High blood cmTreg numbers predict acute rejection of liver transplants
Keywords: acute rejection, liver transplantation, receiver operating characteristic curves, Treg
Abstract
Several studies have analyzed the potential of T regulatory cells (Treg cells) as biomarkers of acute rejection (AR). The aim of the present multicenter study was to correlate the percentage of peripheral Treg cells in liver graft recipients drawn at baseline up to 12 months after transplantation with the presence of AR. The percentage of central memory (cm) Treg cells (CD4+CD25highCD45RO+CD62L+) was monitored at pre-transplant and at 1 and 2 weeks, and 1, 2, 3 and 6 months and 1 year post-transplantation. The same validation standard operating procedures were used in all participating centers. Fifteen patients developed AR (23.4%). Hepatitis C virus recurrence was observed in 16 recipients, who displayed low peripheral blood cmTreg levels compared with patients who did not. A steady increase of cmTregs was observed during the first month after transplantation with statistically significant differences between AR and non-AR patients. The high frequency of memory Treg cells allowed us to monitor rejection episodes during the first month post-transplantation. On the basis of these data, we developed a prediction model for assessing risk of AR that can provide clinicians with useful information for managing patients individually and customizing immunosuppressive therapies.
Introduction
Orthotopic liver transplantation (OLT) is nowadays an accepted and viable therapeutic option for patients with end-stage liver disease. Although allogeneic liver grafts are more readily accepted than other allografts (hepatic tolerogenicity), up to 30% of OLT result in acute rejection (AR), leading to chronic graft dysfunction and decreased graft survival (1). Despite the excellent 1-year graft survival, immunosuppression-related morbidity, chronic rejection and hepatitis C virus (HCV) reinfection contribute to graft loss in the long term (2) and remain as obstacles to successful liver transplantation. Moreover, HCV allograft reinfection after OLT affects 50 to 80% of recipients, causing accelerated progression to graft cirrhosis and final graft loss (3).
The allo-immune response against grafts is a major focus of transplantation research, whose ultimate goal is to deepen our knowledge of transplant tolerance, which, in turn, can be described as a state of immunological non-responsiveness against donor antigens developed by recipients (4, 5). Interestingly, this state has been identified in a substantial number of transplant recipients who did not comply with the physician’s post-transplant recommendations and ceased to use the prescribed immunosuppressive drugs (6).
Research has shown that a particular subset of CD4+ T cells, named T regulatory cells (Treg cells), exert suppressive functions under inflammatory conditions (7). Up to date, a variety of Treg subsets displaying different phenotypes and suppression mechanisms have been identified (8).
The most extensively characterized Treg cells are those that co-express CD4 and the α-chain of IL-2 receptor, also referred to as CD25 (9). These CD4+CD25+ T cells have been shown to express other biomarkers, which allow a more detailed characterization of their suppressive and regulatory functions. Traditionally, Treg cells have been classified into resting Treg (rTreg) and memory Treg (mTreg) cells according to their surface marker expression (10, 11). Moreover, Sanchez Rodriguez et al. (12) have recently described a Treg cell subset displaying memory markers on their surface and named them ‘central memory Treg cells’ (CD4+CD25highCD45RO+CD62L+), hereafter referred to as cmTregs.
Recently, the role of Treg cells in the prevention of AR episodes after liver transplantation has been widely described in several reports. Patients who experienced AR or suffered HCV reinfection (HCVR) after OLT have been described to show decreased (13, 14) and increased number of circulating Treg cells (15), respectively.
Many research centers are nowadays focusing on the role of Treg cells as potential biomarkers of AR. However, these are single-center studies, restricted to a low number of patients. Therefore, we conducted a first-of-its-kind prospective multi-center study in order to demonstrate the role of these cells in liver transplantation. We studied the role of cmTreg cells as potential biomarkers of AR and HCVR in liver transplant recipients who were monitored during the first year after transplantation.
Methods
Study design
This prospective study was conducted at four Spanish centers (University Hospital ‘Virgen de la Arrixaca’ in Murcia, Hospital ‘Clinic’ in Barcelona, Hospital ‘12 de Octubre’ in Madrid and Hospital ‘Marques de Valdecilla’ in Santander) from 2010 to 2012. The study group consisted of 64 consecutive primary liver graft Caucasian recipients [liver graft recipients (LGR), n = 64], who were subjected to transplant from deceased donors. The study was approved by the Ethics Committee of all participating centers, and all patients provided written informed consent for the collection and storage of blood samples. Pediatric, re-transplanted and combined transplant patients were excluded.
The inclusion criteria were primary whole liver transplantation without prior history of other organ transplants, ABO compatibility, immunosuppressive therapy with tacrolimus (TRL) with or without mycophenolate mofetil (MMF) and HIV negativity.
Demographic, clinical and immunological characteristics
The mean donor age was 57.5 years (ranging from 25 to 79 years) and the mean recipient age was 55 years (ranging from 20 to 70 years). The recipients were classified into two groups according to whether they had experienced AR episodes (AR, n = 15, 23.4%) or not [non-AR (NAR), n = 49, 76.6%]. AR episodes occurred at an average of 15±10.5 days after transplantation. Most of the recipients were males (n = 49, 76.6%), 24.5% (n = 12) of whom suffered AR episodes. Three out of the 15 (20%) female patients experienced at least one AR episode.
All patients underwent a standardized treatment protocol. Immunosuppressive therapy consisted of either monotherapy, including TRL and corticosteroids, or double therapy, based on TRL, MMF and corticosteroids. The initial TRL dose was 6.62mg/day administered orally, and the drug level ranged between 2.6 and 17.3ng/ml. The initial dose of MMF was 1300mg/day and the drug level ranged between 0.40 and 4.15 µg/ml. The initial doses were modified in cases of adverse or side effects, such as diarrhea or leucopenia. All demographic, clinical and immunological data are summarized in Table 1.
Table 1.
