Abstract
Schizophrenia is a severe mental illness that afflicts nearly 1% of the world population. Although the exact pathophysiology of schizophrenia is unknown, the N-methyl-d-aspartate receptor (NMDAR), a major glutamate receptor subtype, has received great attention. The NR1 subunit is often considered indispensable for functional NMDAR assemblies, abnormal modulation of which is found in patients with schizophrenia. In this review, we discuss how disrupted function of NR1 subunits in NMDAR leads to the progression and development of symptoms of schizophrenia-like behaviors in a variety of genetically modified mouse models. We also discuss some of the susceptible genes and shared signaling pathways among the schizophrenia, and how their mutations lead to NR1 subunits hypofunction. Finally, we suggest that the subunit-selective modulators of NR1 subunits in NMDA receptors may be promising tools for the therapy of schizophrenia.
Keywords: NR1 subunit, NMDA receptor, schizophrenia
Introduction
Schizophrenia is a severe brain-disabling disorder with a prevalence of 0.5%–1% of the population [1]. Core features of schizophrenia are classified into positive symptoms (e.g. hallucinations and delusions), negative symptoms (e.g. anhedonia, alogia, apathy, and poor self-care), and cognitive symptoms (e.g. executive function deficits, working memory, and recognition memory). Symptoms of schizophrenia typically emerge during adolescence or early adulthood. The positive symptoms often fluctuate, while the negative and cognitive symptoms usually cause great disability and deterioration [2]. In order to develop effective therapies, much effort has been made to further understand the core molecular alterations involved in schizophrenia.
Several abnormal neurotransmitter systems have been implicated in the pathophysiological processes underlying schizophrenia. The prominent theory is the dopamine (DA) dysfunction [3], and current antipsychotics that target DA system have been proved to be effective. Those drugs usually show success in relieving the severity of positive symptoms, but they have limited efficacy in ameliorating negative and cognitive deficits, indicating that mechanisms other than DA are likely to be involved in schizophrenia [4]. In addition to the dopaminergic abnormalities, glutamate system that mediates most of the excitatory neurotransmission in the brain has also been proposed to be involved in schizophrenia, including but not limited to N-methyl-d-aspartate receptors (NMDARs), α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs) [5], vesicular glutamate transporter 1 and 2 (VGLUT1 and VGLUT2) [6,7], glial glutamate and aspartate transporter (GLAST) [8], etc. Each subtype of glutamate receptor has distinct functional and anatomical properties that affect glutamate neurotransmission in a functionally selective manner [9]. Up to now, substantial evidence has supported that NMDAR may be an important factor in schizophrenia pathogenesis, which is widely considered as a disorder of brain development and neural connectivity [10,11]. Due to the complex subunit composition of the receptor, the properties of NMDARs are very diverse and complicated. Subunit-specific molecules are thus important tools for understanding the heterogeneous functional and pharmacological characteristics of NMDARs. The NR1 subunit is often considered indispensable for functional NMDAR assemblies, but the remaining subunits can vary [12]. Recent advances in clinical and preclinical pharmacological, genetic, and brain studies have put forward some direct support for the importance of impaired NMDAR-NR1 subunit-mediated glutamatergic pathways in the pathophysiology of schizophrenia [9,13,14]. These findings have important translational implications that the blockage of NMDAR-NR1 subunits or NR1 subunit gene mutation can potentially increase our understanding of how schizophrenia occurs.
In this review, we describe some significant preclinical and clinically relevant findings related to NR1 subunits in schizophrenia model. These findings reveal that the hypofunction of NR1 subunits contributes to abnormalities in several genes and neurotransmitter systems implicated in the biological mechanism underlying schizophrenia. We would like to recapitulate here the central role of NMDAR-NR1 subunits in mediating the signaling network of schizophrenia. Overall, this review will provide further understanding of how NR1 subunit plays a role in the etiology of schizophrenia and, in turn, give us the possibility to develop new tools that are more selective and specific for schizophrenia treatment.
Structure and Functional Organization of NR1 Subunit in NMDAR
Accumulated evidence substantiates an important role of NR1 subunits in a variety of functions and processes in the brain. NR1 subunits can be found in all NMDARs [15], which are mainly heteromeric complex with two other subunits of NR2 (2A-2D) and/or NR3 (3A-3B) [16,17]. Hence, NR1 subunits are essential components of all NMDARs, and thus important for understanding the distribution and functions of NMDARs. The NR1 family is encoded by a single gene, Grin1. One N terminal (exon 5) and two C terminals (exon 21 and exon 22) of the NR1 subunit gene can undergo alternative splicing, leading to eight NR1 splice variants [18]. Additional heterogeneity is conferred by the eight alternative splice variants of the NR1 subunits that have different expression patterns within the brain. It should be noted that NR1 subunits are distributed ubiquitously in the brain and no single cassettes of NR1 subunits are differentially enriched in the postsynaptic densities. In addition, the NR1 subunit mRNA is expressed throughout the different stages of neurodevelopment, normally in excess in the endoplasmic reticulum (ER) [19–21]. On the subcellular level, C1 terminal cassettes possess several phosphorylation sites and reside primarily in the ER, while splice variants containing N terminal cassettes are equally distributed between the cell surface and the cytoplasm. C2 variants have been observed mainly in the intracellular pools of cells and may participate in long-term potentiation-dependent insertion of NMDAR into the membrane. These splice variants may alter NMDARs under either pathological or physiological condition.
NR1 subunits seem to play a pivotal role in determining the voltage and ligand-gating properties of NMDARs. Assembly of the NR1 subunits with one of the four NR2 subunits is necessary for forming active NMDAR ion channels that only open in the presence of glycine and l-glutamate [22–24]. NR1 subunits also confer different intracellular interactions that affect NMDAR signaling and transport. For example, the d-serine/glycine binding sites on the NR1 subunits must be occupied for enhancing the affinity and efficacy of glutamate to activate the NMDAR [25,26]. Studies have also revealed that activation of d-serine/glycine site delays receptor desensitization, increases the duration and frequency of the open channel state [27], and promotes NMDAR turnover through priming of the receptor for internalization [28]. Additionally, NR1 splice variants also influence other characteristics, such as pH sensitivity [24].
Apart from being related to important characteristics of NMDARs, NR1 splice variants are also indispensable for functional NMDAR complexes under various physiological conditions, such as synaptic plasticity, synapse formation underlying memory and learning, as well as formation of neural networks during development. They are also important for a variety of pathological states, including acute and chronic neurological disorders, psychiatric disorders, and neuropathic pain syndromes, etc. For example, chronic developmental Pb2 + exposure could produce the deficits in synaptic plasticity and in learning in young adult rats by altering the cell surface expression of NMDAR-NR1 subunits (lacking C1 cassettes) [29]. Reduced expression of NR1 splice variants of NMDARs (lacking N terminal splice cassettes) could interfere with spatial reference memory in the aging process [30]. Since NR1 splice variants also affect synaptic plasticity, it is no surprise that changes at their mRNA and protein levels were found in the disease of long-term synaptic plasticity-like chronic pain, as well as in cocaine and alcohol abuse [31,32]. Taken together, NR1 subunits play an important part in processes regulating and controlling the structure and function of synapses. Their dual roles in physiological and pathological functions make it necessary to develop NR1 subunit- and site-specific drugs for precise and selective therapeutic interventions.
Abnormal Modulation of NR1 Subunit in Patients with Schizophrenia
Abnormalities of NR1 subunits in NMDARs have been linked to schizophrenia in a series of studies, among which postmortem studies can provide direct evidence in the brains of schizophrenia patients. The study of NMDARs in postmortem brain samples from schizophrenia patients began in 1989, using radio ligand binding techniques with [3H]MK801 which binds to the phencyclidine (PCP) site of the NMDAR channel. No diagnostic changes were found in the prefrontal cortex or hippocampus when compared with the controls [33]. Since then, a number of studies have continued to probe the idea of abnormal NMDAR function by examining the binding and gene transcription levels of individual NMDAR subunits in various brain regions.
