Gastroenterol 2008, 14: 6458–6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, Rosenberg WM et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut 2012, 61: 1657–1669. [DOI] [PubMed] [Google Scholar]
- 9.Somerset SM, Johannot L. Dietary flavonoid sources in Australian adults. Nutr Cancer 2008, 60: 442–449. [DOI] [PubMed] [Google Scholar]
- 10.Rice-Evans C. Flavonoid antioxidants. Curr Med Chem 2001, 8: 797–807. [DOI] [PubMed] [Google Scholar]
- 11.Kowalski J, Samojedny A, Paul M, Pietsz G, Wilczok T. Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1beta and tumor necrosis factor-alpha genes in J774.2 macrophages. Pharmacol Rep 2005, 57: 390–394. [PubMed] [Google Scholar]
- 12.Chen AY, Chen YC. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem 2013, 138: 2099–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bobe G, Weinstein SJ, Albanes D, Hirvonen T, Ashby J, Taylor PR, Virtamo J et al. Flavonoid intake and risk of pancreatic cancer in male smokers (Finland). Cancer Epidemiol Biomarkers Prevent 2008, 17: 553–562. [DOI] [PubMed] [Google Scholar]
- 14.Cui Y, Morgenstern H, Greenland S, Tashkin DP, Mao JT, Cai L, Cozen W et al. Dietary flavonoid intake and lung cancer—a population-based case-control study. Cancer 2008, 112: 2241–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song W, Dang Q, Xu D, Chen Y, Zhu G, Wu K, Zeng J et al. Kaempferol induces cell cycle arrest and apoptosis in renal cell carcinoma through EGFR/p38 signaling. Oncol Rep 2014, 31: 1350–1356. [DOI] [PubMed] [Google Scholar]
- 16.Choi EJ, Ahn WS. Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells. Nutr Res Pract 2008, 2: 322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song H, Bao J, Wei Y, Chen Y, Mao X, Li J, Yang Z et al. Kaempferol inhibits gastric cancer tumor growth: an in vitro and in vivo study. Oncol Rep 2015, 33: 868–874. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida T, Konishi M, Horinaka M, Yasuda T, Goda AE, Taniguchi H, Yano K et al. Kaempferol sensitizes colon cancer cells to TRAIL-induced apoptosis. Biochem Biophys Res Commun 2008, 375: 129–133. [DOI] [PubMed] [Google Scholar]
- 19.Mylonis I, Lakka A, Tsakalof A, Simos G. The dietary flavonoid kaempferol effectively inhibits HIF-1 activity and hepatoma cancer cell viability under hypoxic conditions. Biochem Biophys Res Commun 2010, 398: 74–78. [DOI] [PubMed] [Google Scholar]
- 20.Leardkamolkarn V, Tiamyuyen S, Sripanidkulchai BO. Pharmacological activity of Kaempferia parviflora extract against human bile duct cancer cell lines. Asian Pac J Cancer Prev 2009, 10: 695–698. [PubMed] [Google Scholar]
- 21.Lee KM, Lee DE, Seo SK, Hwang MK, Heo YS, Lee KW, Lee HJ. Phosphatidylinositol 3-kinase, a novel target molecule for the inhibitory effects of kaempferol on neoplastic cell transformation. Carcinogenesis 2010, 31: 1338–1343. [DOI] [PubMed] [Google Scholar]
- 22.Phromnoi K, Yodkeeree S, Anuchapreeda S, Limtrakul P. Inhibition of MMP-3 activity and invasion of the MDA-MB-231 human invasive breast carcinoma cell line by bioflavonoids. Acta Pharmacol Sin 2009, 30: 1169–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Susnow N, Zeng L, Margineantu D, Hockenbery DM. Bcl-2 family proteins as regulators of oxidative stress. Semin Cancer Biol 2009, 19: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet 2005, 366: 1303–1314. [DOI] [PubMed] [Google Scholar]
- 25.Patel T. Cholangiocarcinoma. Nat Clin Pract Gastroenterol Hepatol 2006, 3: 33–42. [DOI] [PubMed] [Google Scholar]
- 26.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology 2008, 48: 308–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan SA, Miras A, Pelling M, Taylor-Robinson SD. Cholangiocarcinoma and its management. Gut 2007, 56: 1755–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thongprasert S. The role of chemotherapy in cholangiocarcinoma. Ann Oncol 2005, 16(Suppl. 2): ii93–ii96. [DOI] [PubMed] [Google Scholar]