Demographic, clinical and immunological characteristics
| AR (n = 15) | NAR (n = 49) | P value | HCVR (n = 16) | NHCVR (n = 48) | P value | ||
|---|---|---|---|---|---|---|---|
| Gender of recipient, n (%) | Male | 12 (24.49) | 37 (75.51) | 0.488 | 14 (28.57) | 35 (71.43) | 0.666 |
| Female | 3 (20) | 12 (80) | 0.325 | 2 (13.33) | 13 (86.67) | 0.234 | |
| Immunosuppressive regimen, n (%) | TRL+prednisone | 7 (18.42) | 31 (81.58) | 0.344 | 12 (31.58) | 26 (68.42) | 0.025 |
| TRL+MMF+prednisone | 8 (30.77) | 18 (69.23) | 0.026 | 4 (15.38) | 22 (84.62) | 0.086 | |
| A mismatches (0/1/2) | 2 (33.33%)/10 (33.33%)/3 (10.71%) | 4 (66.67%)/20 (66.67%)/25 (89.29%) | 0.882 | 3 (50%)/10 (33.33%)/3 (10.71%) | 3 (50%)/20 (66.67%)/25 (89.29%) | 0.144 | |
| B mismatches (0/1/2) | 3 (33.33%)/10 (43.48%)/2 (6.25%) | 6 (66.67%)/13 (56.52%)/30 (93.75%) | 0.278 | 2 (66.67%)/9 (39.13%)/5 (13.16%) | 1 (33.33%)/14 (60.87%)/33 (86.84%) | 0.207 | |
| DRB1 mismatches (0/1/2) | 1 (16.67%)/9 (36.00%)/5 (15.15%) | 5 (83.34%)/16 (64.00%)/28 (84.85%) | 0.287 | 2 (33.33%)/2 (6.90%)/12 (41.38%) | 4 (66.67%)/27 (93.10%)/17 (58.62%) | 0.077 | |
| Sensitized (no/yes) | 14 (26.92%)/1 (6.6%) | 38 (73.08%)/11 (22.4%) | 0.345 | 12 (26.67%)/4 (21.05%) | 33 (73.33%)/15 (78.95%) | 0.247 | |
| Donor age, years (mean ± SD) | 56±16.81 | 60±14.09 | 0.533 | 63±12.77 | 58±15.33 | 0.483 | |
| Recipient age, years (mean ± SD) | 52±12.13 | 56±9.74 | 0.149 | 58±9.44 | 55±10.67 | 0.304 | |
| Dose TRL, mg/day (mean ± SD) | 7.25±4.20 | 5.98±5.11 | 0.433 | 8.12±2.99 | 6.23±4.94 | 0.633 | |
| Dose MMF, mg/day (mean ± SD) | 1600±1140.17 | 1000±1180.19 | 0.288 | 3000±433.01 | 1052.63±1116.73 | 0.118 | |
| C min TRL, ng/ml (mean±SD) | 9.62±4.85 | 10.29±3.98 | 0.210 | 9.81±3.12 | 10.06±4.71 | 0.667 | |
| Cmin MMF, μg/ml (mean±SD) | 1.83±1.42 | 1.65±0.49 | 0.674 | 1.34±1.43 | 1.76±1.13 | 0.769 | |
| Frequency of lymphocytes (mean±SD) | 13.50±12.84 | 18.94±14.36 | 0.203 | 21.62±11.46 | 16.79±14.66 | 0.101 | |
| Absolute lymphocyte number/mm3 (mean ± SD) | 864.17±1003.89 | 903.95±852.46 | 0.689 | 790.91±372.71 | 920.98±963.06 | 0.529 | |
| Leucocytes (×109/ml) | 6.36±2.24 | 5.49±3.49 | 0.260 | 3.88±0.82 | 6.13±3.49 | 0.006 | |
In our cohort, the primary indications for transplant were as follows: cirrhosis due to HCV (n = 16, 25%), to hepatitis B virus (n = 5, 7.8%) or to alcoholism (n = 9, 15.6%); primary biliary cirrhosis (n = 2, 3.13%); cryptogenic cirrhosis (n = 1, 1.6%); hepatocellular carcinoma (n = 15, 23.4%); and other diseases (n = 15, 23.4%), e.g. hepatorenal polycystic disease, hemangioendothelioma and Budd–Chiari syndrome (Table 2). All 16 patients with cirrhosis caused by HCV developed HCVR after OLT; this group was considered as the hepatitis C positive group with active viral disease. The frequency of cmTreg cells was compared between liver recipients who suffered HCVR (HCVR, n = 16, 25%) and the rest of the patients, who were HCV negative before transplantation [non-HCV-recurrence (NHCVR), n = 48, 75%].
Table 2.
Indication for OLT
| Pre-transplant disease (n, %) | |
|---|---|
| Total (n = 64) | |
| Cirrhosis HCV | 16 (25.0) |
| Cirrhosis HBV | 5 (7.8) |
| Alcoholic cirrhosis | 10 (15.6) |
| Primary biliary cirrhosis | 2 (3.1) |
| Cryptogenic | 1 (1.6) |
| Hepatocellular carcinoma | 15 (23.4) |
| Other diseases | 15 (23.4) |
Other diseases include hepatorenal polycystic disease, hemangioendothelioma and Budd–Chiari syndrome. HBV, hepatitis B virus.
AR diagnosis
The primary clinical endpoint of the study was AR, diagnosed by clinical and laboratory findings and confirmed by histological evaluation of graft biopsies. We assessed the levels of bilirubin and transaminase enzymes (glutamic-oxaloacetic transaminase, glutamate pyruvate transaminase, alkaline phosphatase and gamma glutamyl transferase) in patients presenting clinical signs of rejection, including jaundice. When the levels of liver enzymes were found to be high, Doppler ultrasound was performed in order to exclude hepatic ischemia, caused by occlusion of the hepatic artery or portal vein, and to decide whether liver biopsy should be indicated or not. The histological diagnosis of AR was based on the presence of at least two of the following characteristics: presence of a mixed cellular infiltrate in the portal tracts, infiltration and biliary epithelial damage intra-hepatic bile ducts, and venous endothelium inflammation in portal tracts. The severity of AR was graded according to the Banff classification (16, 17). Severe AR was treated with steroid boluses (500–1000mg methylprednisolone/day, for 3 days) showing a good response to them, moderate AR was treated with increased baseline immunosuppression and mild AR was usually not treated.
Sample collection
The frequency of cmTreg cells from fresh whole peripheral blood (WPB) was determined using flow cytometry. This biomarker was evaluated at baseline, within 1 month before transplantation (pre-transplant profile was performed in 65% of patients during the week before transplantation), before starting immunosuppressive treatment.
According to the protocol of the participating centers, transplantation cannot be performed if a patient has an infection or inflammatory process. For this reason, a complete set of analyses is performed on each potential transplant patient just before transplantation, and if any anomaly is detected, transplantation is postponed until it is resolved.
At post-transplantation, the biomarker panel was analyzed at the first and second week and on the first, second, third, sixth month and 1 year after transplantation. Furthermore, whenever rejection episode or HCVR cases were detected, an extra peripheral blood (PB) sample was taken before the resolving treatment was supplied in order to identify variations on cmTreg cell levels during the clinical event.
Peripheral blood samples were collected at all time points in every center by venipuncture into sterile sodium heparinized tubes (Becton-Dickinson-BD, San Diego, CA, USA). These samples were used immediately after recollection in a flow cytometry assay, using the same standardized methodology and equipment in all centers.
