Abstract
1,25-dihydroxyvitamin D3 (VD3), an active form of Vitamin D, is photosynthesized in the skin of vertebrates in response to solar ultraviolet B radiation (UV-B). VD3 deficiency can cause health problems such as immune disease, metabolic disease, and bone disorders. It has also been demonstrated that VD3 is involved in reproductive functions. Female sex hormones such as estrogen and progesterone are biosynthesized mainly in ovarian granulosa cells as the ovarian follicle develops. The functions of sex hormones include regulation of the estrus cycle and puberty as well as maintenance of pregnancy in females. In this study, we isolated granulosa cells from porcine ovaries and cultured them for experiments. To examine the effects of VD3 on ovarian granulosa cells, the mRNA and protein levels of genes were analyzed by Real-time PCR and Western blotting assay. Production of progesterone from granulosa cells was also measured by ELISA assay. As a result, transcriptional and translational regulation of progesterone biosynthesis-related genes in granulosa cells was significantly altered by VD3. Furthermore, progesterone concentrations in porcine granulosa cell-cultured media decreased in response to VD3. These results show that VD3 was a strong regulator of sex steroid hormone production in porcine granulosa cells, suggesting that vitamin D deficiency may result in inappropriate sexual development of industrial animals and eventually economic loss.
Keywords: Vitamin D3, steroidogenesis, progesterone, granulosa cell, pig
Introduction
Vitamin D refers to a group of fat-soluble secosteroids responsible for enhancing intestinal absorption of calcium, magnesium, and phosphate[1]. Vitamin D obtained from the diet or dermal synthesis due to sunlight is biologically inactive, and its activation requires enzymatic conversion in the liver and kidney. It is assumed that vitamin D is not produced in the body, which means that all vitamin D is obtained from food intake or sunlight[2]. The two major forms of vitamin D are vitamin D2 or ergocalciferol and vitamin D3 or cholecalciferol[3]. In the liver, vitamin D is converted into calcidiol, which is known as calcifediol (25-hydroxycholecalciferol). Vitamin D2 is converted in the liver into 25-hydroxyergocalciferol. These two specific vitamin D metabolites are measured in serum to determine vitamin D status. A part of calcidiol is converted by the kidneys into 1,25-dihydroxyvitamin D3 (VD3), which is the biologically active form of vitamin D. VD3 circulates as a hormone in the blood, regulating the concentration of calcium and phosphate in the bloodstream and promoting healthy growth and bone remodeling. VD3 also affects neuromuscular and immune function[4].
Vitamin D receptor (VDR) is a member of the nuclear receptor family of transcription factors. Upon activation by vitamin D, VDR forms a heterodimer with retinoid-X receptor and binds to hormone response elements on DNA, resulting in expression or transrepression of specific genes[5]. Downstream targets of this nuclear hormone receptor are involved principally in mineral metabolism, although VDR also regulates a variety of other metabolic pathways such as those involved in the immune response and cancer[6].
Vitamin D deficiency induces bone formation and skeletal diseases such as osteoporosis, and osteomalacia since vitamin D helps maintain absorption of calcium and phosphorus. Vitamin D deficiency is also associated with metabolic diseases such as hypertension, diabetes, and obesity as well as immune diseases, including rheumatoid arthritis, atopic dermatitis, and allergic reaction[7-9].
The physiological function of vitamin D related to the female reproductive system has recently been reported. VDR is expressed in the ovaries, uterus, and decidua of the placenta. In the placenta, VDR regulates calcium transfer between trophoblasts and the endometrial decidua, which helps maintain pregnancy by preventing contraction of the uterine muscle. However, the other physiological roles of VDR in reproductive organs are not clear[10-13].
Ovaries are ovum-producing or releasing reproductive organs consisting of granulosa and theca cells, which are flat epithelial cells originated from the surface epithelium covering the ovaries. Granulosa cells surround oocytes and produce female sex steroid hormones, including progesterone and estrogen. Ovarian follicles are basically composed of the cumulus oophorus, membrane granulosa, corona radiate, zona pellucida, and primary oocytes. Follicle growth is based on oocytes and granulosa cells of the follicle in early growth stages, and steroidogenesis is accelerated by follicle development.
