Abstract
Grainyhead genes are involved in wound healing and developmental neural tube closure. Metastasis is a multistep process during which cancer cells disseminate from the site of primary tumors and establish secondary tumors in distant organs. The adhesion protein E-cadherin plays an essential role in metastasis. In light of the high degree of similarity between the epithelial–mesenchymal transition (EMT) occurring in wound-healing processes and the EMT occurring during the acquisition of invasiveness in skin or breast cancer, we investigated the role of the Grainyhead genes in cancer invasion. Here, we show that there is an inverse relationship between Grainyhead-like 3 (Grhl3) and E-cadherin expression in some epithelial tumor cell lines. Overexpression of Grhl3 in the E-cadherin-positive epithelial tumor cell line, characterized by less invasiveness, generated a transcriptional blockage of the E-cadherin gene and promoted cell migration and cell invasion. Conversely, Grhl3 depletion inhibited cell migration and cell invasion and was associated with a gain of E-cadherin expression. To further explore the mechanism by which Grhl3 regulated E-cadherin expression, an E-cadherin promoter report analysis was performed and results showed that Grhl3 repressed E-cadherin gene expression by directly or indirectly binding to the E-boxes present in the proximal E-cadherin promoter. Taken together, our findings define a major role for Grhl3 in the induction of migration and invasion by the downregulation of E-cadherin in cancer cells.
Keywords: Grhl3, migration, invasion, E-cadherin, skin cancer, breast cancer
Introduction
Grainyhead family factors have been shown to play important roles in wound healing, epidermal formation, and the mechanistically related process of embryonic neural tube closure [1–5]. Among these proteins, Grainyhead-like 3 (Grhl3, also known as Get1), a transcription factor expressed in the differentiated suprabasal layers, plays important roles in epidermal differentiation and barrier formation by controlling multiple genes that regulate the differentiation program in skin [2,6]. These proteins also determine some structural proteins, enzymes, and intercellular adhesion molecules. Grhl3 was recently shown to suppress squamous cell carcinoma due to its activation of phosphatase and tensin homolog (PTEN) expression [7], and previous studies have shown that Grhl3 plays important roles in epithelial migration during early embryogenesis [8–10]. Mammalian eyelid development and fusion follows a stereotypical stepwise pathway: first, the primitive eyelid root forms, then, a leading edge of keratinocytes extends centripetally from the rim of the eyelid and ultimately covers the eye, depending on the migration of the keratinocyte sheet [8]. The eye-open phenotype appears in the Grhl3−/− mice because of a defect in the migration of the epithelial sheet. This process is also similar to the upregulation of Grhl3 in wound-front keratinocytes in adult skin.
It has been proposed that the movement and migration of the epithelial cells can be greatly limited by some cellular contacts [11]. E-cadherin, an essential molecule for the formation of adhesion junction, is critical to form the intercellular contacts and frequently downregulated in the later stage of tumorigenesis. Some previous studies indicated that Grainyhead family factors are the activators of E-cadherin in the physiological context [1,5,12–14]. Since Grhl3 also represses the expression of some target genes [6], we hypothesized that Grhl3 may regulate cancer cell migration and invasion by the downregulation of E-cadherin.
In this study, we investigated the effects of Grhl3 in cancer invasion, and showed the inverse relationship between Grhl3 and E-cadherin expression in some epithelial tumor cell lines, which indicated that Grhl3 may play a major role in the induction of migration and invasion by the downregulation of E-cadherin in cancer cells.
Materials and Methods
Cell culture and generation of stable cell lines
A human immortalized keratinocyte cell line (HaCat), a human skin squamous carcinoma cell line (A431) and a human breast cancer cell line (MCF7) with low invasive abilities were grown in high-glucose Dulbecco's modified Eagle's medium (Hyclone, Logan, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone), 100 U/ml penicillin and 100 µg/ml streptomycin. A human breast cancer cell line (MDA-MB-231) with a high invasive ability was cultured in RPMI-1640 medium (Hyclone) supplemented with 10% FBS and 1% antibiotic/antimycotic. Each cell line was maintained at 37°C in a humidified atmosphere with 5% CO2.
Human Grhl3 cDNA was amplified from the human cDNA library using polymerase chain reaction (PCR) and subcloned into pCMV-2B-Vector (Promega, Madison, USA) at the BamHI site by using standard molecular biological methods. The primers for PCR are listed in Supplementary Table S1. The pGV287 vectors containing Grhl3 short hairpin RNA or scrambled control short hairpin RNA (Con-shRNA) were purchased from Genechem Company (Catalog number: grhl3-shRNA 229/65; 229/66; 229/67; 229/68; Shanghai, China). The sequences are listed in Supplementary Table S2. The vectors were transfected into 293T, A431, MCF7 and MAD-MB-231 cells using Lipofectamine 2000 (Invitrogen, Grand Island, USA). Stable transfectants were generated after selection with 400 µg/ml G418 (for A431 cells and MCF7 cells) or with 1 µg/ml puromycin (for MDA-MB-231 cells) for 3–4 weeks, followed by western blot analysis to verify expression.