Monoclonal antibodies and flow-cytometric analysis
The frequency of cmTreg cells in anticoagulated whole blood samples was analyzed by flow cytometry analysis within 1 hour after blood collection. Blood cells were stained with the following panel of fluorochrome-labeled monoclonal antibodies (mAb): anti-CD62L-fluorescein isothiocyanate, clone DREG56; anti-CD45RO-phycoerythrin (PE), clone UCHL-1; anti-CD25-PE, clone 2A3; and anti-CD4-peridinin chlorophyll protein (PerCP), clone L200. All mAb were purchased from Becton-Dickinson Biosciences (BD®). All centers used the same panel of mAb and flow cytometry method to reduce the variability between centers. The panel had been previously tested and validated by the center in Santander (18, 19) with an expertise in Treg cell analysis.
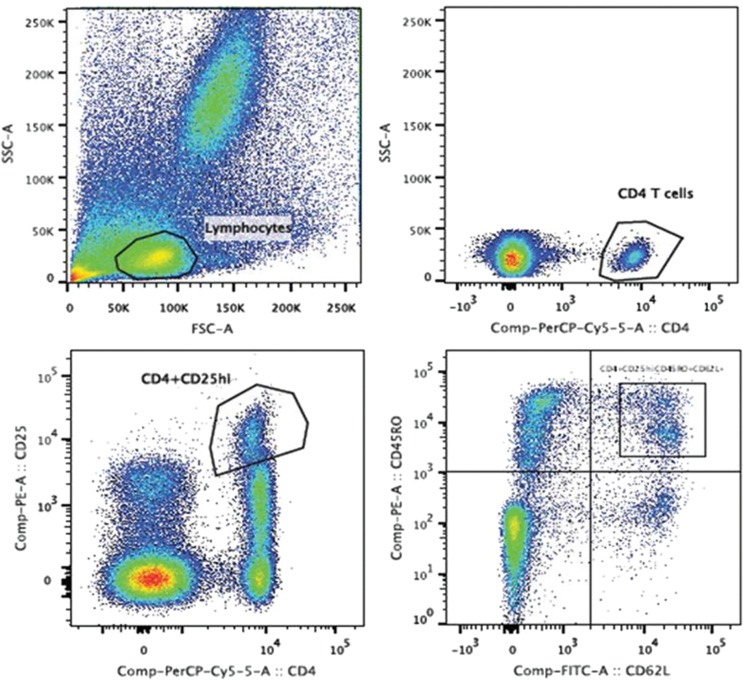
Cells were stained with mAb for 30 minutes in the dark, at room temperature. Erythrocytes were lysed by adding FACS lysing solution (BD). Cells were then washed twice with phosphate buffered saline. Afterwards, 50000 events were acquired within the lymphocyte gate, as determined by forward-scattered light/side-scattered light, using a FACScanto™ II flow cytometer (BD). Data were analyzed with BD FACSDiva™ Software 6.0. cmTreg cells were identified based on the staining of different cell surface biomarkers (Fig. 1). The frequencies (%) of cmTreg cells with respect to the total number of CD4+ peripheral blood T cells were calculated.
Fig. 1.
Peripheral blood memory T regulatory cell gating strategy in orthotopic liver transplant recipients. Peripheral blood lymphocytes were stained with monoclonal antibodies to CD4, CD25, CD62L and CD45RO gated on CD4+ T viable cells.
Statistical analysis
Demographic data and results of the prospective analysis were collected in a unified database. Statistical analysis was performed using SPSS 18.0 software (SPSS Inc, Chicago, IL, USA). To confirm if the samples followed a Gaussian distribution, we performed the non-parametric Kolmogorov–Smirnov test. Samples were adjusted to a non-parametric distribution. Thus, Mann–Whitney test was used to determinate if there were any significant differences between cmTreg cell phenotype and clinical events. Data were presented as the mean ± standard deviation of mean (SD). A two-tailed P value < 0.05 was considered significant.
Receiver operating characteristic (ROC) curves were constructed to determine the optimal cutoff points for biomarkers when comparing patients with and without AR episodes [i.e. the cutoff resulting in the highest Youden-index (sensitivity + specificity-1)] (20). The discrimination capacity was determined by calculating the area under the ROC curves (AUC): an area of 0.7–0.8 was considered acceptable; an area of 0.8–0.9, excellent; an area >0.9, outstanding (21).
Logistic regression analyses were performed using the model-building strategy proposed by Hosmer and Lemeshow (22, 23). The combinations of potential biomarkers with the presence of AR and HCVR were considered as a binary dependent variable. Variables included gender of recipient, donor and recipient age, HLA mismatches, immunosuppressive regimen, absolute number of leukocytes, frequency and absolute number of total lymphocytes, doses of TRL and MMF, C min of TRL and MMF and the frequencies of cmTreg cells. Each potential risk factor was tested independently. All the factors that reached a P < 0.25 in univariate analysis were included in the multivariate model. All models were built using backward stepwise methods to identify possible candidates for the model. The assessment of the importance of each variable included in the model was performed with Wald statistics. In order to compare the models, we used the likelihood ratio test and the predicted probabilities of the model. A tolerance statistic was used to identify colinearity: values lower than 0.10 indicate a serious colinearity problem, and values lower than 0.20, a cause for concern (24). Finally, the variables that did not contribute to the model were deleted, and a new model was achieved.
Results
All the AR episodes occurred within the first month after OLT, and the results of graft biopsies revealed them to be cellular rejections. Of the 15 AR episodes, two were severe, five moderate and eight mild. A clear tendency towards high levels of biomarkers was observed in patients with severe AR compared with those with moderate or mild AR, but the difference was not significant. None of the patients showed multiple episodes of AR during the study follow-up.
No relationship could be established between AR or HCVR and the demographics, or clinical characteristics of patients. The statistically significant difference was found with respect to leukocyte count and immunosuppressant treatment based on TRL between patients who suffered or not HCVR. There was also a statistical difference between patients who developed or not AR with respect to double immunosuppressive therapy based on TRL + MMF (Table 1).
cmTreg cell monitoring in liver transplant recipients within the first year after transplantation
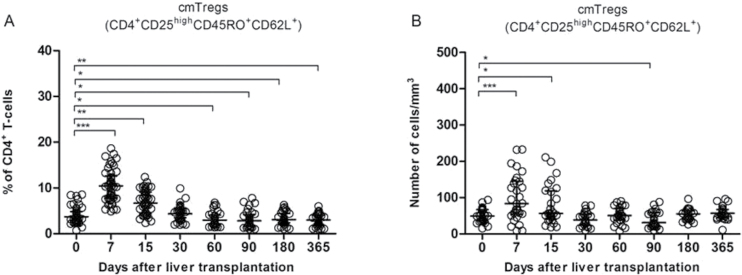
We monitored the frequency of cmTreg cells in WPB of patients who had undergone OLT within 1 year after transplantation. The percentage of cmTreg cells significantly decreased during all time points tested compared with the baseline values before the OLT (Fig. 2A). Interestingly, the percentage of peripheral blood cmTreg cells peaked at the first week post-transplantation (Fig. 2A), decreased afterwards and stabilized over the first year. The absolute number of cmTreg cells significantly increased within 15 days post-transplantation, decreasing afterwards with a partial recovery of their numbers after the second month and going up at the end of the follow-up period (Fig. 2B).