Several studies have identified changes in the binding site (glycine binding site) on NR1 subunits in schizophrenia, which demonstrated binding deficit of NR1 subunits in particular brain areas in schizophrenia. Nudmamud et al. [34] identified an increase in radio ligand binding to the glycine site on the obligate NR1 subunits of the NMDARs in superior temporal cortex in schizophrenia patients. Furthermore, strychnine-insensitive [3H]glycine binding was also increased in six regions of the cerebral cortex [35]. The binding of [3H]L-689,560, a potent antagonist at the glycine site in NR1 subunits, has also been reported to be increased in the putamen and in the superior temporal cortex in a small number of schizophrenia patients [36,37]. In addition to the binding deficit of NR1 subunits, it has also been demonstrated that the brains of individuals with schizophrenia display gene expression alterations of the NR1 subunits [38,39]. Some postmortem studies have revealed changes of NMDAR-NR1 gene expression in the cortex in schizophrenia patients. Although the mRNA level of the NR1 subunits was found to be similar to that in the control subjects in the prefrontal, cerebellum, and parietal–temporal cortices, it is reportedly higher in the left superior temporal cortex, the dorsolateral prefrontal cortex, the occipital cortex, and the substantia nigra in schizophrenia patients [12,40,41]. Significantly increased expression of NR1 subunits was also found in the anterior cingulated cortex at the protein level in postmortem samples from elderly schizophrenia patients [42]. However, the expression of NR1 mRNA was found to be lower in the frontal cortex, hippocampus, and thalamus in schizophrenia patients than those in the controls [39,43,44]. These results derived from postmortem brains suggest that alterations in obligatory NR1 subunit expression may underlie schizophrenic pathology. However, caution should be taken in interpreting these results because the patients may have been chronically treated with antipsychotic drugs, not to mention the varied moribund state, the postmortem delay, and tissue handling. Further studies with a greater number of samples will be necessary.
Recently, the prevalence of several anti-NMDAR antibody subtypes has been found in the serum of patients who were initially diagnosed as schizophrenia. Expressions of functional heteromers (NR1/NR2A and NR1/NR2A/NR2B) are required for antibody binding, and reactivity can hardly be identified when NR1 subunits are expressed individually [45,46]. Thus, the prevalence of those anti-NMDAR antibody subtypes in schizophrenia could be considered as a weak etiologic feature that is related to the glutamate hypothesis. The effect of disease state and the time of serum acquisition may arise; therefore, more studies are needed to quantify anti-NMDAR antibodies in both sera and cerebrospinal fluid by employing standardized assay. Moreover, larger cohorts need to be analyzed by including more patients with the psychiatric diagnoses of schizophrenia to substantiate this conclusion with sufficient statistical power [47]. This might render some schizophrenia patients susceptible to new specific glutamate-modulating therapies.
Behavioral Manifestations of Genetically Modified NR1-mutant Mice as a Model of Schizophrenia
Mice with global deletion of NR1 subunits
The glutamate hypothesis posits a deficit in NMDAR-mediated glutamate neurotransmission underlying much dysfunction seen in schizophrenia. Since the presence of NR1 subunit is indispensable for a functional heteromeric NMDAR, genetic animal models of aberrant NR1 function have been established for gaining insight into their functional role (Table 1). Mice with complete and global loss of the NMDAR NR1 subunits died neonatally (only survive 8–15 h after birth) [48]. To overcome the neonatal lethality of NR1-knockout (KO) mice, mice in which the NR1 KO was partially compensated by ectopic transgenic expression of NR1 splice variant were generated [50–52]. Furthermore, NR1-knockdown mice have also been generated. Previous studies have shown that decreased expression of the NR1 subunits which were required for normal NMDAR function caused a series of schizophrenia-like phenotypes. This phenomenon was consistently observed in different laboratories (Table 1). Mice expressing significantly reduced levels of NR1 subunits (NR1neo−/− mice, expressing 5%–10% of the normal level of NR1 subunit in NMDAR) survived to adulthood and displayed behavioral abnormalities, including increased spontaneous locomotor activity and stereotypy [53]. Subsequent studies revealed that the NR1-knockdown model mice also showed negative and cognitive symptoms in the continuum of schizophrenia. NR1neo−/− mice exhibited deficits in social interaction and mating behaviors; they also displayed deficits in hippocampal and prefrontal cortical-dependent spatial cognitive performance [53–56]. Each of these behaviors was found to have a unique developmental trajectory in mutant mice. For example, abnormalities of working memory and sociability in NR1neo−/− mice were only evident in adult mice. Additionally, hyperlocomotion was most evident in juvenile mice, and stereotypy worsened progressively with aging [89]. Moreover, some schizophrenia-like behavioral alterations can be reversed by antipsychotic treatments, such as clozapine and haloperidol treatment. However, pharmacological data were not consistent or sensitive to amphetamine [53,57,58]. A decrease in prefrontal cortical metabolism was also observed in NR1neo−/− mice, consistent with imaging studies that showed reduced frontal cortical metabolic activity in schizophrenia [59]. Taken together, these studies support that disturbances in the NMDAR-NR1 subunits produce phenotypes that are potentially relevant to the symptoms of schizophrenia. These models have the advantage of reproducing the chronic and developmental nature of NMDAR hypofunction theorized to occur in schizophrenia, representing standard behavioral readouts for the evaluation of animal models of schizophrenia. These studies suggest that NR1neo−/− mice may model some behavioral and physiological aspects of schizophrenia.
Table 1.
Summary of genetic mouse models targeting the NMDAR-NR1 subunits
| NR1-mutant mouse model | Brain alterations | Behavioral phenotypes characterization | References |
|---|---|---|---|
| NR1 KO (NR1−/−) mice | Severe deficit in somatosensory barrel cortex formation in the brain | Perinatal lethality, N/A | [48,49] |
| NR1 KO mice (compensated by ectopic transgene of NR1 splice variant) | Significantly increased life span, altered somatosensory pattern, exuberant axonal arborizations, and altered dendritic differentiation; projection neurons of corpus callosum developed prematurely and faster | N/A | [50–52] |
| NR1 knockdown (90%–95% loss) | Alterations in the regional brain metabolism | Disrupted sensorimotor gating, abnormal ERPs, impaired social and sexual interactions, increased spontaneous locomotor activity, stereotypy | [53–61] |
| DG-NR1 KO (deletion of NR1 in dentate gyrus granule cells of the hippocampus) | N/A | Spatial working memory impairments, decreased ability to distinguish two similar contexts | [62,63] |
| CA3-NR1 KO or knockdown with AAV-Cre (in hippocampal CA3 pyramidal cells) | N/A | Deficits in recalling the associative memory; impairment in adaptive nonspatial learning and excitation, impairment in spatial working memory, decreased social approach behavior | [64–68] |
| CA1-NR1 KO (inducible, reversible, and CA1-specific NR1 KO of hippocampal CA1 pyramidal cells) | N/A | Deficits in spatial and nonspatial learning and memory, spatial memory consolidation and retention of contextual fear associations | [69–73] |
| iFB-KO of NR1 (inducible, reversible, and forebrain-specific NR1 KO of forebrain excitatory neuron with CaMK II promoter) | N/A | 30-Day Dox treatment during the seventh month disrupts the 9-month-old remote fear memories, no influence on normal learning and memory function, locomotor activity and cerebellar coordination, impaired nonspatial memory retention and consolidation | [74–76] |
| Amygdala microinjection of rAAV-GFP-Cre into Floxed NR1 mice | N/A | No deficits in locomotor, somatosensory, or sensory-motor behaviors | [77] |
| Nucleus accumbens (Acb) microinjection of rAAV-GFP-Cre into Floxed NR1 mice | Decrease in the dendritic density in Acb neurons | Diminished social interaction, without affecting novel object recognition or open-field activity | [78] |
| Mice with NR1-specific deletion in GABAergic interneuron | Reduction the inhibitory action in GABAergic interneuron | Disruption in self-care, nest building, social short-term memory, social interactions, mating frequency; increased anxiety-like behaviors in the elevated plus maze and open-field test, impaired PPI, no significant hyperlocomotion | [79–83] |
| Mice with NR1N598 (Qneo/Qneo, Rneo/Rneo, −/Q,−/R,+/R,+/Q) genetic modification | N/A | NR1N598 (Qneo/Qneo, Rneo/Rneo,−/Q,−/R) lethality; NR1+/N598Q(R) increased mortality, impaired maternal behaviors | [84] |
| Mice with Grin1D418N, Grin1D481N/K483Q point mutations | Grin1D481N/K483Q mice: striatal dopaminergic and serotonergic hyperfunction | Grin1D418N mice: deficits in spatial recognition, spatial reference learning and memory, deficits in sociability, reduced anxiety and increased startle reactivity, yet normal PPI Grin1D481N/K483Q mice: locomotor hyperactivity, enhanced stereotypy, increased startle reactivity, impaired nest building | [85–88] |
Disruptions in the prepulse inhibition (PPI) are well-validated behavioral tests that are often used for modeling classic schizophrenia-associated deficits in sensorimotor gating. The NR1neo−/− mice would exhibit the decreased sensorimotor gating through the paradigm of the PPI of acoustic startle responses [54,57,60]. These mice also modeled the deficits of schizophrenia patients in auditory gating in the paired tone paradigm [90,91]. Further studies demonstrated that parvalbumin (PV) interneurons in the NR1 mutants were suggested to be the crucial neuronal populations mediating the sensory gating function involved in the pathophysiology of schizophrenia [79]. Event-related potentials (ERPs) were found to be abnormal in NR1-hypomorphic mice, and thus were used as a paradigm to investigate cognitive processes, such as selective attention [61]. Analysis of ERPs evoked by visual stimuli has also been performed. A similarly increased response to visual stimuli was recorded in NR1neo−/− mice, thereby confirming an abnormal sensory processing across modalities [55]. This result suggests that reductions in NR1 subunits lead to a pattern of dysfunction that is common to multiple sensory modalities. Collectively, data from PPI and ERP tests suggest the model characteristics of the NR1neo−/− mouse strain for schizophrenia. Hence, mice with homozygous constitutive reductions in NR1 subunit expression showed stable behavioral changes, although many of these phenotypes might be more severe than the human disease. In contrast to what has been reported in NR1neo−/− mice, NR1neo+/− mice showed no change in obligatory ERP measurement. Alternatively, they showed a marked reduction in response to a deviant auditory tone, consistent with deficits of deviance-related mismatch negativity among family members of schizophrenia patients and among prodromal patients [92]. Together, these studies provide support for the view that chronic global NR1 dysfunction can induce attention, response inhibition, social interaction deficits, etc.