Western blotting
Western blotting was performed as described previously [15]. Primary antibodies were typically incubated for 2 h up to overnight, and secondary antibodies were incubated for 1 h. The primary antibodies used were rabbit anti-E-cadherin antibody (Cell Signaling Technologies, Danvers, USA), mouse anti-GAPDH antibody (Abcam, Cambridge, USA) and rabbit anti-GRHL3 antibody (Santa Cruz Biotech, Santa Cruz, USA). Secondary antibodies for chemiluminescence detection were either anti-mouse or anti-rabbit immunoglobulin G (IgG) conjugated to horseradish peroxidase (Santa Cruz Biotech) and were used at a 1 : 3000 dilution. Quantitative western blot analysis was performed using Image-Pro.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS) for 10 min. PFA was quenched with 100 mM glycine in PBS. Cells were permeabilized with 0.2% Triton X-100 in PBS at 4°C for 10 min, washed twice with PBS, and blocked for 1 h in PBS with 10% horse serum, 0.1% Tween-20, and 0.1% bovine serum albumin. Primary and secondary antibodies were diluted in blocking buffer. Primary antibodies used were as follows: rabbit anti-E-cadherin antibodies and rabbit anti-Grhl3 antibodies (Cell Signaling Technologies). The secondary antibodies used were anti-rabbit fluorescein isothiocyanate and anti-rabbit Rhodamine (Cell Signaling Technologies). Images were captured with an OPTIKA XDS-3FL4 microscope equipped with an OPTIKA M PRO 5 camera, and analyzed with OPTIKA Vision Lite software (OPTIKA SRL, Ponteranica, Italy).
In vitro migration and invasion assay
Cell migration analysis was performed using a wound-healing assay or the motility assay with Boyden chamber (BD Biosciences, Bedford, USA). For the wound-healing assay, a single scratch wound was created using a p10 micropipette tip in a plate of confluent cells. Cells were washed twice with PBS to remove cell debris, supplemented with regular growth medium, and monitored. Images were captured by phase-contrast microscopy at 0, 24, and 48 h after wounding. The cell migration area (µm2) was measured using Wimasis automated image analysis modules (http://www.wimasis.com). For the Boyden chamber motility assay, 5 × 103 cells were seeded onto the porous membrane in the upper chamber. The top chambers were filled with serum-free medium, and the bottom chambers were filled with medium containing 20% FBS as a chemoattractant. After 16 h of incubation, the number of cells that had migrated to the bottom surfaces of the membranes was counted after staining with Giemsa's solution.
Invasion assay was performed using Matrigel-coated Boyden chamber (BD Biosciences). Cells were serum-starved overnight, and then, 5 × 104 cells were plated into a Matrigel-coated well under the described conditions. After 24 or 48 h, the nonmigrating cells on the upper chamber were removed and the inserts were fixed and stained with Giemsa's solution. Images were captured, and the degree of invasion was determined by the average number of invaded cells in five fields (×100).
Luciferase reporter assays
The E-cadherin promoter fragment (–178 to +92) and various E-box mutant forms were cloned into the pGL3 vector (Addgene, Cambridge, USA). The E-box sequences of 5′-CACCTG-3′ at −24 and 5′-CAGGTG-3′ at −74 were mutated to AACCTA and AAGGTA, respectively. And the third E-box sequence of 5′-CACCTG-3′ sequence located downstream of the transcription start site (at positions +22 to +27) was deleted. Human 293T cells were transiently transfected using Lipofectamine 2000 at a ratio of 1 µg DNA : 2 µl Lipofectamine, according to the manufacturer's instructions. A total of 1.5 µg of DNA per well of a 12-well plate was the maximum amount of DNA found to be tolerable. Firefly luciferase (Luc) and Renilla reniformis luciferase (Rluc) activities were measured using the Dual Luciferase Reporter Assay System (Promega), according to the manufacturer's instructions. Luc activity was always normalized to Rluc activity. In all experiments, the total amount of DNA transfected was standardized with empty pCMV-2B-Vector.
Chromatin immunoprecipitation and quantitative PCR analysis
Chromatin immunoprecipitation (ChIP) was performed with the ChIP Kit (Cell Signaling Technologies), according to the manufacturer's protocol. Briefly, 4 × 107 A431-Grhl3 cells were treated with 1% formaldehyde at room temperature for 10 min, followed by quenching with 0.125 M glycine. Cells were lysed, and the nuclei were sonicated under conditions yielding DNA fragments ranging from 200 to 800 bp. Then 5% of the sonicated lysate was saved as whole-cell extract. Sonicated lysate was divided into three equal volumes and immunoprecipitated with a specific or nonspecific antibody bound to protein A beads, overnight at 4°C with rocking. The antibodies used were rabbit anti-Flag antibody (Sigma, St Louis, USA) or rabbit nonimmune IgG (Cell Signaling Technologies). Five micrograms of antibody were used per 1 × 107 cells. Immunoprecipitated complexes were collected, washed, and eluted using centrifugation at 15,600 g for 30 s. Eluted DNA and whole-cell extracts were incubated at 65°C in a rotating incubator for 8 h to reverse cross-links. DNA samples were sequentially purified using spin columns supplied with the ChIP Kit. ChIP-derived DNA was analyzed by quantitative PCR (ChIP-qPCR). The primers used for ChIP-qPCR were listed in Supplementary Table S1. The ChIP-qPCR conditions were as follows: 1 cycle of 95°C for 10 min, followed by 40 cycles of 95°C for 20 s, 58°C for 30 s, and 60°C for 1 min. The melting curves were conducted to detect the specificity of PCR amplification. Enrichment was calculated as a percentage of input sample compared with an IgG control IP and normalized to a control genomic region.