Steroidogenesis is the biological process by which steroids are generated from cholesterol and transformed into other steroids. Human and mammalians synthesize active steroid hormones from cholesterol in gonads, adrenal glands, and the placenta. The first step of steroidogenesis is transporting cholesterol into mitochondria by steroidogenic acute regulatory protein (StAR). Entered cholesterol is converted into pregnenolone by side-chain cleavage enzyme (CYP11A1), which can be converted into progesterone depending on 3β-hydroxysteroid dehydrogenase (3β-HSD). Estrogen also is synthesized from cholesterol, pregnenolone, 17α-hydroxy progesterone, androstendione and testosterone by 17α hydroxylase (CYP17A1), 17β-hydroxysteroid dehydrogenase (17β-HSD), and aromatase (CYP19)[14].
In steroidogenic ovarian tissue, expression levels of most cell- and tissue-specific steroidogenic enzymes are dependent mainly on trophic hormonal stimulation as a result of ovarian follicle growth. The dependence of enzyme synthesis on transcriptional induction is most conspicuous in ovarian granulosa cells. Hormonal signals such as GnRH, FSH, LH, inhibin, and activin regulate expression of steroidogenic genes positively or negatively.
Stimulated female steroid hormones aid regulation of the estrus cycle, development of reproductive organs, and pregnancy. Progesterone is the most important hormone during pregnancy and has many roles related to development of the fetus. Estrogen play roles in the menstrual and estrus reproductive cycles. Estrogen promotes development of female secondary sexual characteristics such as breasts and is involved in thickening of the endometrium as well as other aspects of the menstrual cycle. Uncontrolled levels of these female hormones cause not only dysregulation of the estrus cycle and puberty but also reproductive disorders such as abortion, breast cancer, and endometriosis.
Although it is well known that VD3 is associated with biosynthesis of progesterone in experimental animals, the function of VD3 in the female reproductive systems of industrial animals has not been explored. In this study, therefore, we examined the effects of VD3 and its mechanism on the biosynthesis of progesterone hormones from porcine ovarian granulosa cells.
Materials and methods
Reagents and chemicals
VD3 was purchased from Merck Millipore Co. (Millipore, MA, USA). Anti-β-actin (Cat# 4967) and CYP11A1 (Cat# 14217) were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). Anti-3β-HSD (Cat# ab55268) was purchased from Abcam (Cambridge, UK). Horseradish peroxidase (HRP)-conjugated anti-rabbit and anti-mouse IgG antibody were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Isolation and treatment of granulosa cells
Granulosa cells were isolated from 3-5 mm-sized follicles of immature pigs. Cells were washed three times by centrifugation (1,500 rpm) in phosphate-buffered saline (PBS) containing 1% streptomycin/penicillin. Granulosa cells were cultured in dulbecco's modified eagle medium (DMEM, Gibco-BRL, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% streptomycin/penicillin (Welgene Inc., Seoul, Korea). Incubation of the granulosa cell suspension was carried out in 6-well dishes at 37°C in an atmosphere of 5% CO2-95% air. Cell density and viability were determined in a hemocytometer by trypan blue exclusion. Cell viability ranged from 70% to 80%. After 24 hours, medium was exchanged to remove red blood cells. After incubation for 48 hours, medium was replaced with phenol-free DMEM supplemented with 10% charcoal-dextran-treated FBS and 1% streptomycin/penicillin 24 hours before treatment. Vitamin D3 was administered at a concentration of 100 nmol/L, and an equal part of ethanol was added to the control group.