Mice with region-specific deletion of NR1 subunits
Alterations of NR1 subunits in certain brain regions may underlie the observed deficits in schizophrenia patients. Region-specific deletions of NR1 subunits may thereby reveal more precisely the contribution of local NR1 subunits to the development of a schizophrenia-like behavior. Working memory deficits, which have been shown to be consistently associated with reduced levels of elementary social skills and learning capacity in schizophrenia patients, were present early in the course of schizophrenia. Recent findings have demonstrated that they are associated with the deletion of NR1 subunits in animal models of schizophrenia. For example, targeted ablation of the NR1 subunits in the dentate gyrus of the hippocampus resulted in spatial working memory impairments [62,63]. Mice with loss of NR1 subunits in hippocampus CA3 pyramidal cells were unable to fully recall memory when recalling cues were degraded [64,65]. They were also impaired in rapid hippocampal encoding of novel information for fast learning of a one-time experience [65,66]. Alternatively, knocking down NR1 subunits with AAV-Cre virus in adult mice resulted in deficient associative memory learning in adult mice, and this kind of learning requires >70% of CA3 neurons and the expression of NR1 subunits [67]. These studies suggest that NR1 subunits in CA3 brain region play an important role in the recall of spatial reference memory by pattern completion and are required for pattern separation of spatial cues. Furthermore, NR1 subunit deletion in CA3 region was found to play a critical role in tracing eye blink conditioning, a form of nonspatial learning. In addition, CA3 NR1 subunit deletion could decrease social approach behavior [68]. Similarly, mice with the deletion of NR1 subunits in CA1 area exhibited significantly impaired spatial (nonspatial) learning and memory [69,70]. NR1 subunits in CA1 area were also crucial for the formation of temporal memories that associate events across time [71]. Results from inducible, reversible CA1-NR1-specific KO mice further indicated that NR1 subunit-dependent synaptic reinforcement was crucial for memory consolidation [72,73]. These results demonstrate that hippocampus-specific deletion of NR1 subunits mainly causes impairment in spatial and nonspatial working memories. However, the underlying cellular mechanisms have not been revealed.
Long-term memory is a multifactorial construct, composed of different stages of information processing and different cognitive operations that are mediated by distinct neural systems, some of which may be responsible for the schizophrenia-associated deficits of memory problems. Apart from the evidence for the essential functions of the NR1 subunit in learning and memory consolidation, its role in the storage of remote memories has also been examined. Inducible and reversible, and forebrain-specific NR1-knockout (iFB-KO) mice, in which NR1 subunits can be temporarily switched off in the forebrain specifically during the information storage stage, have permitted a more precise investigation of long-term memories in a hippocampal-independent manner [74]. By temporarily switching off the forebrain NR1 subunits during the various stages of memory processes, including learning and consolidation stage, the iFB-KO mice showed severe performance deficits in taste memory retention tests. Therefore, NR1 subunits were demonstrated to play a multistage role in dynamically maintaining the long-term synaptic stability of memory storage circuits in the brain [74,75]. On the contrary, overexpression of the NMDAR composed of NR1-NR2B subunits in the mouse forebrain enhanced NMDAR-dependent synaptic potentiation and produced improvements in learning and memory [76]. Other schizophrenia-associated brain regions, such as the amygdala and the nucleus accumbens, were also examined with spatially restricted deletions of the NR1 subunit genes. Amygdala microinjection of rAAV-GFP-Cre produced a decrease in NR1 subunit gene expression, but did not affect locomotor, somatosensory, or sensory-motor behaviors [77]. Spatially restricted NR1 subunit deletion in the nucleus accumbens could induce a diminished social interaction in NR1neo−/− mice [78]. These findings focusing on special brain areas may be particularly relevant in deciphering the mechanisms underlying deficits in schizophrenia.
Mice with cell type-specific deletion of NR1 subunits
Cell type-specific ablation of NR1 subunits may allow a better understanding of the contribution of NR1 subunits in defined cell populations for inducing schizophrenia-like behaviors. Floxed NR1 mice were crossed with Cre transgenic mice, and thus a cell type-specific and partial deletion of NR1 subunits in GABAergic interneurons was obtained [80]. Interneuron-specific ablation of NR1 subunits resulted in a broad variety of behavioral abnormalities, such as disruption in self-care, nest building, social short-term memory, social interactions, and mating frequency in an age-dependent manner [79,81–83]. These mice also showed increased anxiety-like behaviors in the elevated plus maze and open-field test, impaired PPI, but no significant hyperlocomotion [93]. Several of these behavioral disturbances were exacerbated after social isolation stress, mediated by impairment in antioxidant defense mechanisms [80,94]. In those mice models, specific deletion of NR1 subunits might reduce the inhibitory action of GABAergic interneurons. This NR1-mediated deficit could then cause a loss of inhibitory control over multiple projections [95]. In conclusion, these studies demonstrate that selective loss of the obligatory NMDAR subunit NR1 on PV interneurons may reduce the activation of inhibitory GABAergic interneurons and lead to abnormalities similar to negative symptoms of schizophrenia. These findings have led us to hypothesize that NR1 subunits may play a role in schizophrenia by controlling neuronal patterning and some behavioral abnormalities. The disruption of NR1 subunit function may result in either a net reduction of inhibition or an increase of excitation. Impaired NMDAR signaling on GABAergic interneurons may have the potential to mediate several aspects of schizophrenia, such as negative symptoms.
Mice with targeted point mutations in the NR1 subunits
Studies on recombinant NMDARs identified a single amino acid residue in the NR1 subunits, asparagine 598 (N598), as a critical determinant for the key properties of the NMDARs which mediates high Ca2+ permeability and voltage-dependent Mg2+ block. It was subsequently found that mice expressing these mutated NR1 alleles (Table 1) developed a perinatally lethal phenotype or reduced life expectancy and displayed signs of underdevelopment, such as growth retardation and impaired righting reflex [84], indicating that targeted point mutations in NR1N598 could affect the key properties of the NMDAR and induce causal abnormal animal behavior. Mice with targeted point mutations in the d-serine/glycine binding sites of the NR1 subunits have also been studied. NR1D481N mice with reduced affinity for glycine displayed the anxiolytic-like phenotypes and exhibited deficits in spatial recognition, spatial reference learning and memory, reduced anxiety, and increased startle reactivity, but with normal PPI responses [85–87]. Like the NR1-knockdown mice, NR1D481N mice also showed selective deficits in sociability, similar to the social withdrawal aspect of the negative symptom of schizophrenia [86]. Furthermore, in the Grin1D481N/K483Q mice with two-point mutations in the NR1 d-serine/glycine site, more severe reductions in d-serine/glycine occupancy were found to be associated with abnormalities related to psychosis, including locomotor hyperactivity, enhanced stereotypy, increased startle reactivity, impaired nest building as well as striatal dopaminergic and serotonergic hyperfunction [88]. Therapeutic potential of endogenous d-serine/glycine binding sites agonist d-serine has been assessed through the genetic inactivation of its catabolic enzyme d-amino acid oxidase (DAO) in mice. The hypofunctional Dao1(G181R) mutation elevated brain levels of d-serine without affecting performance in the behavioral measures. Compared with animals with only the Grin 1(D481N) mutation, mice with both the Dao1(G181R) and Grin 1(D481N) mutations displayed an improvement in social approach and spatial memory retention, as well as a reversal of abnormally persistent latent inhibition and a partial normalization of startle responses [96]. Collectively, these genetic models with point mutation displayed phenotypes relevant to schizophrenia, particularly to the negative and cognitive symptoms, suggesting that the NR1 subunits may be relevant to the pathophysiology of these symptoms. The utility of these models lies not in how well they reproduce the behavioral abnormalities seen in schizophrenia, but rather in how they improve our understanding of schizophrenia pathogenesis. d-Serine or DAO inhibitors may be used to effectively increase d-serine level in the brain and be exploited for schizophrenia treatment.