Statistical analysis
For in vitro migration and invasion assay, the number of cells in the wells was calculated using the means from five randomly selected fields. Experiments were performed in triplicate and repeated three times. The statistical significance of cell migration was analyzed using a two-tailed Student's t-test. The differences of the protein expressions in tumor tissue specimens were calculated using the two-sample t-test. The results were presented as the mean ± SD of three or more experiments. P value of <0.05 was considered of statistically significant difference.
Results
Grhl3 expression inversely correlates with E-cadherin expression
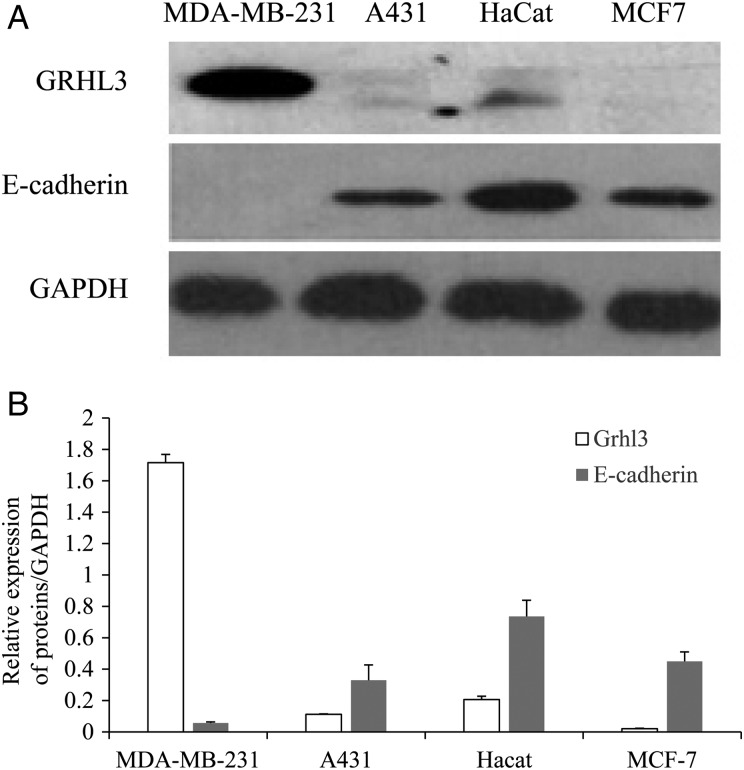
To investigate the relationship between Grhl3 and E-cadherin, the expressions of Grhl3 and E-cadherin were analyzed in human epithelial cell lines by western blotting. The higher expression of E-cadherin protein in most of these cell lines is associated with relatively lower level of Grhl3. And Grhl3 is dominantly expressed in the E-cadherin-negative epithelial tumor cell line, MDA-MB-231, a highly metastatic mammary carcinoma cell line (Fig. 1A). Based on this inverse relationship between Grhl3 and E-cadherin (Fig. 1B), the mechanism by which Grhl3 regulates E-cadherin expression was further explored.
Figure 1.
Grhl3 expression inversely correlates with expression of E-cadherin (A) Detection of Grhl3 and E-cadherin expression in MDA-MB-231, A431, HaCat, and MCF7 cells using western blot analysis. (B) Relative expression of proteins. Data were presented as the mean ± SD of triplicate independently performed experiments.
Exogenous modulation of Grhl3 level alters E-cadherin expression
To investigate the ability of Grhl3 in regulating E-cadherin expression in cancer cells, less invasive cell lines that express higher level of E-cadherin, including A431 and MCF7, were used. An expression vector containing Flag tag fused to the N-terminal of human Grhl3 cDNA was generated and overexpressed in both MCF7 and A431 cell lines. The expression of Grhl3 in stable transfectants was validated by immunoblot analysis using the anti-Grhl3 antibodies or anti-Flag antibodies. The anti-Flag antibody recognizes a specific band of ∼70 kDa in the Grhl3-transfected cells, but not in the vector-transfected cells. Grhl3 overexpression in A431-Grhl3 and MCF7-Grhl3 cells was also observed using the anti-Grhl3 antibodies (Fig. 2A). In Grhl3-overexpressing A431-Grhl3 cells, the expression of E-cadherin was undetectable by western blot analysis (Fig. 2B). Additionally, E-cadherin protein was totally or substantially reduced in MCF7-Grhl3 cells (Fig. 2B). Furthermore, immunofluorescence analysis revealed that E-cadherin staining was restricted to the MCF7-vector cells and completely disappeared in the MCF7-Grhl3 cells (Fig. 2C). In contrast, control cells exhibited a high level of E-cadherin. These effects were also observed in the A431 cell line (Supplementary Fig. S1).
