TSLP and IL-33 are variably required for different manifestations of nasal allergy
Keywords: immunoglobulin E, interleukin-33, mast cells, nose, thymic stromal lymphopoietin
Abstract
Thymic stromal lymphopoietin (TSLP) and IL-33 are epithelium-derived proallergic cytokines that contribute to allergic diseases. Although the involvement of TSLP in allergic rhinitis (AR) is suggested, the exact role of TSLP in AR is poorly understood. Furthermore, the relative contribution of TSLP and IL-33 in nasal allergic responses has not been described. In this study, we examined the roles of TSLP and IL-33 in AR by analyzing acute and chronic AR models. Acute AR mice were intraperitoneally immunized with ragweed, then intranasally challenged with ragweed pollen for four consecutive days. Chronic AR mice were nasally administrated ragweed pollen on consecutive days for 3 weeks. In both models, TSLP receptor (TSLPR)-deficient mice showed defective sneezing responses and reduced serum ragweed-specific IgE levels compared with wild-type (WT) mice. Analyses of bone-marrow chimeric mice demonstrated that hematopoietic cells were responsible for defective sneezing in TSLPR-deficient mice. In addition, FcεRI+-cell-specific TSLPR-deficient mice showed partial but significant reduction in sneezing responses. Of note, Th2 activation and nasal eosinophilia were comparable between WT and TSLPR-deficient mice. ST2- and IL-33-deficient mice showed defective Th2 activation and nasal eosinophilia to acute, but not chronic, ragweed exposure. TSLPR and ST2 double-deficient mice showed defective Th2 activation and nasal eosinophilia even after chronic ragweed exposure. These results demonstrate that TSLPR signaling is critical for the early phase response of AR by controlling the IgE-mast-cell/basophil pathway. The IL-33/ST2 pathway is central to nasal Th2 activation during acute allergen exposure, but both TSLPR and ST2 contribute to Th2 responses in chronically allergen-exposed mice.
Introduction
Allergic rhinitis (AR) is an IgE- and Th2-mediated allergic nasal disease that affects more than 500 million people worldwide (1). Its characteristic symptoms include IgE-mast-cell/basophil-mediated early phase responses (sneezing, rhinorrhea and nasal clotting) and type-2-cytokine-mediated late-phase responses (nasal congestion associated with eosinophilia, mucus production and tissue remodeling) (1, 2). According to the concept of ‘united airway disease’, the prevalence and pathogenesis of AR is associated with that of allergic asthma (3). However, studies have shown that differential immunological events underlie the two disease entities (4–7). Thus, studies to specifically investigate the nasal immune responses that occur in allergic settings are required to fully understand AR pathogenesis and to establish novel clinical interventions.
Thymic stromal lymphopoietin (TSLP), an epithelium-derived pleiotropic cytokine, is considered a master regulator of T helper type-2 (Th2) development. TSLP-activated dendritic cells (DCs) up-regulate surface OX40-ligand expression and down-regulate IL-12 production, thus preferentially differentiating naive CD4+ T cells into a Th2 phenotype (8, 9). In addition, TSLP stimulates several cell types, including Th2 (10) and mast cells (11, 12) to augment their effector functions. Several mouse studies have indicated the critical involvement of TSLP in allergic asthma. The lung epithelia-specific over-expression of TSLP (13) or the epithelium administration of TSLP and antigen (14) induced asthma-like symptoms in mice that were associated with Th2 activation and antigen-specific IgE production. In contrast, allergic asthma symptoms were significantly ameliorated in TSLP receptor (TSLPR)-deficient mice (13, 15). In addition to these animal studies, a proof-of-concept clinical trial demonstrated that asthma patients treated with an anti-TSLP mAb showed attenuated early and late-asthmatic responses induced by allergen challenge (16).
Genetic epidemiological studies predicted that polymorphisms in the TSLP locus were linked to AR (17–20). Moreover, nasal mucosa from AR patients expressed higher levels of TSLP mRNA compared with healthy controls (21–23), which were correlated to disease severity (22, 23). Although these observations suggest the involvement of TSLP in AR pathogenesis, studies regarding the effect of targeting TSLP signaling during the clinical course of AR and the nose-specific role of TSLP are currently lacking. On the basis of previously described role of TSLP in other organs, we hypothesized that TSLP might affect AR symptoms in three different ways. First, TSLP might be essential for inducing Th2 development and allergen-specific IgE production in the nose. Second, TSLP could be involved in stimulating sensory neurons to induce sneezing or itching responses, as TSLP was recently described as a direct activator of sensory neurons in an atopic dermatitis model (24). Third, TSLP might promote the development and/or activation of nasal mucosa mast cells (and perhaps basophils) that are critical effector cells of AR.