Quantitative Real-time PCR
Total RNA was extracted using Trizol reagent (Invitrogen Co., Carlsbad, CA, USA) according to the manufacturer's protocol. The concentration of total RNA was measured by a spectrophotometer. DNA (cDNA) was prepared from total RNA (2 μg) by reverse transcription (RT) using M-MLV reverse transcriptase (Invitrogen) and random primers (9-mers; TaKaRa Bio Inc., Shiga, Japan). Q-PCR was performed using cDNA template (2 μl) and SYBR Green (6 μl; TOYOBOCo. Ltd., Katata, Ohtsu, Japan) containing specific primers. The primers sequences used were as follows : CYP11A1 (left: 5′-AGG CCA ATG TTA CCG AGA TG-3′, right: 5′-ATT GCA GCA TCT TGC TTG TG-3′), 3β-HSD (left: 5′-TCC ACA CCA GCA GCA TAG AG-3′, right: 5′-CAT GTG GGC AAA GAT GAA TG-3′) and β-actin (forward: 5′-TCC CTG GAG AAG AGC TAC GA-3′, reverse: 5′-CGC ACT TCA TGA TCG AGT TG-3′). Q-PCR was carried out for 40 cycles using the following parameters: denaturation at 95°C for 15 seconds, annealing, and extension at 70°C for 60 seconds. Fluorescence intensity was measured at the end of each extension phase. The threshold value for the fluorescence intensity of all samples was set manually. The reaction cycle at which PCR products exceeded this fluorescence intensity threshold during the exponential phase of PCR amplification was considered to be the cycle of threshold (CT). Expression of the target gene was quantified relative to that of β-actin, a housekeeping gene, based on comparison of CTs at constant fluorescence intensity.
Western blotting assay
Protein samples were extracted from granulosa cells with Pro-Prep solution (iNtRON Biotechnology, Seoul, Republic of Korea) following the manufacturer's protocol. A total 30 μg of cytosolic proteins were separated by 8 to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to nitrocellulose membranes (Daeillab Service Co., Ltd, Seoul, Republic of Korea). The membranes were then blocked for 2 hours with 5% skim milk (DifcoTM, Sparks, MD, USA) in PBS with 0.05% Tween-20 (PBS-T). After blocking, membranes were incubated with antibodies specific for CYP11A1 (diluted 1:1,000) and 3β-HSD (diluted 1:2,000) overnight as well as HRP-conjugated anti-rabbit and anti-mouse secondary antibodies (diluted 1:2,000) in blocking buffer with PBS-T for 1 hour. Luminol reagent (Bio-Rad Laboratories Inc., Hercules, CA, USA) was used to visualize antibody binding. Each blot was then stripped by incubation with 2% SDS and 100 mmol/L mercaptoethanol in 62.5 mmol/L Tris-HCl (pH 6.8) for 30 minutes at 50-60°C. Membranes were subsequently probed with antibody against β-actin (diluted 1:3,000, Santa Cruz Biotechnology) as an internal control. Blots were then scanned using Gel Doc 1000, Version 1.5 (Bio-Rad), and band intensities were normalized to β-actin levels.
Measurement of progesterone
After VD3 treatments, cultured media were centrifuged at 1,300 g for 15 minutes. The cultured media was frozen at −80°C until assayed. Progesterone accumulation in media was measured using a competitive enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI, USA) following the manufacturer's protocol.
Statistical analysis
Results were presented as the mean±standard deviation (SD). Data were analyzed using Sigma Plot 10.0 (Systat Software, Inc, San Jose, CA, USA). P-values < 0.05 were considered statistically significant.