Collectively, these studies in transgenic animals support the notion that perturbations in NR1 subunit-mediated signaling contribute to the behavioral phenotypes of schizophrenia. A closer look at individual mutants, however, reveals the specific relationship between mutant NR1 subunits and patient behavioral outcomes. It appears that alterations in NR1 subunit-mediated signaling can lead to different behavioral manifestations. Transgenic studies indeed helped us to evaluate the pathophysiologic roles of NR1 subunits in schizophrenia. However, it is still a challenge to correlate behavioral outcomes of mutant mice with the expression of genetic variants of NR1 subunits in patients. In our opinion, gene alterations in transgenic mice are more pronounced than in patients. Thus, behavioral manifestations of these risk genotypes in patients might be much weaker than those seen in transgenic mice. Hence, we should be very cautious to interpret the results from animal studies.
NR1 Subunits as Potential Mediators of Convergent Molecular Abnormalities for Schizophrenia
Numerous susceptibility genes have been shown to be involved in the pathophysiology of schizophrenia [97,98]. Many of these genetic variants that are associated with schizophrenia are related to the NMDAR-mediated glutamatergic system in the brain. They have been verified to be involved in neurodevelopment, and consequently contributing to the disturbed information processing in brain circuits that mediate the symptoms of schizophrenia [98,99]. The NMDAR-mediated glutamatergic model thus provides an alternate paradigm for interpreting the etiology of schizophrenia. It is therefore of great interest to clarify how hypofunction of NR1 subunits is involved in the abnormal signaling pathways and neurotransmission system in schizophrenia.
The changes of DA function in the brain might be secondary to reduced activity of NR1 subunits [100]. There is a physical interaction between DA receptor 1 and NR1 subunits, in which NR1 subunits increase plasma membrane insertion of D1 receptors and modify D1 receptor trafficking [101,102]. Thus, a decrease in NR1 subunits function can produce the DA disturbance in schizophrenia. Particularly, NR1 subunits can regulate DA neurons and DA transmission, and hypofunction of NR1 subunits may be responsible for the abnormal DA activity associated with the symptoms of schizophrenia [100,103]. Furthermore, disruption of phasic DA neuron activity by selective genetic inactivation of NR1 subunits impaired the burst firing and dramatically attenuated cognitive function-related behavioral responses together with acoustic startle behavior [78,104,105]. In addition, it has been demonstrated that the glutamatergic disturbances may lead to hypofunction of NR1 subunits on the GABAergic interneurons in the limbic circuit [106]. Therefore, it appears that NR1 subunits in NMDAR could be the convergent point underlying major neurotransmissions associated with schizophrenia. Accumulated evidence shows that convergent mechanisms targeting NR1 subunits of NMDAR may contribute to symptoms and neurocognitive dysfunction in schizophrenia.
In the following sections, we will focus on the current literatures and explain that the NR1 subunit hypofunction might be a downstream joint point for some susceptible genes in schizophrenia. We will also attempt to elucidate the possible signaling pathways related to the regulation of NR1 subunit function by high-risk genes for schizophrenia.
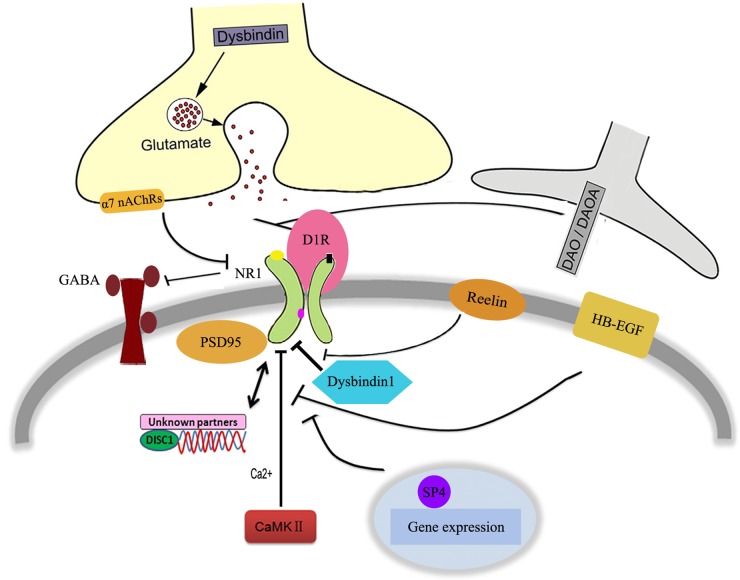
There are many risk genes associated with schizophrenia. These genes include Dysbindin, Disrupted in schizophrenia 1 (DISC1), etc. [107–109], most of which appear to converge at synaptic sites, and are indirectly or directly involved in modulating NR1 subunit-mediated function through a variety of mechanisms (Fig. 1). In addition to the molecular and physiologic effects of reduced Dysbindin in the DA system [110], Dysbindin was demonstrated to regulate NR1 subunit expression and degradation in the glutamate system which correlates with impairments in spatial working memory performance [111]. Furthermore, genetic disruption of Bloc1s8 subunit in Dysbindin was sufficient to downregulate the expression of NR1 subunits in the hippocampus [112]. Cell surface but not the cytoplasmic expression of the NR1 subunits of the NMDAR was decreased, suggesting a dysregulation of NMDAR trafficking in a mouse model carrying a large genomic deletion exclusively within the Dysbindin gene [113]. Thus, the above studies establish a link between Dysbindin expression level and the activity of NR1 subunits. The relationship between DISC1 and NR1 subunits also attracted much attention. In humanized DISC1-Boymaw mice which displayed abnormal information processing of acoustic startle, the protein translation activity of NR1 subunits was decreased [114]. On the other hand, sub-chronic administration of MK801 produced reductions in both spine density and DISC1 expression. These results indicate that synaptic levels of DISC1 and NMDAR function are reciprocally regulated [115]. However, so far none of other partners have been identified to be related to the NR1–DISC1 interaction, which means that other unknown factors may be involved in this signaling pathway.
Figure 1.
Schizophrenia-susceptible genes related to the hypofunction of NR1 subunits in NMDAR
 represents positive function,
represents positive function,  represents negative function, and
represents negative function, and  represents interaction.
represents interaction.
Some other genes that are associated with the functions of NR1 subunits in schizophrenia model have also been briefly reviewed (Fig. 1). Genetically induced reduction in the NR1 subunits expression was also observed in α7 nicotinic acetylcholine receptors (α7 nAChRs) null mice, which may underlie the cortical dysfunction in schizophrenia. Data showed that glutamatergic synapse formation and GABAergic deficits were both impaired in α7 nAChRs' deletion model [116,117]. Evidence also supported the involvement of Reelin, an extracellular matrix (ECM) glycoprotein, in the regulation of NR1 subunits in the pathophysiology of schizophrenia, thus strongly affecting synaptic maturation and stabilization. Reelin has been shown to mediate a switch in the subunits composition of NMDARs, which was considered to be the hallmark of synaptic maturation [118]. In the frontal cortex, Reelin heterozygous mice showed significant downregulation of NR1 subunits. This genotype effect was male-specific for locomotor hyperactivity [119]. In neonatal ventral hippocampus (NVH)-lesioned rats (a neurodevelopmental impairment model of schizophrenia), reduced Ca2+/calmodulin-dependent protein kinase II (CaMK II) autophosphorylation and NR1 (Ser896) phosphorylation were confirmed. This result demonstrated that impaired cognition observed in NVH-lesioned rat is associated with decreased CaMK II and NR1 subunits activities in memory-related brain regions [120]. In line with this, the infusion of a CaMK II inhibitor and NR1 antisense oligonucleotide into the prefrontal cortex produced an impairment of latent learning, indicating a dysfunction of NR1-CaMK II signaling in the mouse model of schizophrenia [121].