Figure 2.
Overexpression of Grhl3 downregulates E-cadherin expression (A) Western blot analysis. Left: individual cell clones were isolated after selection and were analyzed by western blotting for the expression of the Flag-tagged transgene. Right: immunoblotting analysis of Grhl3 and E-cadherin expression in A431 and MCF7 transfected by pCMV-2B-Vector or pCMV-2B-Grhl3 (A431-Vector/-Grhl3 and MCF7-Vector/-Grhl3). (B) Relative expression of proteins. Data were presented as the mean ± SD of triplicate experiments (**P< 0.01 vs. controls). (C) Immunofluorescence detection of Grhl3 and E-cadherin expression in MCF7 cells (magnification, ×100).
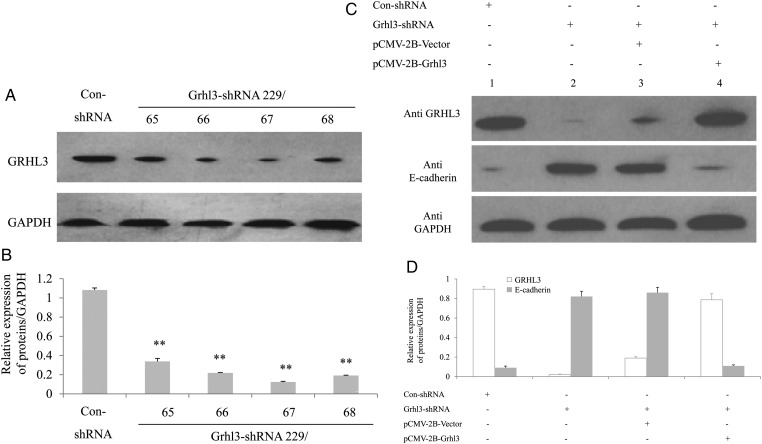
To determine whether knockdown of Grhl3 affects the expression of E-cadherin in the invasive MDA-MB-231 cells, recombinant GV287 construct with a GRHL3 short hairpin RNA cassette (Grhl3-shRNA 229/65, 66, 67, or 68) or a scrambled control short hairpin RNA cassette (Con-shRNA) was transfected into 293T cells. Western blot analysis showed that the expression of Grhl3 was significantly reduced when compared with the control (Fig. 3A,B). Stable transfectants of MDA-MB-231 were generated using these two constructs (Grhl3-shRNA and Con-shRNA). Grhl3 knockdown significantly increased the expression of E-cadherin in MDA-MB-231 cells, and Grhl3 expression could be rescued and E-cadherin expression could also be inversely decreased after transiently transfected pCMV-2B-Grhl3 into the stable transfectants (Fig. 3C,D).
Figure 3.
Knockdown of Grhl3 upregulates E-cadherin expression (A) Immunoblotting analysis of Grhl3 expression in 293T cells transiently transfected with shRNA for Grhl3 to detect interfering efficiency. (B) Relative expression of proteins in MDA-MB-231 cells. Data were presented as the mean ± SD of triplicate experiments (**P< 0.01 vs. Con-shRNA). (C) Immunoblotting analysis of Grhl3 and E-cadherin expression in MDA-MB-231 cells following stable transfection with shRNA for Grhl3. Lane 1, stable transfectant with Con-shRNA; Lane 2, stable transfectant with Grhl3-shRNA; Lane 3, stable transfectant with Grhl3-shRNA following transiently transfected by pCMV-2B-Vector; Lane 4, stable transfectant with Grhl3-shRNA following transiently transfected with pCMV-2B-Grhl3. (D) Relative expression of proteins in MDA-MB-231 cells. Data were presented as the mean ± SD of triplicate experiments.
Grhl3 overexpression promotes motility and invasion of cancer cells
Wound-healing assay was performed to qualitatively determine the effect of Grhl3 on cell motility. The scratch was made in cell culture at a 90% confluence. After 24 or 48 h, an increase in the number of cells that moved into the scratch wound was observed. Compared with the controls, Grhl3-transfected A431 cells or MCF7 cells showed a significant (P< 0.01) increase in motility (Fig. 4A,B). To investigate the invasive ability of Grhl3-transfected cells, an in vitro transwell invasion assay was performed. The Grhl3-transfected cells, including A431 cells and MCF7 cells, showed a significant (P< 0.05) increase in the number of invading cells compared with the controls (Fig. 4C).