- 29.Briggs CD, Neal CP, Mann CD, Steward WP, Manson MM, Berry DP. Prognostic molecular markers in cholangiocarcinoma: a systematic review. Eur J Cancer 2009, 45: 33–47. [DOI] [PubMed] [Google Scholar]
- 30.Luo H, Rankin GO, Li Z, Depriest L, Chen YC. Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway. Food Chem 2011, 128: 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labbe D, Provencal M, Lamy S, Boivin D, Gingras D, Beliveau R. The flavonols quercetin, kaempferol, and myricetin inhibit hepatocyte growth factor-induced medulloblastoma cell migration. J Nutr 2009, 139: 646–652. [DOI] [PubMed] [Google Scholar]
- 32.Li C, Zhao Y, Yang D, Yu Y, Guo H, Zhao Z, Zhang B et al. Inhibitory effects of kaempferol on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Biochem Cell Biol 2015, 93: 16–27. [DOI] [PubMed] [Google Scholar]
- 33.Lin CW, Chen PN, Chen MK, Yang WE, Tang CH, Yang SF, Hsieh YS. Kaempferol reduces matrix metalloproteinase-2 expression by down-regulating ERK1/2 and the activator protein-1 signaling pathways in oral cancer cells. PLoS One 2013, 8: e80883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen HJ, Lin CM, Lee CY, Shih NC, Peng SF, Tsuzuki M, Amagaya S et al. Kaempferol suppresses cell metastasis via inhibition of the ERK-p38-JNK and AP-1 signaling pathways in U-2 OS human osteosarcoma cells. Oncol Rep 2013, 30: 925–932. [DOI] [PubMed] [Google Scholar]
- 35.Deryugina EI, Quigley JP. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol 2015, 44–46C: 94–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shirabe K, Shimada M, Kajiyama K, Hasegawa H, Gion T, Ikeda Y, Takenaka K et al. Expression of matrix metalloproteinase-9 in surgically resected intrahepatic cholangiocarcinoma. Surgery 1999, 126: 842–846. [PubMed] [Google Scholar]
- 37.Blain EJ. Mechanical regulation of matrix metalloproteinases. Front Biosci 2007, 12: 507–527. [DOI] [PubMed] [Google Scholar]
- 38.Ehrenfeld P, Conejeros I, Pavicic MF, Matus CE, Gonzalez CB, Quest AF, Bhoola KD et al. Activation of kinin B1 receptor increases the release of metalloproteases-2 and -9 from both estrogen-sensitive and -insensitive breast cancer cells. Cancer Lett 2011, 301: 106–118. [DOI] [PubMed] [Google Scholar]
- 39.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci 2002, 115: 3719–3727. [DOI] [PubMed] [Google Scholar]
- 40.Huang WW, Tsai SC, Peng SF, Lin MW, Chiang JH, Chiu YJ, Fushiya S et al. Kaempferol induces autophagy through AMPK and AKT signaling molecules and causes G2/M arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human hepatic cancer cells. Int J Oncol 2013, 42: 2069–2077. [DOI] [PubMed] [Google Scholar]
- 41.Xie F, Su M, Qiu W, Zhang M, Guo Z, Su B, Liu J et al. Kaempferol promotes apoptosis in human bladder cancer cells by inducing the tumor suppressor, PTEN. Int J Mol Sci 2013, 14: 21215–21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leelawat K, Leelawat S, Narong S, Hongeng S. Roles of the MEK1/2 and AKT pathways in CXCL12/CXCR4 induced cholangiocarcinoma cell invasion. World J Gastroenterol 2007, 13: 1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanno S, Yanagawa N, Habiro A, Koizumi K, Nakano Y, Osanai M, Mizukami Y et al. Serine/threonine kinase AKT is frequently activated in human bile duct cancer and is associated with increased radioresistance. Cancer Res 2004, 64: 3486–3490. [DOI] [PubMed] [Google Scholar]
- 44.Al-Bahrani R, Tuertcher D, Zailaie S, Abuetabh Y, Nagamori S, Zetouni N, Bahitham W et al. Differential SIRT1 expression in hepatocellular carcinomas and cholangiocarcinoma of the liver. Ann Clin Lab Sci 2015, 45: 3–9. [PubMed] [Google Scholar]
- 45.Johnston J, Al-Bahrani R, Abuetabh Y, Chiu B, Forsman CL, Nagamori S, Leng R et al. Twisted gastrulation expression in cholangiocellular and hepatocellular carcinoma. J Clin Pathol 2012, 65: 945–948. [DOI] [PubMed] [Google Scholar]