Neurotrophic factors and cytokines have been shown to be associated with schizophrenia. A multiplatform profiling study identified peripheral changes that are potentially linked to central alterations in synaptic plasticity and neuronal function associated with NMDAR-NR1 hypofunction [122]. Besides the abnormal behaviors similar to those described in other schizophrenia mouse model [123], reduction of NR1 subunits was observed in mice with heparin-binding epidermal growth factor (HB-EGF) disruption. Additionally, NR1 subunits were considered as mediators of reduced Specificity Protein (Sp) transcription factors associated with negative symptoms in schizophrenia. As Sp may play a role in schizophrenia by controlling neuronal patterning and some behavioral abnormalities [124], an imbalance in NR1 subunit-mediated function has thus been proposed as the underlying mechanism for the complex negative symptoms in this disorder.
Taken together, these susceptible genes function to affect NR1 subunits activities through a variety of mechanisms. Those pathways might regulate NR1 subunit-dependent signaling through both presynaptic and postsynaptic mechanisms, and likely are or contribute to the downstream consequence of the NR1 deficiency. These findings provide convincing evidence for an involvement of NR1 subunits in schizophrenia, and lead to the idea that NR1 subunits may be a mediator of convergent molecular abnormalities for schizophrenia.
Perspective
In the past few years, diverse investigations including developmental studies, genetic manipulations, and postmortem analysis have identified molecular and cellular mechanisms that link the NR1 subunits in NMDAR to the etiology of schizophrenia. These studies have so far enhanced our understanding of both the structural and neurochemical bases of glutamate system that leads to symptoms of schizophrenia, and have led to the discovery of potentially novel treatments for this disorder.
Genetically modified mouse models represent valuable tools for studying the role of NR1 subunits in schizophrenia. However, all rodent models of schizophrenia suffer from the fact that the definition of a schizophrenia-like phenotype in rodents is unclear in the absence of any conclusively demonstrated neurobiological basis of this disease. Future studies should focus on identifying NR1 subunit-mediated developmental deficits associated with schizophrenia at cellular levels.
Much of our knowledge of NR1 subunits in NMDAR was from rodent models based on loss of function via genetic deletion of NR1 subunits. Although some supporting evidence from human postmortem brain analyses was obtained, it is still not known if NR1 subunits for the pathophysiology of schizophrenia can lead to cellular deficits and impaired signaling in patients' neurons. It has been widely accepted that an induced pluripotent stem cell (iPS) model for schizophrenia patients at the cellular level should be established to gain new insight into its pathophysiology. Neurons from human induced pluripotent stem cells (hiPSCs) were presumably glutamatergic. Schizophrenia hiPSC-derived neural progenitor cells and neurons showed significant perturbations of glutamate receptor signaling pathway [125,126]. Thus, we propose to use the iPS model to confirm the function of NR1 subunits derived from existing hypotheses or to verify previous results from animal models.
Due to the complex subunit composition of the receptor, the pharmacology of NMDARs is very diverse and complicated. Although several broad-spectrum competitive antagonists and channel blockers have been developed, molecules with a broad-spectrum effect on all subunit types are not likely to be clinically useful for schizophrenia. Approaches targeting the glycine binding site in NR1 subunits seem more promising because they can induce cognitive enhancement in animal models without producing excitotoxicity [127]. Clinical data showed that agonists for d-serine/glycine site in NR1 subunits (e.g. glycine, d-cycloserine, and d-serine) positively modulate NMDAR channel function and significantly improve cognitive deficits and negative symptoms when given in combination with antipsychotics [128–130]. Therefore, increasing the activation of d-serine/glycine site in NR1 subunits may offer a safer alternative with an increased therapeutic efficiency and a decreased side effect.
Funding
This work was supported by the grants from the Natural Science Foundation of Shanghai, China (No. 14ZR1435900), Qihang Personnel Training Program of Shanghai Mental Health Center (Nos. 2013-QH-01 and 2013-QH-01-X), the National Natural Science Foundation of China (Nos. 81271481, 81171266, and 81501153) and the Shanghai Key Laboratory of Psychotic Disorders (No. 13dz2260500).
References
- 1.Insel TR. Rethinking schizophrenia. Nature 2010, 468: 187–193. [DOI] [PubMed] [Google Scholar]
- 2.Yamauchi K, Aki H, Tomotake M, Iga J, Numata S, Motoki I, Izaki Y et al. . Predictors of subjective and objective quality of life in outpatients with schizophrenia. Psychiatry Clin Neurosci 2008, 62: 404–411. [DOI] [PubMed] [Google Scholar]
- 3.Abi-Dargham A. Schizophrenia: overview and dopamine dysfunction. J Clin Psychiatry 2014, 75: e31. [DOI] [PubMed] [Google Scholar]
- 4.Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron 2006, 52: 139–153. [DOI] [PubMed] [Google Scholar]
- 5.Wiedholz LM, Owens WA, Horton RE, Feyder M, Karlsson RM, Hefner K, Sprengel R et al. . Mice lacking the AMPA GluR1 receptor exhibit striatal hyperdopaminergia and ‘schizophrenia-related’ behaviors. Mol Psychiatry 2008, 13: 631–640. [DOI] [PubMed] [Google Scholar]
- 6.Wallen-Mackenzie A, Nordenankar K, Fejgin K, Lagerstrom MC, Emilsson L, Fredriksson R, Wass C et al. . Restricted cortical and amygdaloid removal of vesicular glutamate transporter 2 in preadolescent mice impacts dopaminergic activity and neuronal circuitry of higher brain function. J Neurosci 2009, 29: 2238–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inta D, Vogt MA, Perreau-Lenz S, Schneider M, Pfeiffer N, Wojcik SM, Spanagel R et al. . Sensorimotor gating, working and social memory deficits in mice with reduced expression of the vesicular glutamate transporter VGLUT1. Behav Brain Res 2012, 228: 328–332. [DOI] [PubMed] [Google Scholar]
- 8.Karlsson RM, Tanaka K, Saksida LM, Bussey TJ, Heilig M, Holmes A. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology 2009, 34: 1578–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bondi C, Matthews M, Moghaddam B. Glutamatergic animal models of schizophrenia. Curr Pharm Des 2012, 18: 1593–1604. [DOI] [PubMed] [Google Scholar]
- 10.Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet 2014, 383: 1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 2011, 14: 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dracheva S, Marras SA, Elhakem SL, Kramer FR, Davis KL, Haroutunian V. N-methyl-D-aspartic acid receptor expression in the dorsolateral prefrontal cortex of elderly patients with schizophrenia. Am J Psychiatry 2001, 158: 1400–1410. [DOI] [PubMed] [Google Scholar]
- 13.Ramsey AJ. NR1 knockdown mice as a representative model of the glutamate hypothesis of schizophrenia. Prog Brain Res 2009, 179: 51–58. [DOI] [PubMed] [Google Scholar]
- 14.Sacchi S, Rosini E, Pollegioni L, Molla G. D-Amino acid oxidase inhibitors as a novel class of drugs for schizophrenia therapy. Curr Pharm Des 2013, 19: 2499–2511. [DOI] [PubMed] [Google Scholar]
- 15.Monaghan DT, Jane DE. Pharmacology of NMDA receptors. In: Van Dongen AM. ed. Biology of the NMDA Receptor. Boca Raton: CRC Press; 2009. [PubMed] [Google Scholar]
- 16.Qiu S, Hua YL, Yang F, Chen YZ, Luo JH. Subunit assembly of N-methyl-d-aspartate receptors analyzed by fluorescence resonance energy transfer. J Biol Chem 2005, 280: 24923–24930. [DOI] [PubMed] [Google Scholar]
- 17.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 2001, 11: 327–335. [DOI] [PubMed] [Google Scholar]
- 18.Zukin RS, Bennett MV. Alternatively spliced isoforms of the NMDARI receptor subunit. Trends Neurosci 1995, 18: 306–313. [DOI] [PubMed] [Google Scholar]
- 19.Gonda X. Basic pharmacology of NMDA receptors. Curr Pharm Des 2012, 18: 1558–1567. [DOI] [PubMed] [Google Scholar]