Results
Regulation of progesterone biosynthesis-related genes in granulosa cells
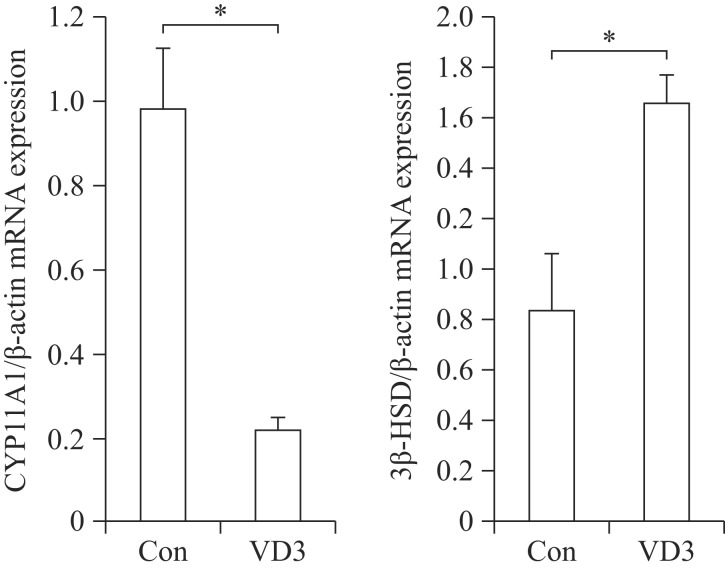
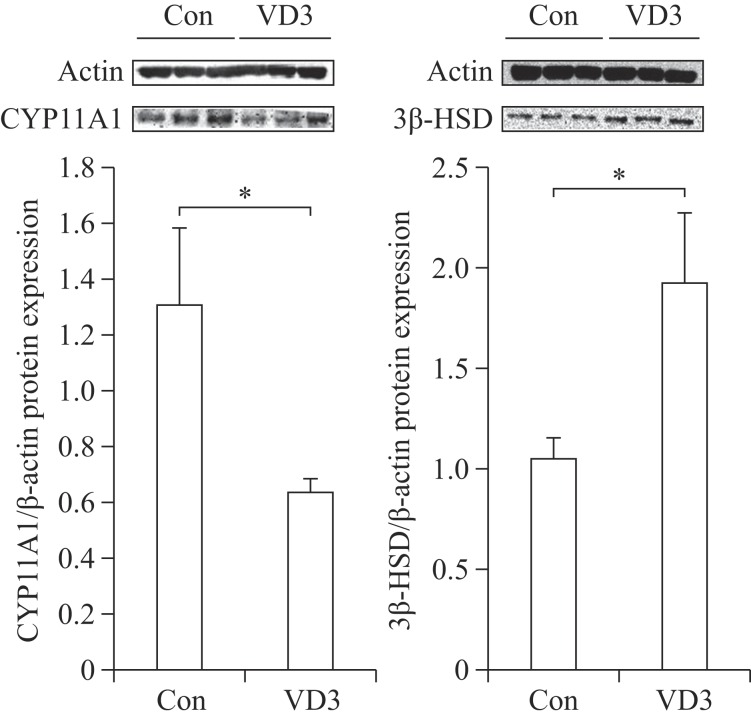
We examined the effects of VD3 on expression of progesterone biosynthesis-related enzymes such as CYP11A1 and 3β-HSD. Since ovarian granulosa cells biosynthesize female hormones, we isolated granulosa cells from porcine ovarian follicles. Expression of steroidogenesis-associated enzymes was controlled at the transcriptional level. Therefore, we first examined mRNA expression of CYP11A1 and 3β-HSD in granulosa cells. The mRNA expression of CYP11A1 and 3β-HSD in granulosa cells was significantly altered by VD3. VD3 significantly down-regulated mRNA expression of CYP11A1, whereas mRNA expression of 3β-HSD increased (Fig. 1). The protein levels of CYP11A1 and 3β-HSD were regulated in a similar manner as the mRNA levels (Fig. 2). Treatment with VD3 significantly up-regulated protein expression of 3β-HSD. VD3 down-regulated protein levels of CYP11A1, although the magnitude of regulation was not significant.
Fig. 1. Transcriptional regulation of progesterone biosynthesis-related genes in granulosa cells treated with VD3.
CYP11A1 and 3β-HSD were analyzed by Real-time PCR. Gene expression level was normalized to the level of β-actin. Data are expressed as mean ±SD. *P<0.05 compared to the control group.
Fig. 2. Translational regulation of progesterone biosynthesis-related genes in granulosa cells treated with VD3.
CYP11A1 and 3β-HSD were analyzed by Western blotting assay. Gene expression level was normalized β-actin. Data are expressed as mean±SD. *P<0.05 compared to the control group.