Fig. 2.
Longitudinal changes in cmTregs within the first year after OLT. (A) cmTreg cell frequency expressed as a % of total number of CD4+ T lymphocytes; (B) absolute number of cmTreg expressed as a number of CD4+ T-lymphocytes/mm3. The lines reflect the median and interquartile range at any time point. Only significant P values are shown. *P < 0.05; **P < 0.01 and ***P < 0.001.
Recipients who suffered early AR showed higher levels of Treg cells in peripheral blood
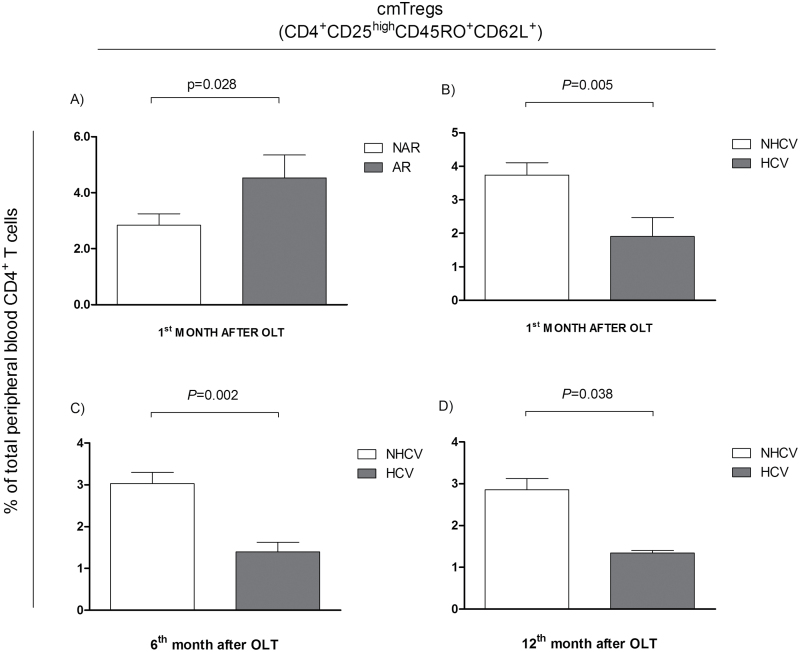
During the first year after OLT, the frequency of circulating cmTreg cells was higher in AR than that in NAR recipients. At baseline, the percentage of cmTreg cells was already higher in rejectors than in non-rejectors. Although these differences were not significant (Table 3), the percentage of cmTreg cells in rejectors persistently increased within the first month after transplantation showing significantly higher levels in AR patients at 1 month after transplantation (Fig. 3A; NAR = 2.84±2.29; AR = 4.53±2.70; P = 0.028). No significant differences in the absolute number of cmTreg cells were observed between the study groups (data not shown). Since the frequency of cmTreg cells increased distinctively in rejectors as compared with non-rejectors straight after the surgery up to first month, an additional study was performed considering the cmTreg cell percentages obtained at 1 week + 2 week + 1 month as a single value to assess its overall increase within the first month. The differences on the frequency of cmTreg cells between rejectors and non-rejectors were significant (P = 0.019; Fig. 4A).
Table 3.
Frequency of cmTreg cells during the first year after liver transplantation
| Biomarker | % CD4+CD25highghCD45RO+CD62L+ | % CD4+CD25highghCD45RO+CD62L+ | ||||
|---|---|---|---|---|---|---|
| Profile | NAR (n = 44) | AR (n = 15) | P value | NHCVR (n = 46) | HCVR (n = 16) | P value |
| Basal | 3.19±1.79 | 3.91±1.94 | 0.144 | 3.60±1.94 | 2.61±1.21 | 0.100 |
| First week | 5.71±4.09 | 8.47±5.82 | 0.145 | 6.84±4.63 | 5.00±5.75 | 0.162 |
| Second week | 4.88±3.15 | 5.95±4.04 | 0.475 | 5.34±3.19 | 4.43±4.58 | 0.245 |
| First month | 2.84±2.29 | 4.53±2.71 | 0.028* | 3.74±2.49 | 1.91±2.01 | 0.005** |
| Second month | 1.80±0.93 | 2.66±1.85 | 0.376 | 2.18±1.13 | 1.77±1.47 | 0.141 |
| Third month | 2.12±1.54 | 4.09±2.87 | 0.166 | 2.90±2.21 | 1.66±1.29 | 0.176 |
| Sixth month | 2.36±1.72 | 2.90±1.78 | 0.433 | 3.03±1.80 | 1.40±0.82 | 0.002** |
| First year | 2.59±1.76 | 1.49±0.50 | 0.201 | 2.86±1.80 | 1.34±0.22 | 0.038* |
The frequency of cmTreg cells from patients with AR (n = 15) and NAR (n = 49) were monitored during the first year after OLT and are represented as mean ± SD. The frequency of cmTreg cells from patients who had suffered HCVR (n = 16) or NHCVR (n = 48) are represented as mean ± SD.
*P < 0.05; **P < 0.01.
Fig. 3.
Percentage of medium fluorescence intensity (MFI) as frequency of peripheral blood cmTreg cells with respect to the total number of peripheral blood CD4+ T cells in liver transplant recipients. (A) % of cmTreg cells in AR and NAR patients at 1 month after transplant. (B) % of cmTreg in HCV and NHCV patients at 1 month post-transplant. (C) % of cmTreg cells in in HCV and NHCV patients at 6 months post-transplant. (D) % of cmTreg cells in HCV and NHCV patients at 12 months post-transplant. Data show mean ± SD.
Fig. 4.