- 20.Huh KH, Wenthold RJ. Turnover analysis of glutamate receptors identifies a rapidly degraded pool of the N-methyl-D-aspartate receptor subunit, NR1, in cultured cerebellar granule cells. J Biol Chem 1999, 274: 151–157. [DOI] [PubMed] [Google Scholar]
- 21.Awobuluyi M, Lipton SA, Sucher NJ. Translationally distinct populations of NMDA receptor subunit NR1 mRNA in the developing rat brain. J Neurochem 2003, 87: 1066–1075. [DOI] [PubMed] [Google Scholar]
- 22.Karakas E, Furukawa H. Crystal structure of a heterotetrameric NMDA receptor ion channel. Science 2014, 344: 992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mori H, Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology 1995, 34: 1219–1237. [DOI] [PubMed] [Google Scholar]
- 24.Yamakura T, Shimoji K. Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog Neurobiol 1999, 59: 279–298. [DOI] [PubMed] [Google Scholar]
- 25.Fadda E, Danysz W, Wroblewski JT, Costa E. Glycine and D-serine increase the affinity of N-methyl-D-aspartate sensitive glutamate binding sites in rat brain synaptic membranes. Neuropharmacology 1988, 27: 1183–1185. [DOI] [PubMed] [Google Scholar]
- 26.Hirai H, Kirsch J, Laube B, Betz H, Kuhse J. The glycine binding site of the N-methyl-D-aspartate receptor subunit NR1: identification of novel determinants of co-agonist potentiation in the extracellular M3-M4 loop region. Proc Natl Acad Sci USA 1996, 93: 6031–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vyklicky L Jr, Benveniste M, Mayer ML. Modulation of N-methyl-D-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol 1990, 428: 313–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, Salter MW. Glycine binding primes NMDA receptor internalization. Nature 2003, 422: 302–307. [DOI] [PubMed] [Google Scholar]
- 29.Guilarte TR, McGlothan JL. Selective decrease in NR1 subunit splice variant mRNA in the hippocampus of Pb2+-exposed rats: implications for synaptic targeting and cell surface expression of NMDAR complexes. Brain Res Mol Brain Res 2003, 113: 37–43. [DOI] [PubMed] [Google Scholar]
- 30.Das SR, Jensen R, Kelsay R, Shumaker M, Bochart R, Brim B, Zamzow D et al. . Reducing expression of GluN1(0XX) subunit splice variants of the NMDA receptor interferes with spatial reference memory. Behav Brain Res 2012, 230: 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loftis JM, Janowsky A. Cocaine treatment- and withdrawal-induced alterations in the expression and serine phosphorylation of the NR1 NMDA receptor subunit. Psychopharmacology (Berl) 2002, 164: 349–359. [DOI] [PubMed] [Google Scholar]
- 32.Raeder H, Holter SM, Hartmann AM, Spanagel R, Moller HJ, Rujescu D. Expression of N-methyl-D-aspartate (NMDA) receptor subunits and splice variants in an animal model of long-term voluntary alcohol self-administration. Drug Alcohol Depend 2008, 96: 16–21. [DOI] [PubMed] [Google Scholar]
- 33.Kornhuber J, Mack-Burkhardt F, Riederer P, Hebenstreit GF, Reynolds GP, Andrews HB, Beckmann H. [3H]MK-801 binding sites in postmortem brain regions of schizophrenic patients. J Neural Transm 1989, 77: 231–236. [DOI] [PubMed] [Google Scholar]
- 34.Nudmamud S, Reynolds GP. Increased density of glutamate/N-methyl-D-aspartate receptors in superior temporal cortex in schizophrenia. Neurosci Lett 2001, 304: 9–12. [DOI] [PubMed] [Google Scholar]
- 35.Ishimaru M, Kurumaji A, Toru M. Increases in strychnine-insensitive glycine binding sites in cerebral cortex of chronic schizophrenics: evidence for glutamate hypothesis. Biol Psychiatry 1994, 35: 84–95. [DOI] [PubMed] [Google Scholar]
- 36.Aparicio-Legarza MI, Davis B, Hutson PH, Reynolds GP. Increased density of glutamate/N-methyl-D-aspartate receptors in putamen from schizophrenic patients. Neurosci Lett 1998, 241: 143–146. [DOI] [PubMed] [Google Scholar]
- 37.Grimwood S, Slater P, Deakin JF, Hutson PH. NR2B-containing NMDA receptors are up-regulated in temporal cortex in schizophrenia. Neuroreport 1999, 10: 461–465. [DOI] [PubMed] [Google Scholar]
- 38.Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP, Potkin SG et al. . Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci 1996, 16: 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao XM, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry 2000, 157: 1141–1149. [DOI] [PubMed] [Google Scholar]
- 40.Le Corre S, Harper CG, Lopez P, Ward P, Catts S. Increased levels of expression of an NMDARI splice variant in the superior temporal gyrus in schizophrenia. Neuroreport 2000, 11: 983–986. [DOI] [PubMed] [Google Scholar]
- 41.Mueller HT, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of the ionotropic glutamate receptor subunits and NMDA receptor-associated intracellular proteins in the substantia nigra in schizophrenia. Brain Res Mol Brain Res 2004, 121: 60–69. [DOI] [PubMed] [Google Scholar]
- 42.Kristiansen LV, Beneyto M, Haroutunian V, Meador-Woodruff JH. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol Psychiatry 2006, 11: 737–747, 705. [DOI] [PubMed] [Google Scholar]
- 43.Humphries C, Mortimer A, Hirsch S, de Belleroche J. NMDA receptor mRNA correlation with antemortem cognitive impairment in schizophrenia. Neuroreport 1996, 7: 2051–2055. [DOI] [PubMed] [Google Scholar]
- 44.Ibrahim HM, Hogg AJ Jr, Healy DJ, Haroutunian V, Davis KL, Meador-Woodruff JH. Ionotropic glutamate receptor binding and subunit mRNA expression in thalamic nuclei in schizophrenia. Am J Psychiatry 2000, 157: 1811–1823. [DOI] [PubMed] [Google Scholar]
- 45.Dalmau J, Tuzun E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM et al. . Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007, 61: 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steiner J, Walter M, Glanz W, Sarnyai Z, Bernstein HG, Vielhaber S, Kastner A et al. . Increased prevalence of diverse N-methyl-D-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: specific relevance of IgG NR1a antibodies for distinction from N-methyl-D-aspartate glutamate receptor encephalitis. JAMA Psychiatry 2013, 70: 271–278. [DOI] [PubMed] [Google Scholar]
- 47.Pearlman DM, Najjar S. Meta-analysis of the association between N-methyl-d-aspartate receptor antibodies and schizophrenia, schizoaffective disorder, bipolar disorder, and major depressive disorder. Schizophr Res 2014, 157: 249–258. [DOI] [PubMed] [Google Scholar]
- 48.Forrest D, Yuzaki M, Soares HD, Ng L, Luk DC, Sheng M, Stewart CL et al. . Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron 1994, 13: 325–338. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell 1994, 76: 427–437. [DOI] [PubMed] [Google Scholar]
- 50.Iwasato T, Erzurumlu RS, Huerta PT, Chen DF, Sasaoka T, Ulupinar E, Tonegawa S. NMDA receptor-dependent refinement of somatotopic maps. Neuron 1997, 19: 1201–1210. [DOI] [PubMed] [Google Scholar]
- 51.Lee LJ, Lo FS, Erzurumlu RS. NMDA receptor-dependent regulation of axonal and dendritic branching. J Neurosci 2005, 25: 2304–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elberger AJ, Deng J. Corpus callosum and visual cortex of mice with deletion of the NMDA-NR1 receptor: I. Accelerated development of callosal projection neurons. Brain Res Dev Brain Res 2003, 144: 121–133. [DOI] [PubMed] [Google Scholar]
- 53.Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 1999, 98: 427–436. [DOI] [PubMed] [Google Scholar]
- 54.Duncan GE, Moy SS, Perez A, Eddy DM, Zinzow WM, Lieberman JA, Snouwaert JN et al. . Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav Brain Res 2004, 153: 507–519. [DOI] [PubMed] [Google Scholar]
- 55.Halene TB, Ehrlichman RS, Liang Y, Christian EP, Jonak GJ, Gur TL, Blendy JA et al. . Assessment of NMDA receptor NR1 subunit hypofunction in mice as a model for schizophrenia. Genes Brain Behav 2009, 8: 661–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dzirasa K, Ramsey AJ, Takahashi DY, Stapleton J, Potes JM, Williams JK, Gainetdinov RR et al. . Hyperdopaminergia and NMDA receptor hypofunction disrupt neural phase signaling. J Neurosci 2009, 29: 8215–8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duncan GE, Moy SS, Lieberman JA, Koller BH. Typical and atypical antipsychotic drug effects on locomotor hyperactivity and deficits in sensorimotor gating in a genetic model of NMDA receptor hypofunction. Pharmacol Biochem Behav 2006, 85: 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moy SS, Perez A, Koller BH, Duncan GE. Amphetamine-induced disruption of prepulse inhibition in mice with reduced NMDA receptor function. Brain Res 2006, 1089: 186–194. [DOI] [PubMed] [Google Scholar]