Time- and dose-dependent gene regulation of CYP11A1
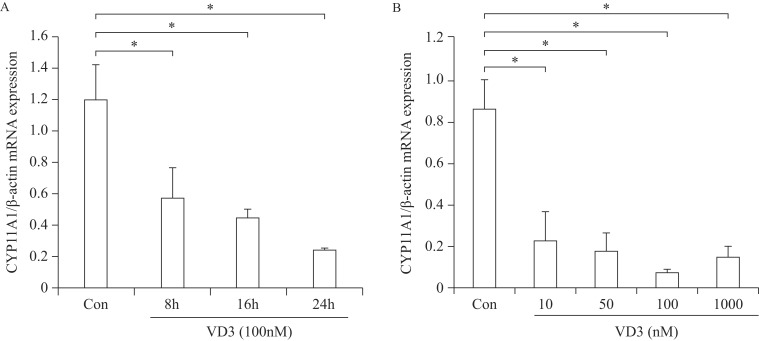
Regulation of CYP11A1 by VD3 was further explored in a time- and a dose-dependent manner, as CYP11A1 was critically regulated by VD3. The mRNA expression of CYP11A1 was reduced after VD3 treatment from 8 to 24 hours gradually in a time-dependent manner (Fig. 3A). In a dose-dependent experiment, CYP11A1 mRNA expression was significantly reduced but reached a maximum at 100 nmol/L (Fig. 3B).
Fig. 3. Real-time PCR analysis of granulosa cells treated with VD3 in a time and a dose-dependent manner.
After treatment of granulosa cells with VD3, mRNA was obtained. Transcriptional levels of CYP11A1 in granulosa cells treated in a time-dependent (A) and a dose-dependent (B) manner with VD3 were analyzed by Real-time PCR. Data are expressed as mean ±SD. *P<0.05 compared to the control group.
Analysis of steroid hormone levels after vitamin D3 treatment
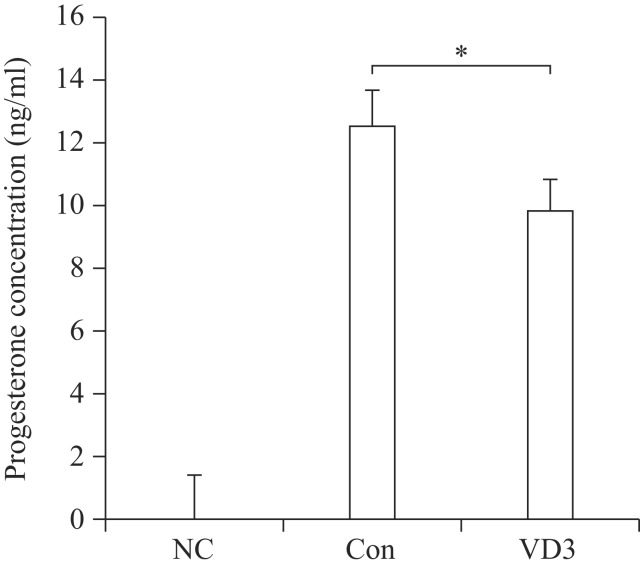
Since progesterone biosynthesis-related enzymes were significantly altered by VD3, we examined progesterone secretion in granulosa cell-cultured media. Basal concentration of progesterone secreted in conditioned culture media collected from the control group was 12.5 ng/mL (Fig. 4). Consistent with the results of progesterone-metabolizing enzymes, VD3 decreased the concentration of progesterone in cultured media.
Fig. 4. Effects of VD3 on progesterone secretion in granulosa cells.
After treatment of granulosa cells with VD3, medium was harvested. Progesterone concentration in cell growth medium was analyzed by ELISA assay. NC which is no treated group stands for negative control. Data are expressed as mean ±SD. *P<0.05 compared to the control group.
