Abstract
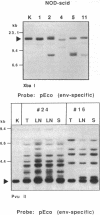
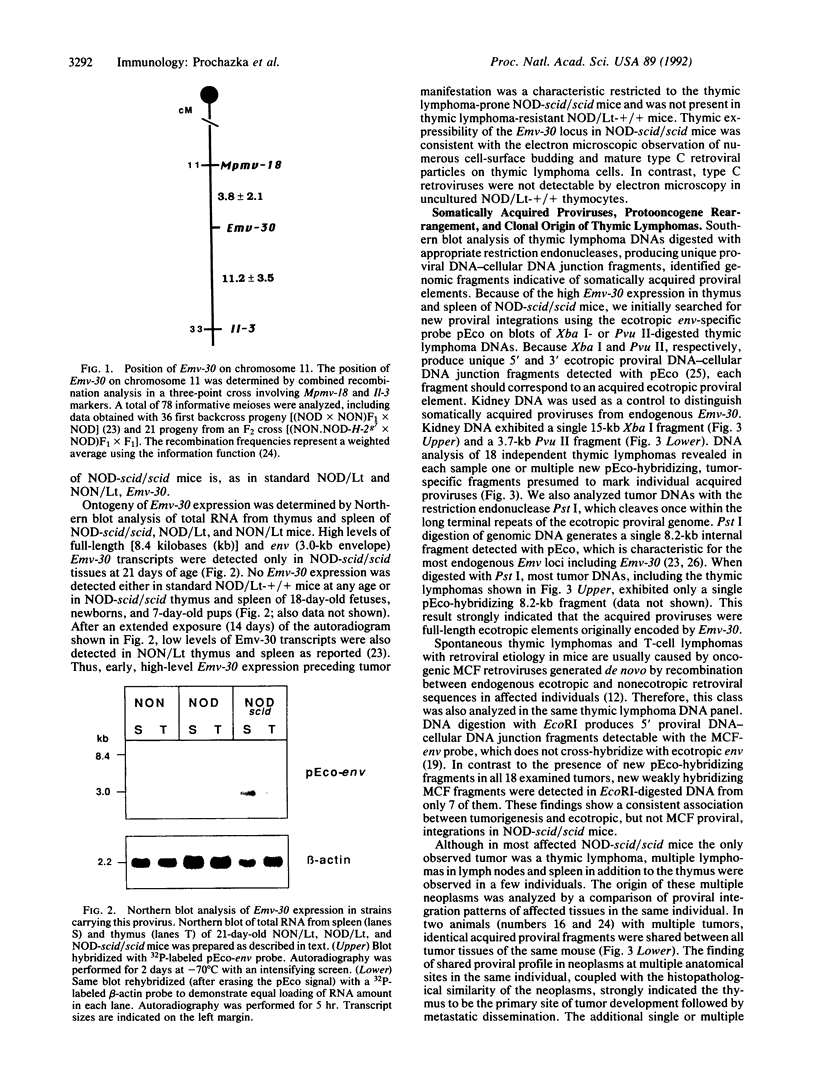
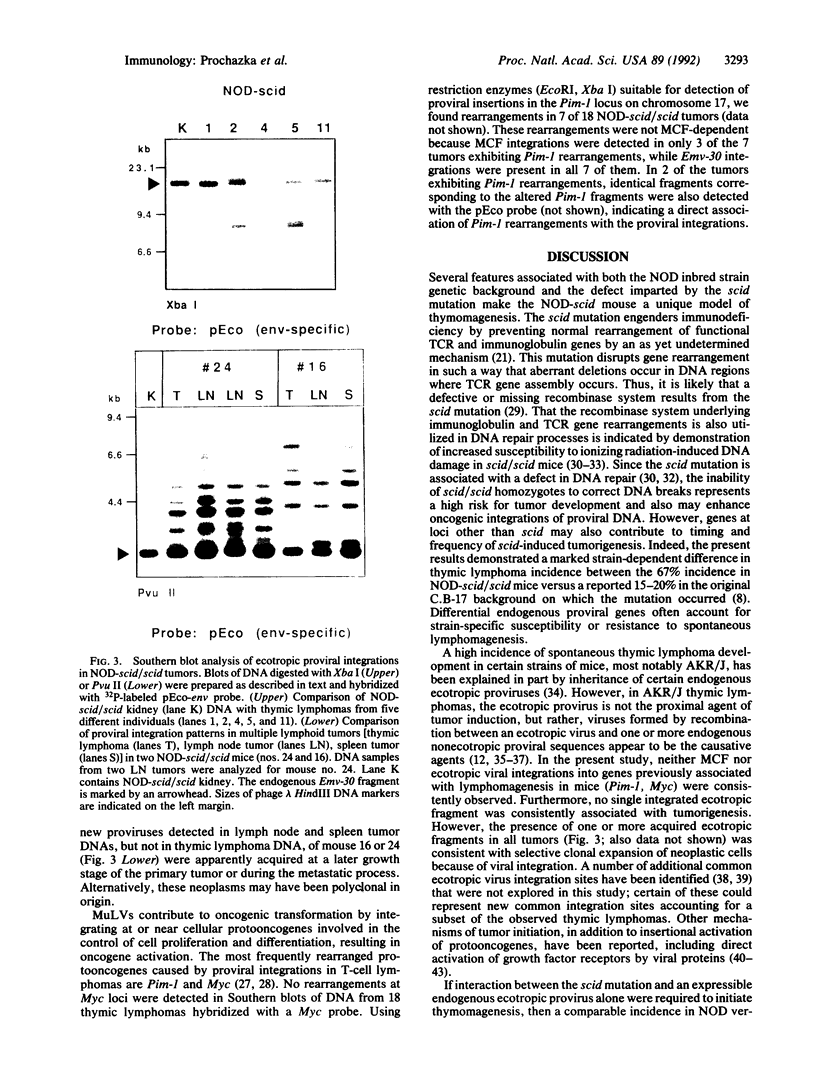
Homozygosity for the severe combined immunodeficiency (scid) mutation results in a block in T- and B-lymphocyte development. An unusually high incidence of spontaneous thymic lymphoma development was observed after transfer of this mutation from the C.B-17 congenic strain background onto the diabetes-susceptible nonobese diabetic (NOD) background. Thymomagenesis in the NOD-scid/scid mouse was associated with expression of an NOD mouse-unique endogenous ecotropic murine leukemia provirus locus (Emv-30, mapped to proximal region of chromosome 11) not expressed in the standard substrain NOD/Lt thymus. All tumors exhibited insertions of ecotropic proviruses, whereas only a subset also exhibited proviral integrations of mink cell focus-forming retrovirus. Neither class of retrovirus was associated with consistent integration into genes previously associated with activation of oncogenesis. We propose that the unusual features of T-cell ontogeny characteristic of the NOD inbred strain synergize with the scid-imparted block in thymocyte development, leading to activation of the NOD-unique Emv-30 to initiate thymomagenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biedermann K. A., Sun J. R., Giaccia A. J., Tosto L. M., Brown J. M. scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1394–1397. doi: 10.1073/pnas.88.4.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilliant M. H., Gondo Y., Eicher E. M. Direct molecular identification of the mouse pink-eyed unstable mutation by genome scanning. Science. 1991 Apr 26;252(5005):566–569. doi: 10.1126/science.1673574. [DOI] [PubMed] [Google Scholar]
- Buchberg A. M., Bedigian H. G., Taylor B. A., Brownell E., Ihle J. N., Nagata S., Jenkins N. A., Copeland N. G. Localization of Evi-2 to chromosome 11: linkage to other proto-oncogene and growth factor loci using interspecific backcross mice. Oncogene Res. 1988;2(2):149–165. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W., Hartley J. W., Rowe W. P. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980 Mar 1;151(3):542–552. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossman J. Molecular genetic basis of the diagnosis of hematopoietic neoplasms. J Natl Cancer Inst Monogr. 1990;(10):3–6. [PubMed] [Google Scholar]