Figure 4.
Exogenous modulation of Grhl3 level alters cell migration and invasion (A) Wound-healing assay was performed to visualize the differences in motility of Grhl3- and Vector-transfected A431 cells or MCF7 cells. (B) Grhl3 promotes cell migration. A431 cells and MCF7 cells overexpressing Grhl3 were induced to migrate to the scratch wound for 24 and 48 h. The migration examined is shown as a percentage of wound closure from 0 h. Data were presented as the mean ± SD of triplicate experiments (**P< 0.01 vs. A431-Vector). (C) Grhl3 promotes cell invasion. A Matrigel-coated Boyden chamber invasion assay was performed for A431 cells and MCF7 cells transfected with either Grhl3 or empty vector. The invasive ability is presented as the average number of invading cells per field. Data were presented as the mean ± SD of triplicate experiments (*P< 0.05 vs. A431-Vector). (D) Knockdown of Grhl3 inhibits cell migration. A Boyden chamber motility assay was performed for MDA-MB-231 cells transfected with either Grhl3-shRNA or Con-shRNA. The migration ability is presented as the average number of migrating cells per field. Data were presented as the mean ± SD of triplicate experiments (**P < 0.01 vs. Con-shRNA). (E) Knockdown of Grhl3 inhibits cell invasion. A Matrigel-coated Boyden chamber invasion assay was used to detect the number of invading cells per field. Columns indicate the number of invading cells. Data were presented as the mean ± SD of triplicate experiments (***P< 0.001 vs. Con-shRNA).
To further detect whether knockdown of Grhl3 inhibits cell migration or cell invasion, the Boyden chamber motility assay and transwell invasion assay were performed with MDA-MB-231 cells. The number of migrating cells (P < 0.01) or invading cells (P < 0.001) was significantly decreased in cells transfected with Grhl3-shRNA compared with cells transfected with Con-shRNA (Fig. 4D,E).
Grhl3 represses the activity of the E-cadherin proximal promoter
To determine the mechanism of E-cadherin repression by Grhl3, and in particular, whether it interacts directly with a specific region of the E-cadherin promoter, the ability of this transcription factor to interfere with the function of the −178 to +92 fragment of the human E-cadherin promoter was first analyzed. It has been reported that this proximal promoter fragment confers specific epithelial expression to a reporter gene [16,17]. We transiently transfected 293T cells with a reporter construct containing the luciferase gene under the control of the −178 to +92 fragment from the human E-cadherin promoter. Luc activity was efficiently repressed when cotransfected with the Grhl3 expression vector (Fig. 5A, P < 0.001). A similar degree of repression of Luc activity was observed in A431 cells that have high E-cadherin expression (data not shown). To further define the elements inside the human E-cadherin promoter that are involved in this repression, the −178 to +92 fragment was analyzed for the presence of putative Grhl3-binding sites. There were three consensus sequence repeats (CACCTG) previously described as E-boxes. The abilities of Grhl3 to repress different reporter constructs carrying combinations of mutated putative Grhl3-binding sites were compared (Fig. 5B, P < 0.001). The results from these experiments prompt us to conclude that the three boxes cooperate in the Grhl3-mediated E-cadherin repression and that the furthest downstream sequence (E-box 3) has the strongest repressive activity.
Figure 5.
Grhl3 represses the E-cadherin gene expression (A) Grhl3 blocks the activity of the human E-cadherin promoter. Human 293T cells were transfected with 50 ng of pGL3 vector containing the Luciferase gene under the control of the −178/+92 fragment of the human E-cadherin promoter [pGL3-E-cad(–178/+92)] plus 5 ng of the control reporter vector (pRLtk) and either pCMV-2B-Grhl3 or empty pCMV-2B-Vector (25, 50, and 100 ng). The Luc activity levels were normalized in all cases by Rluc activity. Data were presented as the mean ± SD of triplicate experiments (***P< 0.001 vs. 0 ng pCMV-2B-Grhl3). (B) Mutation of the E-boxes impairs repression of the E-cadherin promoter by Grhl3. ProE-cad178-luc (–178/+92) (WT) or the same plasmid containing mutations in different E-boxes (MUT) was cotransfected into 293T cells together with pRLtk and with either pCMV-2B-Grhl3 or empty pCMV-2B-Vector (50 ng). Data are expressed as relative luc activity normalized to the Rluc control. Data were presented as the mean ± SD of triplicate experiments (***P< 0.001 vs. 50 ng empty pCMV-2B-Vector). (C) ChIP-qPCR assays. Chromatin from A431-Grhl3 cells was immunoprecipitated with anti-Flag antibody or nonimmune antibody. Primer pairs amplify a region in the promoter of each gene. PCR for E-cadherin promoter is located at position of −78 to +30. The other unrelated DNA regions, such as afp gene promoter (at positions −1700 to −1589) and gapdh gene promoter (at positions −395 to −228), were used to detect nonspecific binding of Grhl3 (n = 3).