Discussion
Female sex hormones made by the ovaries play an important role in female life. Especially, progesterone influences all aspects of life from birth to menopause, including puberty and pregnancy. Therefore, progesterone can be linked to disorders affecting female reproductive organs, such as breast cancer, infertility, ovarian cancer, uterine cancer, and etc[15]. Due to its physiological function in reproductive organs, regulation of progesterone biosynthesis has been highlighted. Furthermore, reproductive function is critically more important in farm animals including sows and cows.
A recent research has shown that VD3 alters expression of genes involved in follicular development and steroidogenesis in human granulosa cells. However, this study demonstrated that VD3 did not significantly alter the expression of StAR, CYP11A1, CYP19A1, or 3β-HSD[16]. In the present study, we examined progesterone biosynthesis-related gene expression in porcine granulosa cells after VD3 treatment. To explore the effect of VD3 on ovarian granulosa cells, the mRNA and protein levels of genes were analyzed by Real-time PCR and Western blotting assay. Transcriptional and translational expression levels of progesterone-synthesizing enzymes 3β-HSD and CYP11A1 were differentially regulated by VD3 in porcine granulosa cells. VD3 significantly increased expression of 3β-HSD, whereas it reduced levels of CYP11A1. In case of 3β-HSD, expression level was increased up to 2-fold, while CYP11A1 was decreased in granulosa cells after VD3 treatment. According to these results, we assumed that 3β-HSD is associated with estrogen as well as progesterone production. Since CYP11A1 is a powerful regulator on progesterone biosynthesis in porcine granulosa cells, we examined the transcriptional levels of CYP11A1 depending on time and dose dependent manners. As we expected, the transcripts of CYP11A1 was reduced by time and dose dependent manners. In addition, VD3 slightly reduced secretion of progesterone in cultured media of porcine granulosa cells. These results are different from those of a previous study in which VD3 had no effect on steroidogenic protein expression in human granulosa cells. These new results may be due to species-specific regulation of VD3 during steroidogenesis, and VD3 may function more sensitively in porcine granulosa cells than in humans.
Most people nowadays are not exposed to a sufficient amount of UV-B due to indoor lifestyles, resulting in lack of VD3 synthesis in the skin. Recent studies have shown that VD3 deficiency has increased and is associated with illness, including cancer and immune-related disease[17-19]. VD3 also has reproductive functions. In a previous study, VDR was reported to be expressed in the ovaries, and VD3 was shown to increase estrogen and progesterone in human ovarian cells[16]. It was also revealed that VD3 increased estrogen biosynthesis in mice[20].
In particular, industrial animals such as pigs, cows, and chickens are bred in captivity to increase body weight, resulting in insufficient sunlight exposure. In the case of pigs, a higher body weight necessitates greater VD3 levels. Health problems and low birth rate can be caused by lack of VD3 in pigs. It has been reported that indoor breeding pigs have a lower birth rate and litter size than grazing pigs. Pigs are the main source of animal meat for humans and are suitable for production of artificial organs for human use. Recently, it was suggested that animal epidemic diseases such as foot and mouse disease and swine fever are associated with weak immunity due to lack of VD3.
In summary, we examined the effects of VD3 on synthesis of progesterone in porcine granulosa cells. Our results demonstrate that VD3 is a powerful regulator of sex steroid hormone production in porcine granulosa cells, suggesting that VD3 deficiency may result in inappropriate sexual development of industrial animals and eventually economic loss.
Acknowledgment
This work was supported by a 2-Year Research Grant of Pusan National University.