- 59.Duncan G, Miyamoto S, Gu H, Lieberman J, Koller B, Snouwaert J. Alterations in regional brain metabolism in genetic and pharmacological models of reduced NMDA receptor function. Brain Res 2002, 951: 166–176. [DOI] [PubMed] [Google Scholar]
- 60.Fradley RL, O'Meara GF, Newman RJ, Andrieux A, Job D, Reynolds DS. STOP knockout and NMDA NR1 hypomorphic mice exhibit deficits in sensorimotor gating. Behav Brain Res 2005, 163: 257–264. [DOI] [PubMed] [Google Scholar]
- 61.Bickel S, Lipp HP, Umbricht D. Impaired attentional modulation of auditory evoked potentials in N-methyl-D-aspartate NR1 hypomorphic mice. Genes Brain Behav 2007, 6: 558–568. [DOI] [PubMed] [Google Scholar]
- 62.Niewoehner B, Single FN, Hvalby O, Jensen V, Meyer zum Alten Borgloh S, Seeburg PH, Rawlins JN et al. . Impaired spatial working memory but spared spatial reference memory following functional loss of NMDA receptors in the dentate gyrus. Eur J Neurosci 2007, 25: 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB et al. . Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 2007, 317: 94–99. [DOI] [PubMed] [Google Scholar]
- 64.Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A et al. . Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 2002, 297: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci 2004, 5: 361–372. [DOI] [PubMed] [Google Scholar]
- 66.Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron 2003, 38: 305–315. [DOI] [PubMed] [Google Scholar]
- 67.Rajji T, Chapman D, Eichenbaum H, Greene R. The role of CA3 hippocampal NMDA receptors in paired associate learning. J Neurosci 2006, 26: 908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Finlay JM, Dunham GA, Isherwood AM, Newton CJ, Nguyen TV, Reppar PC, Snitkovski I et al. . Effects of prefrontal cortex and hippocampal NMDA-NR1 subunit deletion on complex cognitive and social behaviors. Brain Res 2014, 1600: 70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rondi-Reig L, Libbey M, Eichenbaum H, Tonegawa S. CA1-specific N-methyl-D-aspartate receptor knockout mice are deficient in solving a nonspatial transverse patterning task. Proc Natl Acad Sci USA 2001, 98: 3543–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 1996, 87: 1327–1338. [DOI] [PubMed] [Google Scholar]
- 71.Huerta PT, Sun LD, Wilson MA, Tonegawa S. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron 2000, 25: 473–480. [DOI] [PubMed] [Google Scholar]
- 72.Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science 2000, 290: 1170–1174. [DOI] [PubMed] [Google Scholar]
- 73.Cheli V, Adrover M, Blanco C, Ferrari C, Cornea A, Pitossi F, Epstein AL et al. . Knocking-down the NMDAR1 subunit in a limited amount of neurons in the rat hippocampus impairs learning. J Neurochem 2006, 97(Suppl. 1): 68–73. [DOI] [PubMed] [Google Scholar]
- 74.Cui Z, Wang H, Tan Y, Zaia KA, Zhang S, Tsien JZ. Inducible and reversible NR1 knockout reveals crucial role of the NMDA receptor in preserving remote memories in the brain. Neuron 2004, 41: 781–793. [DOI] [PubMed] [Google Scholar]
- 75.Cui Z, Lindl KA, Mei B, Zhang S, Tsien JZ. Requirement of NMDA receptor reactivation for consolidation and storage of nondeclarative taste memory revealed by inducible NR1 knockout. Eur J Neurosci 2005, 22: 755–763. [DOI] [PubMed] [Google Scholar]
- 76.Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G et al. . Genetic enhancement of learning and memory in mice. Nature 1999, 401: 63–69. [DOI] [PubMed] [Google Scholar]
- 77.Glass MJ, Hegarty DM, Oselkin M, Quimson L, South SM, Xu Q, Pickel VM et al. . Conditional deletion of the NMDA-NR1 receptor subunit gene in the central nucleus of the amygdala inhibits naloxone-induced conditioned place aversion in morphine-dependent mice. Exp Neurol 2008, 213: 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Glass MJ, Robinson DC, Waters E, Pickel VM. Deletion of the NMDA-NR1 receptor subunit gene in the mouse nucleus accumbens attenuates apomorphine-induced dopamine D1 receptor trafficking and acoustic startle behavior. Synapse 2013, 67: 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Billingslea EN, Tatard-Leitman VM, Anguiano J, Jutzeler CR, Suh J, Saunders JA, Morita S et al. . Parvalbumin cell ablation of NMDA-R1 causes increased resting network excitability with associated social and self-care deficits. Neuropsychopharmacology 2014, 39: 1603–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM et al. . Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci 2010, 13: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pozzi L, Dorocic IP, Wang X, Carlen M, Meletis K. Mice lacking NMDA receptors in parvalbumin neurons display normal depression-related behavior and response to antidepressant action of NMDAR antagonists. PLoS One 2014, 9: e83879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gandal MJ, Sisti J, Klook K, Ortinski PI, Leitman V, Liang Y, Thieu T et al. . GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Transl Psychiatry 2012, 2: e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bodarky CL, Halene TB, Ehrlichman RS, Banerjee A, Ray R, Hahn CG, Jonak G et al. . Novel environment and GABA agonists alter event-related potentials in N-methyl-D-aspartate NR1 hypomorphic and wild-type mice. J Pharmacol Exp Ther 2009, 331: 308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Single FN, Rozov A, Burnashev N, Zimmermann F, Hanley DF, Forrest D, Curran T et al. . Dysfunctions in mice by NMDA receptor point mutations NR1(N598Q) and NR1(N598R). J Neurosci 2000, 20: 2558–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kew JN, Koester A, Moreau JL, Jenck F, Ouagazzal AM, Mutel V, Richards JG et al. . Functional consequences of reduction in NMDA receptor glycine affinity in mice carrying targeted point mutations in the glycine binding site. J Neurosci 2000, 20: 4037–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Labrie V, Lipina T, Roder JC. Mice with reduced NMDA receptor glycine affinity model some of the negative and cognitive symptoms of schizophrenia. Psychopharmacology (Berl) 2008, 200: 217–230. [DOI] [PubMed] [Google Scholar]
- 87.Labrie V, Clapcote SJ, Roder JC. Mutant mice with reduced NMDA-NR1 glycine affinity or lack of D-amino acid oxidase function exhibit altered anxiety-like behaviors. Pharmacol Biochem Behav 2009, 91: 610–620. [DOI] [PubMed] [Google Scholar]
- 88.Ballard TM, Pauly-Evers M, Higgins GA, Ouagazzal AM, Mutel V, Borroni E, Kemp JA et al. . Severe impairment of NMDA receptor function in mice carrying targeted point mutations in the glycine binding site results in drug-resistant nonhabituating hyperactivity. J Neurosci 2002, 22: 6713–6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Milenkovic M, Mielnik CA, Ramsey AJ. NMDA receptor-deficient mice display sexual dimorphism in the onset and severity of behavioural abnormalities. Genes Brain Behav 2014, 13: 850–862. [DOI] [PubMed] [Google Scholar]
- 90.Bickel S, Lipp HP, Umbricht D. Early auditory sensory processing deficits in mouse mutants with reduced NMDA receptor function. Neuropsychopharmacology 2008, 33: 1680–1689. [DOI] [PubMed] [Google Scholar]
- 91.Boutros NN, Belger A, Campbell D, D'Souza C, Krystal J. Comparison of four components of sensory gating in schizophrenia and normal subjects: a preliminary report. Psychiatry Res 1999, 88: 119–130. [DOI] [PubMed] [Google Scholar]
- 92.Featherstone RE, Shin R, Kogan JH, Liang Y, Matsumoto M, Siegel SJ. Mice with subtle reduction of NMDA NR1 receptor subunit expression have a selective decrease in mismatch negativity: implications for schizophrenia prodromal population. Neurobiol Dis 2014, 73C: 289–295. [DOI] [PubMed] [Google Scholar]
- 93.Inta D, Monyer H, Sprengel R, Meyer-Lindenberg A, Gass P. Mice with genetically altered glutamate receptors as models of schizophrenia: a comprehensive review. Neurosci Biobehav Rev 2010, 34: 285–294. [DOI] [PubMed] [Google Scholar]