To determine whether the E-cadherin promoter is a direct target for repression by Grhl3, ChIP analysis was conducted. Chromatin from A431-Grhl3 was immunoprecipitated with an anti-Flag antibody or nonimmune IgG and then analyzed by qPCR using the indicated ChIP primers. The data showed a strong enrichment of the PCR signal using the anti-Flag antibody compared with nonimmune IgG or primers set that represented an unrelated region of the genome, such as promoter of gapdh (at positions −1700 to −1589) and promoter of afp (at positions −395 to −228) (Fig. 5C). These results indicate that Grhl3 represses E-cadherin expression and interacts with the E-cadherin promoter.
Discussion
Grhl3 regulates many types of terminal differentiation genes that are required for epidermal barrier formation in Drosophila and in mice [2,6,8]. The integral function of Grhl3 in this process may be associated with the maintenance of the balance between keratinocyte differentiation and proliferation. In a recent study, mice with a Grhl3 deletion in keratinocytes were likely to generate SCC [7], and the Grhl3 gene was thus defined as a potent suppressor of SCC in the skin of mammals, acting through the direct transcriptional regulation of PTEN. However, in contrast to in the wild-type mice that were resistant to chemical-induced tumor formation, tumors were easier to be induced in the Grhl3-knockout mice by chemical agents. Therefore, Grhl3 deletion in keratinocytes may increase the possibility of chemical-induced tumor formation by disrupting the balance between differentiation and proliferation and may be important in early tumorigenesis. In breast cancer, previous studies have shown that early stage cancers, especially non-triple-negative breast cancers, have augmented expression of Grhl3, suggesting an association between symmetric division and gain of tumor mass [18,19]. Although Grhl3 expression is associated with long survival in a lymph node positive breast cancer group [19], there is no correlation between Grhl3 expression and survival in all stages. With regard to the early or advanced stage cancer cells, it is still unknown whether Grhl3 regulates cancer migration, invasion, and even metastasis.
Previous studies have shown that Grhl3 plays an important role in wound healing during epidermal barrier formation and that this function is highly conserved in evolution across multiple species. For example, Grainyhead is required for wound healing in Drosophila [20], and a mutation in Grhl3 impairs embryonic wound healing in mice [2]. Some studies demonstrated that Grhl3 mainly plays roles in regulating the differentiation and migration of ectoderm cells, especially epithelial cells. For example, Grhl3−/− mice have eye-open and neural tube closure defects at birth phenotype [3,6]. Therefore, Grhl3 executes the function of endepidermis cells migration, which closes the epithelial defect during the early epithelial closure process. In this regard, its function may be consistent with cancer cell migration, invasion, or both. When comparing the loss of epithelial characteristics during tumorigenesis with the EMT that occurs during development, a common event is the downregulation of E-cadherin. In the current study, we used several cancer cell lines, including skin cancer cells and breast cancer cells that were derived from ectoderm, to investigate the functions of Grhl3. Based on measurements at the protein level, we observed that there was an inverse relationship between Grhl3 and E-cadherin expression in some epithelial tumors. Most importantly, exogenous modulation of Grhl3 levels inversely altered the expression of E-cadherin in epithelial tumor cells. For both A431 cells and MCF7 cells with less mobility, overexpression of Grhl3 significantly increased cell migration and invasion. In contrast, knockdown of Grhl3 in invasive breast cancer cells inhibited cancer cell migration and invasion. Although previous studies have proved that E-cadherin is upregulated by Grhls in nontumorigenic cells, e.g. human immortalization breast cell line (HMLE) [1,12], mouse inner medullar collecting duct (mIMCD-3) [5], primary keratinocytes or human immortalization keratinocytes (HaCat) [13], and nontumorigenic mouse mammary gland cell line (NmuMG) [14], Grhls may play some roles in regulating mesenchymal-epithelial transition under physiological conditions. Though Alotaibi et al. [14] demonstrated that Grhl3 could also activate E-cadherin expression in the hepatoma carcinoma cells derived from mesoderm, it has not been reported the Grhl3 functions during mesoderm development. In fact, Grhl3 can act as an activator or repressor on the target genes [6,21]. Therefore, Grhl3 may play different roles in regulating migration or invasion of epithelial cells derived from the different blastoderms. Cieply et al. [1] reported that MDA-MB-231 cells had a characteristic with gain of Grhl2 and activation of E-cadherin; however, Grhl2 is unable to replace Grhl3 in the establishment of the epidermal barrier during development [3].