- Custer R. P., Bosma G. C., Bosma M. J. Severe combined immunodeficiency (SCID) in the mouse. Pathology, reconstitution, neoplasms. Am J Pathol. 1985 Sep;120(3):464–477. [PMC free article] [PubMed] [Google Scholar]
- Cuypers H. T., Selten G., Quint W., Zijlstra M., Maandag E. R., Boelens W., van Wezenbeek P., Melief C., Berns A. Murine leukemia virus-induced T-cell lymphomagenesis: integration of proviruses in a distinct chromosomal region. Cell. 1984 May;37(1):141–150. doi: 10.1016/0092-8674(84)90309-x. [DOI] [PubMed] [Google Scholar]
- Disney J. E., Barth A. L., Shultz L. D. Defective repair of radiation-induced chromosomal damage in scid/scid mice. Cytogenet Cell Genet. 1992;59(1):39–44. doi: 10.1159/000133196. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Gautsch J. W., Jensen F. C., Lerner R. A., Hartley J. W., Rowe W. P. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4676–4680. doi: 10.1073/pnas.74.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop G. M., Phillips R. A. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990 Oct 4;347(6292):479–482. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- Gjerset R. A., Yeargin J., Volkman S. K., Vila V., Arya J., Haas M. Insulin-like growth factor-I supports proliferation of autocrine thymic lymphoma cells with a pre-T cell phenotype. J Immunol. 1990 Nov 15;145(10):3497–3501. [PubMed] [Google Scholar]
- Hamaguchi K., Leiter E. H. Comparison of cytokine effects on mouse pancreatic alpha-cell and beta-cell lines. Viability, secretory function, and MHC antigen expression. Diabetes. 1990 Apr;39(4):415–425. doi: 10.2337/diab.39.4.415. [DOI] [PubMed] [Google Scholar]
- Harris H. The role of differentiation in the suppression of malignancy. J Cell Sci. 1990 Sep;97(Pt 1):5–10. doi: 10.1242/jcs.97.1.5. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson E. A., Qin X. Q., Bump E. A., Schatz D. G., Oettinger M., Weaver D. T. A link between double-strand break-related repair and V(D)J recombination: the scid mutation. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4061–4065. doi: 10.1073/pnas.88.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- Lilly F., Duran-Reynals M. L., Rowe W. P. Correlation of early murine leukemia virus titer and H-2 type with spontaneous leukemia in mice of the BALB/c times AKR cross: a genetic analysis. J Exp Med. 1975 Apr 1;141(4):882–889. [PMC free article] [PubMed] [Google Scholar]