References
- [1].Holick MF. (2006) High prevalence of vitamin D inadequacy and implications for health[J]. JCR, 81(3):353-373. [DOI] [PubMed] [Google Scholar]
- [2].Wolf G. (2004) The discovery of vitamin D: the contribution of Adolf Windaus[J]. J Nutr, 134(8):1299-1302. [DOI] [PubMed] [Google Scholar]
- [3].Dorland WAN. (2011) Dorland's Illustrated Medical Dictionary32: Dorland's Illustrated Medical Dictionary. Elsevier Health Sciences. [Google Scholar]
- [4].Voet D, Voet JG. (2004) Biochemistry: Biomolecules, Mechanisms of Enzyme Action, and Metabolism. Volume One, Recording for the Blind & Dyslexic. [Google Scholar]
- [5].Adorini L, Daniel KC, Penna G. (2006) Vitamin D receptor agonists, cancer and the immune system: an intricate relationship[J]. Curr Top Med Chem, 6(12):1297-1301. [DOI] [PubMed] [Google Scholar]
- [6].Zittermann A. (2003) Vitamin D in preventive medicine: are we ignoring the evidence?[J] Br J Nutr, 89(5):552-572. [DOI] [PubMed] [Google Scholar]
- [7].Cantorna MT, Zhu Y, Froicu M, et al. (2004) Vitamin D status, 1, 25-dihydroxyvitamin D3, and the immune system[J]. Am J Clin Nutr, 80(6 Suppl):1717S-1720S. [DOI] [PubMed] [Google Scholar]
- [8].Kang EJ, Lee JE, An SM, et al. (2015) The effects of vitamin D3 on lipogenesis in the liver and adipose tissue of pregnant rats[J]. Int J Mol Med, 36(4):1151-1158. [DOI] [PubMed] [Google Scholar]
- [9].Parikh G, Varadinova M, Suwandhi P, et al. (2010) Vitamin D regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells[J]. Horm Metab Res, 42(10):754-757. [DOI] [PubMed] [Google Scholar]
- [10].Zarnani AH, Shahbazi M, Salek-Moghaddam A, et al. (2010) Vitamin D3 receptor is expressed in the endometrium of cycling mice throughout the estrous cycle[J]. Fertil Steril, 93(8):2738-2743. [DOI] [PubMed] [Google Scholar]
- [11].Avila E, Díaz L, Halhali A, et al. (2004) Regulation of 25-hydroxyvitamin D 3 1α-hydroxylase, 1, 25-dihydroxyvitamin D 3 24-hydroxylase and vitamin D receptor gene expression by 8-bromo cyclic AMP in cultured human syncytiotrophoblast cells[J]. J Steroid Biochem Mol Biol, 89(1-5):115-119. [DOI] [PubMed] [Google Scholar]
- [12].Belkacemi L, Gariépy G, Mounier C, et al. (2003) Expression of calbindin-D28k (CaBP28k) in trophoblasts from human term placenta[J]. Biol Reprod, 68(6): 1943-1950. [DOI] [PubMed] [Google Scholar]
- [13].Ross MH, Pawlina W. Histology. Lippincott Williams & Wilkins, 2006. [Google Scholar]
- [14].Payne AH, Hales DB. (2004) Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones[J]. Endocr Rev, 25(6):947-970. [DOI] [PubMed] [Google Scholar]
- [15].Ho S-M. (2003) Estrogen, progesterone and epithelial ovarian cancer[J]. Reprod Biol Endocrinol, 1:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Merhi Z, Doswell A, Krebs K, et al. (2014) Vitamin D alters genes involved in follicular development and steroidogenesis in human cumulus granulosa cells[J]. J Clin Endocrinol Metab, 99(6):E1137-E1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gorham ED, Garland CF, Garland FC, et al. (2005) Vitamin D and prevention of colorectal cancer[J]. J Steroid Biochem Mol, 97:179-194. [DOI] [PubMed] [Google Scholar]
- [18].Giovannucci E, Liu Y, Rimm EB, et al. (2006) Prospective study of predictors of vitamin D status and cancer incidence and mortality in men[J]. J Natl Cancer Inst, 98(7):451-459. [DOI] [PubMed] [Google Scholar]
- [19].Holick MF. (2007) Vitamin D deficiency[J]. NEJM, 357(3):266-281. [DOI] [PubMed] [Google Scholar]
- [20].Kinuta K, Tanaka H, Moriwake T, et al. (2000) Vitamin D Is an Important Factor in Estrogen Biosynthesis of Both Female and Male Gonads 1[J]. Endocr, 141(4): 1317-1324. [DOI] [PubMed] [Google Scholar]