It is important to understand the mechanism by which Grhl3 represses E-cadherin expression. Therefore, we tried to analyze the gene regulatory elements in the E-cadherin gene. Previous studies have proved that the proximal E-boxes in the E-cadherin promoter region, as the important cis-acting elements, are involved in transcriptional inhibition in many different cellular contexts [6,22–25]. Luc analysis showed that Luc activity was efficiently repressed when cotransfected with the Grhl3 expression vector. The ChIP-qPCR results further showed that Grhl3 specifically interacted with the E-boxes present in the proximal E-cadherin promoter. As identified by many research groups, Grainyhead transcription factors bind to the specific motif 5′-AACCGGTT-3′ or 5′-AACCTGTT-3′ [3,6] which can downregulate the target gene expression. However, we are unable to demonstrate these binding sites of the Grhl3 in the E-cadherin regulatory elements. Based on the Grhl3-binding site analysis in promoters from upregulated genes in Grhl3−/− epidermis, Yu et al. [6] also found that GRHL3 can bind to another specific motif 5′-AACAGGTT-3′. This finding indicates that Grhl3 may act as a repressor on the target genes when it binds to the sequence of 5′-AACAGGTT-3′. Interestingly, this consensus sequence is similar to the E3-box sequence of 5′-CAGGTG-3′ at positions +22 to +27, which was deleted in this study. So, Grhl3 may directly bind to this motif to repress E-cadherin expression. Additionally, several E-cadherin transcriptional repressors, such as Twist, Zeb1, Snail, Slug, Loxl2, and E47 that directly interact with the proximal E-boxes of the promoter, have been identified in recent years [17,26–29]. Grhl3 may also interact with these factors and bind to the E-boxes, and indirectly repress E-cadherin expression. Therefore, we conclude that the transcription factor Grhl3 expressed by keratinocytes and some epithelial tumor cells may directly or indirectly repress E-cadherin gene expression.
Taken together, the current study addresses the importance of Grhl3 in the downregulation of E-cadherin expression and in cancer cell migration and invasion. To the best of our knowledge, this is the first report demonstrating this novel role of Grhl3 in the induction of migration and invasion in cancer cells.
Supplementary Data
Funding
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 81172591 and 81372911) and the Science & Technology Development Fund of Macao SAR (No. FDCT 025/2014/A1, to L.D.).
Supplementary Material
Acknowledgement
We would like to thank Prof. Jiahuai Han (Xiamen University, Xiamen, China) for providing us with human Grhl3 cDNA.
References
- 1.Cieply B, Farris J, Denvir J, Ford HL, Frisch SM. Suppression of the epithelial–mesenchymal transition by Grainyhead-like-2. Cancer Res 2012, 72: 2440–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ting SB, Caddy J, Hislop N, Wilanowski T, Auden A, Zhao LL, Ellis S et al. . A homolog of Drosophila grainy head is essential for epidermal integrity in mice. Science 2005, 308: 411–413. [DOI] [PubMed] [Google Scholar]
- 3.Boglev Y, Wilanowski T, Caddy J, Parekh V, Auden A, Darido C, Hislop NR et al. . The unique and cooperative roles of the Grainy head-like transcription factors in epidermal development reflect unexpected target gene specificity. Dev Biol 2011, 349: 512–522. [DOI] [PubMed] [Google Scholar]
- 4.Pyrgaki C, Liu A, Niswander L. Grainyhead-like 2 regulates neural tube closure and adhesion molecule expression during neural fold fusion. Dev Biol 2011, 353: 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werth M, Walentin K, Aue A, Schönheit J, Wuebken A, Pode-Shakked N, Vilianovitch L et al. . The transcription factor grainyhead-like 2 regulates the molecular composition of the epithelial apical junctional complex. Development 2010, 137: 3835–3845. [DOI] [PubMed] [Google Scholar]
- 6.Yu Z, Lin KK, Bhandari A, Spencer JA, Xu X, Wang N, Lu Z et al. . The Grainyhead-like epithelial transactivator Get-1/Grhl3 regulates epidermal terminal differentiation and interacts functionally with LMO4. Dev Biol 2006, 299: 122–136. [DOI] [PubMed] [Google Scholar]
- 7.Darido C, Georgy SR, Wilanowski T, Dworkin S, Auden A, Zhao Q, Rank G et al. . Targeting of the tumour suppressor GRHL3 by a miR-21-dependent proto-oncogenic network results in PTEN loss and tumorigenesis. Cancer Cell 2011, 20: 635–648. [DOI] [PubMed] [Google Scholar]
- 8.Yu Z, Bhandari A, Mannik J, Pham T, Xu X, Andersen B. Grainyhead-like factor Get1/Grhl3 regulates formation of the epidermal leading edge during eyelid closure. Dev Biol 2008, 319: 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hopkin AS, Gordon W, Klein RH, Espitia F, Daily K, Zeller M, Baldi P et al. . GRHL3/GET1 and trithorax group members collaborate to activate the epidermal progenitor differentiation program. PLoS Genet 2012, 8: e1002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustavsson P, Greene ND, Lad D, Pauws E, de Castro SC, Stanier P, Copp AJ. Increased expression of Grainyhead-like-3 rescues spina bifida in a folate-resistant mouse model. Hum Mol Genet 2007, 16: 2640–2646. [DOI] [PubMed] [Google Scholar]