- Mucenski M. L., Bedigian H. G., Shull M. M., Copeland N. G., Jenkins N. A. Comparative molecular genetic analysis of lymphomas from six inbred mouse strains. J Virol. 1988 Mar;62(3):839–846. doi: 10.1128/jvi.62.3.839-846.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski M. L., Gilbert D. J., Taylor B. A., Jenkins N. A., Copeland N. G. Common sites of viral integration in lymphomas arising in AKXD recombinant inbred mouse strains. Oncogene Res. 1987;2(1):33–48. [PubMed] [Google Scholar]
- O'Neill R. R., Khan A. S., Hoggan M. D., Hartley J. W., Martin M. A., Repaske R. Specific hybridization probes demonstrate fewer xenotropic than mink cell focus-forming murine leukemia virus env-related sequences in DNAs from inbred laboratory mice. J Virol. 1986 May;58(2):359–366. doi: 10.1128/jvi.58.2.359-366.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka M., Gaskins H. R., Leiter E. H., Koch-Nolte F., Haag F., Thiele H. G. Chromosomal localization, DNA polymorphism, and expression of Rt-6, the mouse homologue of rat T-lymphocyte differentiation marker RT6. Immunogenetics. 1991;33(2):152–156. doi: 10.1007/BF00210829. [DOI] [PubMed] [Google Scholar]
- Prochazka M., Leiter E. H., Serreze D. V., Coleman D. L. Three recessive loci required for insulin-dependent diabetes in nonobese diabetic mice. Science. 1987 Jul 17;237(4812):286–289. doi: 10.1126/science.2885918. [DOI] [PubMed] [Google Scholar]
- Prochazka M., Serreze D. V., Worthen S. M., Leiter E. H. Genetic control of diabetogenesis in NOD/Lt mice. Development and analysis of congenic stocks. Diabetes. 1989 Nov;38(11):1446–1455. doi: 10.2337/diab.38.11.1446. [DOI] [PubMed] [Google Scholar]
- Savino W., Boitard C., Bach J. F., Dardenne M. Studies on the thymus in nonobese diabetic mouse. I. Changes in the microenvironmental compartments. Lab Invest. 1991 Mar;64(3):405–417. [PubMed] [Google Scholar]
- Sawyers C. L., Denny C. T., Witte O. N. Leukemia and the disruption of normal hematopoiesis. Cell. 1991 Jan 25;64(2):337–350. doi: 10.1016/0092-8674(91)90643-d. [DOI] [PubMed] [Google Scholar]
- Schatz D. G., Baltimore D. Stable expression of immunoglobulin gene V(D)J recombinase activity by gene transfer into 3T3 fibroblasts. Cell. 1988 Apr 8;53(1):107–115. doi: 10.1016/0092-8674(88)90492-8. [DOI] [PubMed] [Google Scholar]