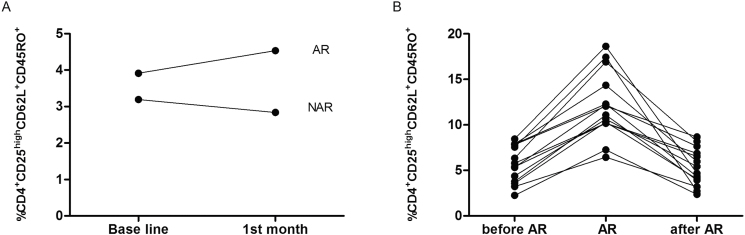
(A) Changes in the frequency of cmTreg cells within the first month after transplantation. The differences in the peripheral levels of cmTreg cells between patients with AR (upper line) and without AR (lower line) were significant (P = 0.019) when comparing the levels together corresponding to the first week + second week + first month. (B) During acute allograft rejection episodes, cmTreg cell levels were higher than observed before rejection or after successful anti-rejection treatment (P = 0.022).
During AR episodes, cmTreg cell levels were higher than observed before rejection or after successful anti-rejection treatment (P = 0.022; Fig. 4B). After AR cmTreg cell levels diminished as previously demonstrating a complete recovery of its levels after successful anti-rejection treatment.
In order to establish whether cmTreg cells in rejectors independently increased of the immunosuppressant regimen, since it is a well-known fact that, based on mTOR signaling, immunosuppressors control the differentiation and functions of Treg cells (25, 26); we performed a further study comparing the frequency of cmTreg cells between AR patients who received TRL and MMF as well as with regard to average drug level with non-significant differences.
HCVR patients showed a low number of peripheral blood cmTreg cells after OLT
Sixteen patients developed HCVR during the first year after OLT. When we analyzed cmTreg cells with respect to the total number of CD4+ T cells, the data showed that the percentage of cmTreg cells was significantly higher in NHCVR than in HCVR patients at 1 month (3.7±2 versus 1.9±2; P = 0.005; Fig. 3B), 6 months (3±1.8 versus 1.4±0.8; P = 0.002; Fig. 3C) and 1 year (2.9±1.8 versus 1.3±0.2; P = 0.038; Fig. 3D) after OLT. Data are summarized in Table 3. cmTreg cell frequencies did not discriminate patients at risk of HCVR and were not used for the subsequent analysis.
In patients with HCVR during the study period, 14 (87.5%) did not develop AR and only 2 (12.5%) suffered AR within the first month after transplantation. Although the AR rate in HCVR patients was small, we performed a further study to investigate whether the percentage of cmTreg cells on HCVR patients might have affected in both AR and NAR patients. No significant differences were found between them at all time points within the first month after transplantation.
Frequencies of peripheral blood cmTreg cells discriminate patients at high risk of early AR
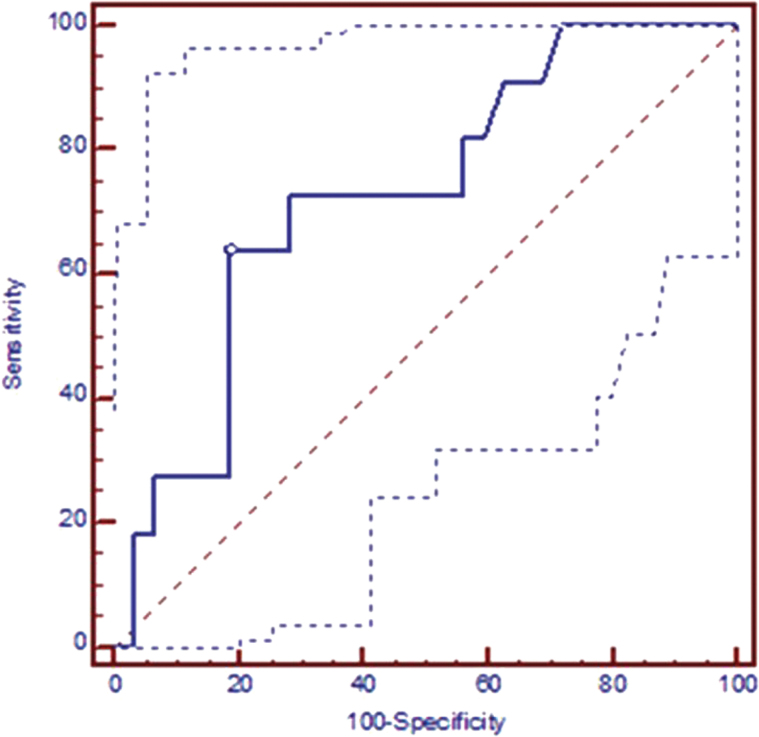
The cmTreg cells showing significant differences in the aforementioned tests were used in an additional analysis based on ROC curves. Cutoff values for early AR were determined based on the AUC for the percentage of cmTreg cells with significantly higher levels in patients who rejected. Cutoff values for the percentage of cmTreg cells with respect to the total number of CD4+ T cells within 1 month after transplantation accurately discerned between rejectors and non-rejectors (Fig. 5). The AUC for cmTreg cells expressed as total PB CD4+ T cells was 0.724 (P = 0.0106). ROC curve analysis showed that patients with a percentage of cmTreg cells higher than 3.7%, sensitivity of 72.73% and specificity of 81.25% had high risk of early AR. Data are summarized in Table 4.
Fig. 5.
Post-transplantation ROC curves for discriminating between rejectors and non-rejectors in liver transplant recipients. In liver patients, cutoff values for % cmTreg (represented as a percentage of total number of CD4+ peripheral blood T-cells) within 1 month after transplantation, AUC = 0.724 (95% CI: 0.567–0.849); P = 0.0106. CI, confidence interval.
Table 4.
Post-transplantation cutoff value, sensitivity and specificity for potential biomarkers of AR allow discrimination between AR and NAR patients
| Orthotopic liver transplant recipients | AUC | P value | Cutoff | Sensitivity (95% CI)a | Specificity (95% CI)a | +PV (%) | -PV (%) |
|---|---|---|---|---|---|---|---|
| Profile: first month post-transplantation | |||||||
| (%)CD4+CD25highghCD45RO+CD62L+ | 0.724 | 0.0106 | 3.70 | 72.73 (39.0–94.0) | 81.25 (63.6–92.8) | 3.39 | 0.45 |
The percentage of cmTreg cells at 1 month after transplantation, with respect to the total number of CD4+ peripheral blood T cells accurately discriminated between AR and NAR patients. CI, confidence interval; +PV, positive predictive value; -PV, negative predictive value.
aUnits are expressed as percentages.
The high percentage of peripheral cmTreg cells had an associated increased risk for early AR within the first month after liver transplantation
On the basis of previous findings for the percentage of cmTreg cells at all time points within 1 month after transplantation, we carried out a logistic regression model to evaluate the odd ratio (OR) for early AR, taking into account clinical parameters. The logistic regression coefficients of these variables can be used to calculate the OR of AR for an individual patient, which can be used to determine the probability of AR with the formula elogit AR/1 + elogit AR. The model was constructed for the first month after OLT as well as within first month, when the incidence of AR was the highest of the study period. First, we modeled cmTreg cells individually at 1 week, 2 weeks and 1 month after OLT. Afterwards, we joined these time points together (first week + second week + first month) into what we have called ‘first month post-transplantation’ profile.