- 11.Hennig G, Löwrick O, Birchmeier W, Behrens J. Mechanisms identified in the transcriptional control of epithelial gene expression. J Biol Chem 1996, 271: 595–602. [DOI] [PubMed] [Google Scholar]
- 12.Cieply B, Farris J, Denvir J, Ford HL, Frisch SM. Epithelial-mesenchymal transition and tumor suppression are controlled by a reciprocal feedback loop between ZEB1 and Grainyhead-like-2. Cancer Res 2013, 73: 6299–6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhandari A, Gordon W, Dizon D, Hopkin AS, Gordon E, Yu Z, Andersen B. The Grainyhead transcription factor Grhl3/Get1 suppresses miR-21 expression and tumorigenesis in skin: modulation of the miR-21 target MSH2 by RNA-binding protein DND1. Oncogene 2013, 32: 1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alotaibi H, Basilicata MF, Shehwana H, Kosowan T, Schreck I, Braeutigam C, Konu O et al. . Enhancer cooperativity as a novel mechanism underlying the transcriptional regulation of E-cadherin during mesenchymal to epithelial transition. Biochim Biophys Acta 2015, 1849: 731–742. [DOI] [PubMed] [Google Scholar]
- 15.Zha X, Hu Z, Ji S, Jin F, Jiang K, Li C, Zhao P et al. . NFκB up-regulation of glucose transporter 3 is essential for hyperactive mammalian target of rapamycin-induced aerobicglycolysis and tumor growth. Cancer Lett 2015, 359: 97–106. [DOI] [PubMed] [Google Scholar]
- 16.Giroldi LA, Bringuier PP, de Weijert M, Jansen C, van Bokhoven A, Schalken JA. Role of E boxes in the repression of E-cadherin expression. Biochem Biophys Res Commun 1997, 241: 453–458. [DOI] [PubMed] [Google Scholar]
- 17.Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, García De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000, 2: 84–89. [DOI] [PubMed] [Google Scholar]
- 18.Panis C, Pizzatti L, Herrera AC, Cecchini R, Abdelhay E. Putative circulating markers of the early and advanced stages of breast cancer identified by high-resolution label-free proteomics. Cancer Lett 2013, 330: 57–66. [DOI] [PubMed] [Google Scholar]
- 19.Xu H, Liu C, Zhao Z, Gao N, Chen G, Wang Y, Cui J. Clinical implications of GRHL3 protein expression in breast cancer. Tumour Biol 2014, 35: 1827–1831. [DOI] [PubMed] [Google Scholar]
- 20.Mace KA, Pearson JC, McGinnis W. An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science 2005, 308: 381–385. [DOI] [PubMed] [Google Scholar]
- 21.Kudryavtseva EI, Sugihara TM, Wang N, Lasso RJ, Gudnason JF, Lipkin SM, Andersen B. Identification and characterization of Grainyhead-like epithelial transactivator (GET-1), a novel mammalian Grainyhead-like factor. Dev Dyn 2003, 226: 604–617. [DOI] [PubMed] [Google Scholar]
- 22.Bolós V, Peinado H, Pérez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci 2003, 116: 499–511. [DOI] [PubMed] [Google Scholar]
- 23.Hennig G, Behrens J, Truss M, Frisch S, Reichmann E, Birchmeier W. Progression of carcinoma cells is associated with alterations in chromatin structure and factor binding at the E-cadherin promoter in vivo. Oncogene 1995, 11: 475–484. [PubMed] [Google Scholar]
- 24.Faraldo ML, Rodrigo I, Behrens J, Birchmeier W, Cano A. Analysis of the E-cadherin and P-cadherin promoters in murine keratinocyte cell lines from different stages of mouse skin carcinogenesis. Mol Carcinog 1997, 20: 33–47. [DOI] [PubMed] [Google Scholar]
- 25.Hajra KM, Ji X, Fearon ER. Extinction of E-cadherin expression in breast cancer via a dominant repression pathway acting on proximal promoter elements. Oncogene 1999, 18: 7274–7279. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigo I, Cato AC, Cano A. Regulation of E-cadherin gene expression during tumour progression: the role of a new Ets-binding site and the E-pal element. Exp Cell Res 1999, 248: 358–371. [DOI] [PubMed] [Google Scholar]
- 27.Canesin G, Cuevas EP, Santos V, López-Menéndez C, Moreno-Bueno G, Huang Y, Csiszar K et al. . Lysyl oxidase-like 2 (LOXL2) and E47 EMT factor: novel partners in E-cadherin repression and early metastasis colonization. Oncogene 2014, 23: 1–14. [DOI] [PubMed] [Google Scholar]
- 28.Villarejo A, Cortés-Cabrera A, Molina-Ortíz P, Portillo F, Cano A. Differential role of Snail1 and Snail2 zinc fingers in E-cadherin repression and epithelial to mesenchymal transition. J Biol Chem 2014, 289: 930–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vannier C, Mock K, Brabletz T, Driever W. Zeb1 regulates E-cadherin and Epcam (epithelial cell adhesion molecule) expression to control cell behavior in early zebrafish development. J Biol Chem 2013, 288: 18643–18659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.