- 94.Jiang Z, Rompala GR, Zhang S, Cowell RM, Nakazawa K. Social isolation exacerbates schizophrenia-like phenotypes via oxidative stress in cortical interneurons. Biol Psychiatry 2013, 73: 1024–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Farber NB. The NMDA receptor hypofunction model of psychosis. Ann N Y Acad Sci 2003, 1003: 119–130. [DOI] [PubMed] [Google Scholar]
- 96.Labrie V, Wang W, Barger SW, Baker GB, Roder JC. Genetic loss of D-amino acid oxidase activity reverses schizophrenia-like phenotypes in mice. Genes Brain Behav 2010, 9: 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, Tanzi RE et al. . Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet 2008, 40: 827–834. [DOI] [PubMed] [Google Scholar]
- 98.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 2005, 10: 40–68; Image 45. [DOI] [PubMed] [Google Scholar]
- 99.Papaleo F, Lipska BK, Weinberger DR. Mouse models of genetic effects on cognition: relevance to schizophrenia. Neuropharmacology 2012, 62: 1204–1220. [DOI] [PubMed] [Google Scholar]
- 100.Laruelle M. Schizophrenia: from dopaminergic to glutamatergic interventions. Curr Opin Pharmacol 2014, 14: 97–102. [DOI] [PubMed] [Google Scholar]
- 101.Pei L, Lee FJ, Moszczynska A, Vukusic B, Liu F. Regulation of dopamine D1 receptor function by physical interaction with the NMDA receptors. J Neurosci 2004, 24: 1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fiorentini C, Gardoni F, Spano P, Di Luca M, Missale C. Regulation of dopamine D1 receptor trafficking and desensitization by oligomerization with glutamate N-methyl-D-aspartate receptors. J Biol Chem 2003, 278: 20196–20202. [DOI] [PubMed] [Google Scholar]
- 103.Ferris MJ, Milenkovic M, Liu S, Mielnik CA, Beerepoot P, John CE, Espana RA et al. . Sustained N-methyl-d-aspartate receptor hypofunction remodels the dopamine system and impairs phasic signaling. Eur J Neurosci 2014, 40: 2255–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M et al. . Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci USA 2009, 106: 7281–7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Komendantov AO, Komendantova OG, Johnson SW, Canavier CC. A modeling study suggests complementary roles for GABAA and NMDA receptors and the SK channel in regulating the firing pattern in midbrain dopamine neurons. J Neurophysiol 2004, 91: 346–357. [DOI] [PubMed] [Google Scholar]
- 106.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci 2008, 31: 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Williams NM, O'Donovan MC, Owen MJ. Is the dysbindin gene (DTNBP1) a susceptibility gene for schizophrenia? Schizophr Bull 2005, 31: 800–805. [DOI] [PubMed] [Google Scholar]
- 108.Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci 2011, 12: 707–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ghiani CA, Dell'Angelica EC. Dysbindin-containing complexes and their proposed functions in brain: from zero to (too) many in a decade. ASN Neuro 2011, 3: e00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Papaleo F, Weinberger DR. Dysbindin and Schizophrenia: it's dopamine and glutamate all over again. Biol Psychiatry 2011, 69: 2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karlsgodt KH, Robleto K, Trantham-Davidson H, Jairl C, Cannon TD, Lavin A, Jentsch JD. Reduced dysbindin expression mediates N-methyl-D-aspartate receptor hypofunction and impaired working memory performance. Biol Psychiatry 2011, 69: 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Larimore J, Zlatic SA, Gokhale A, Tornieri K, Singleton KS, Mullin AP, Tang J et al. . Mutations in the BLOC-1 subunits dysbindin and muted generate divergent and dosage-dependent phenotypes. J Biol Chem 2014, 289: 14291–14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jeans A, Malins R, Padamsey Z, Reinhart M, Emptage N. Increased expression of dysbindin-1A leads to a selective deficit in NMDA receptor signaling in the hippocampus. Neuropharmacology 2011, 61: 1345–1353. [DOI] [PubMed] [Google Scholar]
- 114.Ji B, Higa KK, Kim M, Zhou L, Young JW, Geyer MA, Zhou X. Inhibition of protein translation by the DISC1-Boymaw fusion gene from a Scottish family with major psychiatric disorders. Hum Mol Genet 2014, 23: 5683–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ramsey AJ, Milenkovic M, Oliveira AF, Escobedo-Lozoya Y, Seshadri S, Salahpour A, Sawa A et al. . Impaired NMDA receptor transmission alters striatal synapses and DISC1 protein in an age-dependent manner. Proc Natl Acad Sci USA 2011, 108: 5795–5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin H, Hsu FC, Baumann BH, Coulter DA, Anderson SA, Lynch DR. Cortical parvalbumin GABAergic deficits with alpha7 nicotinic acetylcholine receptor deletion: implications for schizophrenia. Mol Cell Neurosci 2014, 61: 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin H, Hsu FC, Baumann BH, Coulter DA, Lynch DR. Cortical synaptic NMDA receptor deficits in alpha7 nicotinic acetylcholine receptor gene deletion models: implications for neuropsychiatric diseases. Neurobiol Dis 2014, 63: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Berretta S. Extracellular matrix abnormalities in schizophrenia. Neuropharmacology 2012, 62: 1584–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van den Buuse M, Halley P, Hill R, Labots M, Martin S. Altered N-methyl-D-aspartate receptor function in Reelin heterozygous mice: male-female differences and comparison with dopaminergic activity. Prog Neuropsychopharmacol Biol Psychiatry 2012, 37: 237–246. [DOI] [PubMed] [Google Scholar]
- 120.Yabuki Y, Nakagawasai O, Moriguchi S, Shioda N, Onogi H, Tan-No K, Tadano T et al. . Decreased CaMK II and PKC activities in specific brain regions are associated with cognitive impairment in neonatal ventral hippocampus-lesioned rats. Neuroscience 2013, 234: 103–115. [DOI] [PubMed] [Google Scholar]
- 121.Mouri A, Noda Y, Noda A, Nakamura T, Tokura T, Yura Y, Nitta A et al. . Involvement of a dysfunctional dopamine-D1/N-methyl-d-aspartate-NR1 and Ca2+/calmodulin-dependent protein kinase II pathway in the impairment of latent learning in a model of schizophrenia induced by phencyclidine. Mol Pharmacol 2007, 71: 1598–1609. [DOI] [PubMed] [Google Scholar]
- 122.Wesseling H, Guest PC, Lee CM, Wong EH, Rahmoune H, Bahn S. Integrative proteomic analysis of the NMDA NR1 knockdown mouse model reveals effects on central and peripheral pathways associated with schizophrenia and autism spectrum disorders. Mol Autism 2014, 5: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oyagi A, Oida Y, Kakefuda K, Shimazawa M, Shioda N, Moriguchi S, Kitaichi K et al. . Generation and characterization of conditional heparin-binding EGF-like growth factor knockout mice. PLoS One 2009, 4: e7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pinacho R, Villalmanzo N, Roca M, Iniesta R, Monje A, Haro JM, Meana JJ et al. . Analysis of Sp transcription factors in the postmortem brain of chronic schizophrenia: a pilot study of relationship to negative symptoms. J Psychiatr Res 2013, 47: 926–934. [DOI] [PubMed] [Google Scholar]
- 125.Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y et al. . Modelling schizophrenia using human induced pluripotent stem cells. Nature 2011, 473: 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brennand K, Savas JN, Kim Y, Tran N, Simone A, Hashimoto-Torii K, Beaumont KG et al. . Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Mol Psychiatry 2015, 20: 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bennett M. Positive and negative symptoms in schizophrenia: the NMDA receptor hypofunction hypothesis, neuregulin/ErbB4 and synapse regression. Aust N Z J Psychiatry 2009, 43: 711–721. [DOI] [PubMed] [Google Scholar]
- 128.Goff DC, Tsai G, Manoach DS, Coyle JT. Dose-finding trial of D-cycloserine added to neuroleptics for negative symptoms in schizophrenia. Am J Psychiatry 1995, 152: 1213–1215. [DOI] [PubMed] [Google Scholar]
- 129.Javitt DC, Zylberman I, Zukin SR, Heresco-Levy U, Lindenmayer JP. Amelioration of negative symptoms in schizophrenia by glycine. Am J Psychiatry 1994, 151: 1234–1236. [DOI] [PubMed] [Google Scholar]
- 130.Tsai G, Yang P, Chung LC, Lange N, Coyle JT. D-serine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry 1998, 44: 1081–1089. [DOI] [PubMed] [Google Scholar]