A percentage of cmTreg cells, referred to as total CD4+ T cells, higher than 3.7% was associated with a 1.1-fold increase in the risk of AR (P = 0.05) at 1 month after transplantation. Similarly, the risk for early AR increased 1.3-fold (P = 0.008) when we evaluated the percentage of cmTreg cells throughout the first month after transplantation. All the logistic regression model coefficients are summarized in Table 5.
Table 5.
Post-transplantation prediction model for assessing risk of AR in liver transplant recipients
| Biomarker | Coefficient | P value | OR | 95% CI |
|---|---|---|---|---|
| Univariate logistic regression model | ||||
| Profile: first month post-transplantation | ||||
| CD4+CD25highghCD45RO+CD62L+ | 0.261 | 0.05 | 1.131 | 1.233–1.363 |
| Constant | −2.001 | 0.002 | 0.135 | − |
| Logit AR = −2.001 + (CD4+CD25highghCD45RO+CD62L+ × 0.261) | ||||
| Profile: first week–first month post-transplantation | ||||
| CD4+CD25highghCD45RO+CD62L+ | 0.123 | 0.008 | 1.298 | 1.074–1.188 |
| Constant | −1.584 | 0.000 | 0.205 | − |
| Logit AR = −1.584 + (CD4+CD25highghCD45RO+CD62L+ × 0.123) | ||||
The formula for logit AR derived from the coefficients is also shown. Logit AR can be used to predict the probability of AR with the formula elogit AR/1+elogit AR. CI, confidence interval.
The clinical, demographic and pharmacokinetic parameters, as assessed by univariate logistic regression model, were not significant (Table 6). Therefore, these parameters were not included in the multivariate regression model, because its predictive potential was not affected by these variables.
Table 6.
Univariate logistic regression analysis of the effect of demographic, immunological and pharmacynetic parameters on patients who developed AR after OLT
| Variable | Wald | OR | 95% CI | P |
|---|---|---|---|---|
| Gender of recipient | 0.488 | 0.520 | 0.083–3.259 | 0.485 |
| Donor age, years | 0.118 | 0.992 | 0.944–1.041 | 0.731 |
| Recipient age, years | 0.593 | 0.971 | 0.902–1.046 | 0.441 |
| HLA-A mismatches | 0.023 | 1.120 | 0.256–4.905 | 0.880 |
| HLA-B mismatches | 0.471 | 1.664 | 0.388–7.129 | 0.493 |
| HLA-DR mismatches | 1.145 | 2.400 | 0.483–11.931 | 0.285 |
| Leukocytes (×109/ml) | 0.002 | 1.004 | 0.826–1.221 | 0.968 |
| Frequency of lymphocytes | 1.074 | 0.926 | 0.800–1.071 | 0.284 |
| Absolute lymphocyte number/mm3 | 1.149 | 0.998 | 0.996–1.000 | 0.188 |
| TRL treatment | 0.023 | 0.893 | 0.204–3.910 | 0.880 |
| TRL + MMF treatment | 0.618 | 1.857 | 0.397–8.689 | 0.432 |
| Dose TRL, mg/day | 0.422 | 0.935 | 0.764–1.145 | 0.516 |
| Dose MMF, mg/day | 0.031 | 1.000 | 0.998–1.002 | 0.861 |
| C min TRL, ng/ml | 0.664 | 1.026 | 0.964–1.092 | 0.415 |
| C min MMF, μg/ml | 0.617 | 1.261 | 0.707–2.249 | 0.432 |
CI, confidence interval.
Discussion
In recent years, Treg cells have been reported to have a positive effect on the induction of tolerance in animal models (27) and in kidney graft recipients (19). However, these reports are based in most cases either on animal experimental studies or on single-center studies with relatively small cohorts of patients.
This is the first prospective multi-center study in which variations in the number of cmTreg cells in LGR have been monitored at pre- and post-transplant times in order to discriminate patients with high risk of early AR. Four Spanish centers have participated in the present study, following previously validated identical protocols in order to keep inter-laboratory variability to a minimum.
Nowadays, LGR are assessed either at baseline or in the early post-transplant period in order to efficiently identify patients with high risk of AR and to adjust the immunosuppressive treatment. In our study, doses were adjusted according to blood concentration and clinical complications in order to overcome AR; however, the analysis of cmTreg cell frequencies was performed before diagnosing AR and before the immunosuppressive treatment was given in order to prevent changes in the cmTreg cell levels.
Although we could not find significantly different frequencies of cmTreg cells able to discriminate patients for risk of AR before OLT, we observed that the percentage of cmTreg cells at baseline was already higher in patients who suffered AR. Then, cmTreg cells constantly increased straight after transplantation and up to the first month with significantly different cmTreg cell kinetics for rejectors and non-rejectors. Moreover, we analyzed the cmTreg cell levels in both study groups, and we found that at 1 month after transplantation the differences were significant and also the highest of the period.
One week after OLT, the frequency of cmTreg cells peaked, gradually went down and stabilized 2 months after OLT. In a recent report, Demirkiran et al. (28) suggested that functional CD4+CD25+ Treg cells are transferred with the donor graft and migrate from liver to PB soon after OLT. However, these CD4+CD25+ donor Treg cells that peak in the circulation at 1 week after transplantation rapidly declined thereafter. This could explain the increased number of peripheral Treg cells observed 1 week after OLT.
Just after the first week after OLT, the number of cmTreg cells in LGR started to decrease rapidly until the second month after OLT and stabilized for the remainder of the study period. These results are consistent with several reports showing that the number of Treg cells decreases over the first year after liver or kidney transplantation (29, 30), which could be explained by the immunosuppressant therapy, since the use of certain immunosuppressive agents, such as calcineurin inhibitors, may lead to lower numbers of Treg cells, as compared with other immunosuppressants such as rapamycin (18, 31).
In addition, we have also observed higher post-transplant frequencies of circulating cmTreg cells in total peripheral blood CD4 T cells within as well as at 1 month after OLT in AR patients than in NAR recipients. We presume that higher peripheral cmTreg cell levels are an indication of risk of rejection. This has been recently suggested for lung (32) and kidney transplant recipients (33), in which AR patients showed an increase in circulating Treg cells. Using the cutoff values based on the AUC of the ROC analysis, we have found that levels of cmTreg cells above 3.7% were associated with a 1.1- and a 1.3-fold increase in the risk of AR at 1 month as well as within 1 month, respectively, after transplantation. These data are in concordance with data recently published by our group showing that the frequency of aTreg was able to discriminate patients undergoing kidney transplantation with high risk of AR at baseline (33). Although differences before OLT were not sustained, we found correlation between AR and the increment of circulating cmTreg cells within the first month after transplantation.