- Schuler W., Weiler I. J., Schuler A., Phillips R. A., Rosenberg N., Mak T. W., Kearney J. F., Perry R. P., Bosma M. J. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell. 1986 Sep 26;46(7):963–972. doi: 10.1016/0092-8674(86)90695-1. [DOI] [PubMed] [Google Scholar]
- Selten G., Cuypers H. T., Berns A. Proviral activation of the putative oncogene Pim-1 in MuLV induced T-cell lymphomas. EMBO J. 1985 Jul;4(7):1793–1798. doi: 10.1002/j.1460-2075.1985.tb03852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selten G., Cuypers H. T., Zijlstra M., Melief C., Berns A. Involvement of c-myc in MuLV-induced T cell lymphomas in mice: frequency and mechanisms of activation. EMBO J. 1984 Dec 20;3(13):3215–3222. doi: 10.1002/j.1460-2075.1984.tb02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serreze D. V., Leiter E. H. Defective activation of T suppressor cell function in nonobese diabetic mice. Potential relation to cytokine deficiencies. J Immunol. 1988 Jun 1;140(11):3801–3807. [PubMed] [Google Scholar]
- Serreze D. V., Leiter E. H., Worthen S. M., Shultz L. D. NOD marrow stem cells adoptively transfer diabetes to resistant (NOD x NON)F1 mice. Diabetes. 1988 Feb;37(2):252–255. doi: 10.2337/diab.37.2.252. [DOI] [PubMed] [Google Scholar]
- Shultz L. D., Schweitzer P. A., Hall E. J., Sundberg J. P., Taylor S., Walzer P. D. Pneumocystis carinii pneumonia in scid/scid mice. Curr Top Microbiol Immunol. 1989;152:243–249. doi: 10.1007/978-3-642-74974-2_29. [DOI] [PubMed] [Google Scholar]
- Stoye J. P., Moroni C., Coffin J. M. Virological events leading to spontaneous AKR thymomas. J Virol. 1991 Mar;65(3):1273–1285. doi: 10.1128/jvi.65.3.1273-1285.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip M. T., Chen I. S. Modes of transformation by the human T-cell leukemia viruses. Mol Biol Med. 1990 Feb;7(1):33–44. [PubMed] [Google Scholar]
- Zipris D., Crow A. R., Delovitch T. L. Altered thymic and peripheral T-lymphocyte repertoire preceding onset of diabetes in NOD mice. Diabetes. 1991 Apr;40(4):429–435. doi: 10.2337/diab.40.4.429. [DOI] [PubMed] [Google Scholar]
- Zipris D., Lazarus A. H., Crow A. R., Hadzija M., Delovitch T. L. Defective thymic T cell activation by concanavalin A and anti-CD3 in autoimmune nonobese diabetic mice. Evidence for thymic T cell anergy that correlates with the onset of insulitis. J Immunol. 1991 Jun 1;146(11):3763–3771. [PubMed] [Google Scholar]