In our cohort, the number of cmTreg cells in HCVR patients after OLT displayed lower frequencies of Treg cells than NHCVR patients did. The reason for a low number in these patients could be that Treg cells are being recruited to the allograft. Recently, Riezu-Boj et al. (34) reported that intrahepatic levels of CCL17 and CCL22 chemokines were higher in HCV patients than in controls and that these higher levels of chemokines enhanced Treg cell migration favoring the accumulation of Treg cells cell in liver. These results might suggest that cmTreg activity is lower in presence of viral infection as previously described (35).
We also investigated the potential role of cmTreg cell levels as a tool for predicting early AR episodes in patients who suffer HCVR. First, it has to be noted that should HCVR have an effect on the frequency of cmTreg cells, one would expect this to be decreased overall in rejectors, because patients are exposed to a response against HCV migrating out into the peripheral circulation, as previously described. In our study, however, we found no correlation between the frequency of cmTreg cells on HCVR patients and rejection episodes, which we believe is a further indication of the limited impact of cmTreg cell kinetics in other events different from rejection. Second, it is a well-known fact that the frequency of AR episodes in patients with HCVR is most times underestimated because clinical and laboratory manifestations of AR are usually nonspecific, and histopathologic assessment is one of the mainstays for its diagnosis and treatment. Nevertheless, diagnosis of AR may be hard, since the histological picture of AR may reflect a component of recurrent hepatitis C (36, 37).
In the field of post-transplantation immune-vigilance, an increasing number of studies are focusing on the potential role of certain molecules and cells in responses as pharmacodynamic biomarkers of immune responses against grafts. Millán et al. (38) demonstrated in recent work that monitoring the intracellular expression of IFN-γ and IL-2 in liver transplant recipients within the first year after transplantation can help in managing patients with high risk of AR. Interestingly, not only IFN-γ or IL-2, but also IL-17 has been related with high risk of AR (39), indicating that these three cytokines can be used as predictive biomarkers of AR after kidney or liver transplantation.
In the present study, we decided not to perform intra-graft cmTreg cell analysis on biopsies since the aim of this study was to implement a non-invasive method; however, the intra-graft expression might be included in future investigations in order to corroborate our findings in peripheral blood.
Regardless of the relatively low number of monitored transplant recipients, although this is common to many reported transplantation studies, and the lack of an external validation cohort preferably with non-Caucasian patients, we have obtained significant differences between tested groups. Despite all the limitations, this observational prospective multi-center study provides preliminary indications that the post-transplantation analysis of the frequency of circulating cmTreg cells may help identify liver transplant recipients with high risk of AR. Nevertheless, our present study is based on phenotypic data, and it must be completed with functional data in our future studies.
The main aim of the present work was to establish the potential role of cmTreg cells as a concomitant factor for early AR in liver recipients. We found that the higher peripheral cmTreg cell levels could be useful for both predicting patients with high risk of early AR as well as for monitoring the immune-response against the graft within the first month after OLT in order to adjust immunosuppressive treatment.
Therefore, we conclude that although we did not find differences in cmTreg cell levels at baseline, patients who suffered AR during the first month after OLT also showed higher frequencies of peripheral CD4+CD25highCD45RO+CD62L+, which could be useful for assessing the immune status of graft recipients.
Funding
CIBERehd is funded by the Instituto de Salud Carlos III. The present study was partially supported by grants from the Fondo de Investigaciones Sanitarias - Instituto de Salud Carlos III (ISCIII) (PI080300, PI080157, PI080160, PI080446, PI1102686, PI1109900 and PI1501370).
Acknowledgements
The authors would like to thank the physicians and patients from the four participating centers: Hospital Clinic, Barcelona; Hospital Universitario Marques de Valdecilla, Santander; Hospital 12 de Octubre, Madrid; and Hospital Clinico Universitario Virgen de la Arrixaca, Murcia. O. M., M. B., M. M., E. P. and M. L. H. planned the experiments; P. M. C. and A. R. performed the experiments; E. M., J. M. B., L. A., F. B., D. S., and A. R. analyzed the data; E. F., L. R. V., A. M., S. L. and A. R. contributed other essential data and material; M. M., F. B., M. L. H. and A. M. wrote the paper.
Conflict of interest statement: The authors declared no conflict of interests.
Footnotes
The original version was incorrect. Errors were included within Figures 3 and 4. The legend for Table 4 was also incorrect.
References
- 1. Wiesner R. H., Demetris A. J., Belle S. H., et al. 1998. Acute hepatic allograft rejection: incidence, risk factors, and impact on outcome. Hepatology 28:638. [DOI] [PubMed] [Google Scholar]
- 2. Asfar S., Metrakos P., Fryer J., et al. 1996. An analysis of late deaths after liver transplantation. Transplantation 61:1377. [DOI] [PubMed] [Google Scholar]
- 3. König V., Bauditz J., Lobeck H., et al. 1992. Hepatitis C virus reinfection in allografts after orthotopic liver transplantation. Hepatology 16:1137. [DOI] [PubMed] [Google Scholar]
- 4. Maloy K. J. and Powrie F. 2001. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2:816. [DOI] [PubMed] [Google Scholar]
- 5. Mason D. and Powrie F. 1998. Control of immune pathology by regulatory T cells. Curr. Opin. Immunol. 10:649. [DOI] [PubMed] [Google Scholar]
- 6. Pillai A. A. and Levitsky J. 2009. Overview of immunosuppression in liver transplantation. World J. Gastroenterol. 15:4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sakaguchi S., Ono M., Setoguchi R., et al. 2006. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212:8. [DOI] [PubMed] [Google Scholar]
- 8. Jonuleit H. and Schmitt E. 2003. The regulatory T cell family: distinct subsets and their interrelations. J. Immunol. 171:6323. [DOI] [PubMed] [Google Scholar]
- 9. Sakaguchi S. Sakaguchi N. Asano M. Itoh M. and Toda M. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151. [PubMed] [Google Scholar]
- 10. Valmori D. Merlo A. Souleimanian N. E. Hesdorffer C. S. and Ayyoub M. 2005. A peripheral circulating compartment of natural naive CD4 Tregs. J. Clin. Invest. 115:1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miyara M., Yoshioka Y., Kitoh A., et al. 2009. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30:899. [DOI] [PubMed] [Google Scholar]
- 12. Sanchez Rodriguez R., Pauli M. L., Neuhaus I. M., et al. 2014. Memory regulatory T cells reside in human skin. J. Clin. Invest. 124:1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Demirkiran A., Kok A., Kwekkeboom J., et al. 2006. Low circulating regulatory T-cell levels after acute rejection in liver transplantation. Liver Transpl. 12:277. [DOI] [PubMed] [Google Scholar]
- 14. Stenard F., Nguyen C., Cox K., et al. 2009. Decreases in circulating CD4+CD25hiFOXP3+ cells and increases in intragraft FOXP3+ cells accompany allograft rejection in pediatric liver allograft recipients. Pediatr. Transplant. 13:70. [DOI] [PubMed] [Google Scholar]
- 15. Carpentier A., Conti F., Stenard F., et al. 2009. Increased expression of regulatory Tr1 cells in recurrent hepatitis C after liver transplantation. Am. J. Transplant. 9:2102. [DOI] [PubMed] [Google Scholar]
- 16. Clavien P. Medical Care of the Liver Transplant Patient. 4th edn John Wiley & Sons, Ltd, Oxford, 2012. [Google Scholar]
- 17.Anonymous. 1997. Banff schema for grading liver allograft rejection: an international consensus document. Hepatology 25:658. [DOI] [PubMed] [Google Scholar]
- 18. Segundo D. S., Ruiz J. C., Izquierdo M., et al. 2006. Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplant recipients. Transplantation 82:550. [DOI] [PubMed] [Google Scholar]
- 19. San Segundo D., Fernández-Fresnedo G., Ruiz J. C., et al. 2010. Two-year follow-up of a prospective study of circulating regulatory T cells in renal transplant patients. Clin. Transplant. 24:386. [DOI] [PubMed] [Google Scholar]
- 20. Youden W. J. 1950. Index for rating diagnostic tests. Cancer 3:32. [DOI] [PubMed] [Google Scholar]
- 21. Hanley J. A. and McNeil B. J. 1982. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29. [DOI] [PubMed] [Google Scholar]
- 22.Hosmer, D. and Lemeshow, S. 2000. Model-building strategies and methods for logistic regression. In: Hosmer D, Lemeshow S, (eds). Applied Logistic Regression. 2nd edn Wiley, New York, 91. [Google Scholar]
- 23. Hilbe J. 2009. Model development. In: Hilbe, J., ed. Logistic Regression Models. Chapman & Hall/CRC Press, Boca Raton, 73. [Google Scholar]
- 24. Harrell F. E. 2001. Regression Modeling Strategies: With Applications to Linear Models Logistic Regression, and Survival Analysis. Springer-Verlag, New York. [Google Scholar]
- 25. Chapman N. M. and Chi H. 2014. mTOR signaling, Tregs and immune modulation. Immunotherapy 6:1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shan, J., Feng, L., Sun, G. et al 2014. Interplay between mTOR and STAT5 signaling modulates the balance between regulatory and effective T cells. Immunobiology S0171–2985:00211. [DOI] [PubMed] [Google Scholar]
- 27. Graca L. Thompson S. Lin C. Y. Adams E. Cobbold S. P. and Waldmann H. 2002. Both CD4(+)CD25(+) and CD4(+)CD25(-) regulatory cells mediate dominant transplantation tolerance. J. Immunol. 168:5558. [DOI] [PubMed] [Google Scholar]
- 28. Demirkiran A., Bosma B. M., Kok A., et al. 2007. Allosuppressive donor CD4+CD25+ regulatory T cells detach from the graft and circulate in recipients after liver transplantation. J. Immunol. 178:6066. [DOI] [PubMed] [Google Scholar]
- 29. Demirkiran A. Kok A. Kwekkeboom J. Metselaar H. J. Tilanus H. W. and van der Laan L. J. 2005. Decrease of CD4+CD25+ T cells in peripheral blood after liver transplantation: association with immunosuppression. Transplant. Proc. 37:1194. [DOI] [PubMed] [Google Scholar]
- 30. Wekerle T. 2008. T-regulatory cells-what relationship with immunosuppressive agents? Transplant. Proc. 40(Suppl. 10):S13. [DOI] [PubMed] [Google Scholar]
- 31. Qu Y., Zhang B., Zhao L., et al. 2007. The effect of immunosuppressive drug rapamycin on regulatory CD4+CD25+Foxp3+T cells in mice. Transpl. Immunol. 17:153. [DOI] [PubMed] [Google Scholar]
- 32. San Segundo D., Brunet M., Ballesteros M. A., et al. 2012. Prospective study of biomarkers of immune response in lung transplant recipients. Transplant. Proc. 44:2666. [DOI] [PubMed] [Google Scholar]
- 33. San Segundo D., Millán O., Muñoz-Cacho P., et al. 2014. High proportion of pretransplantation activated regulatory T cells (CD4+CD25highCD62L+CD45RO+) predicts acute rejection in kidney transplantation: results of a multicenter study. Transplantation 98:1213. [DOI] [PubMed] [Google Scholar]
- 34. Riezu-Boj J. I., Larrea E., Aldabe R., et al. 2011. Hepatitis C virus induces the expression of CCL17 and CCL22 chemokines that attract regulatory T cells to the site of infection. J. Hepatol. 54:422. [DOI] [PubMed] [Google Scholar]
- 35. Utsumi M., Takaki A., Umeda Y., et al. 2014. Frequency of regulatory T-cell and hepatitis C viral antigen-specific immune response in recurrent hepatitis C after liver transplantation. Transpl. Immunol. 31:33. [DOI] [PubMed] [Google Scholar]
- 36. Regev A., Molina E., Moura R., et al. 2004. Reliability of histopathologic assessment for the differentiation of recurrent hepatitis C from acute rejection after liver transplantation. Liver Transpl. 10:1233. [DOI] [PubMed] [Google Scholar]
- 37. Petrovic L. M. Villamil F. G. Vierling J. M. Makowka L. and Geller S. A. 1997. Comparison of histopathology in acute allograft rejection and recurrent hepatitis C infection after liver transplantation. Liver Transpl. Surg. 3:398. [DOI] [PubMed] [Google Scholar]
- 38. Millán O., Rafael-Valdivia L., Torrademé E., et al. 2013. Intracellular IFN-γ and IL-2 expression monitoring as surrogate markers of the risk of acute rejection and personal drug response in de novo liver transplant recipients. Cytokine 61:556. [DOI] [PubMed] [Google Scholar]
- 39. Millán O., Rafael-Valdivia L., San Segundo D., et al. 2014. Should IFN-γ, IL-17 and IL-2 be considered predictive biomarkers of acute rejection in liver and kidney transplant? Results of a multicentric study. Clin. Immunol. 154:141. [DOI] [PubMed] [Google Scholar]