In addition to TSLP, other epithelium-derived proallergic cytokines are also considered to participate in AR pathogenesis (25). We previously demonstrated the critical role of IL-33 in a ragweed-specific acute AR model (26). In IL-33-deficient mice, ragweed-challenge-induced sneezing and nasal eosinophilia were attenuated with reduced Th2 responses and serum IgE levels (26). These observations suggested that IL-33 is central to nasal Th2 responses. However, it is unknown whether targeting IL-33-signaling prevents AR symptoms even in chronically allergen-exposed mice. In addition, although nasal TSLP and IL33 levels were correlated in a human nasal allergy study (27), the relative contribution of the two cytokines in nasal allergic responses is currently unknown.
In this study, we investigated the role of TSLPR and IL-33/ST2 pathways in murine AR models. TSLPR-deficient (Crlf2 −/−) mice showed ameliorated early phase responses (sneezing) in ragweed-specific AR models. In contrast, Crlf2 −/− mice showed normal late-phase responses and nasal Th2 activation. This study demonstrates the contribution of TSLPR- and ST2-signaling pathways in nasal Th2 development and late-phase responses of AR in acute and chronic allergen-exposed mice.
Methods
Mice
Wild-type (WT) BALB/c mice were purchased from Charles River Laboratories Japan, Inc. (Yokohama, Japan). BALB/c-background Fcer1a mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). BALB/c-background Crlf2 −/− (13, 28) and Il33 −/− (26) mice were previously described. Il1rl1 −/− (29) mice were backcrossed onto BALB/c mice over 11 generations. Crlf2 −/− and Il1rl1 −/− mice were intercrossed to generate Crlf2 −/− Il1rl1 −/− mice. All mice were maintained under specific pathogen-free conditions in the animal facilities of the Hyogo College of Medicine. All animal experiments were performed with approval of and in accordance with the guidelines of the Institutional Animal Care Committee of Hyogo College of Medicine (no. A11-235, no. 14-007 and no. 28028).
Reagents
FITC-anti-mouse-IgE mAb (23G3) was purchased from Southern Biotechnology Associates, Inc. (Birmingham, AL, USA). FITC-anti-mouse-CCR3 mAb (83101), PE-anti-mouse-Siglec-F mAb (E50-2440) and recombinant mouse IL-3 were purchased from R&D Systems (Minneapolis, MN, USA). PerCP/Cy5.5-anti-mouse-c-Kit mAb (2B8), Pacific-Blue-anti-mouse-CD45 mAb (30-F11), APC-anti-mouse-FcεRI mAb (MAR-1) and APC-anti-mouse-CD49b mAb (DX5) were purchased from Bio Legend (San Diego, CA, USA). Recombinant mouse TSLP was purchased from eBioscience (San Diego, CA, USA). Ragweed pollen was purchased from PolyScience (Niles, IL, USA). Ragweed extract was purchased from LSL Co Ltd (Tokyo, Japan). Histamine dihydrochloride was purchased from Wako (Osaka, Japan). Mouse monoclonal IgE against ovalbumin (OVA) was purchased from Chondrex, Inc. (Redmond, WA, USA). Recombinant human IL-2 was purchased from PeproTech (Hamburg, Germany). Aluminum hydroxide hydrate, neomycin, OVA Grade V, polymyxin B and p-nitrophenyl-N-acetyl-β-d-glucosaminide (PNAG) were purchased from Sigma-Aldrich Japan (Tokyo, Japan).
Murine models of AR
Acute AR model.
The acute AR mouse model was induced as previously described (26). Briefly, mice were immunized against ragweed by intraperitoneal (i.p.) injection of ragweed pollen (100 μg) and aluminum hydroxide hydrate gel (1mg) in 200 μl of PBS on day 0 and with ragweed pollen (100 μg) in 200 μl of PBS on day 7. These mice were then intranasally (i.n.) challenged with ragweed pollen (1mg in 20 μl of PBS) or PBS alone (20 μl) for four consecutive days (days 14–17).
Chronic AR model.
The chronic AR mouse model was induced as previously described (30). Briefly, mice were i.n. administrated ragweed pollen (1mg in 20 μl of PBS) or PBS alone (20 μl) for six consecutive days in the first and second week, and four consecutive days in the third week (days 0–5, 7–12 and 14–17).
In both models, the frequency of sneezing was counted immediately after each nasal challenge for 10min. In some experiments, mice were i.n. challenged with ragweed pollen (1mg in 20 μl of PBS) for three or seven consecutive days.
Generation of bone-marrow chimeric mice
To generate reciprocal bone-marrow (BM) chimeric mice using WT or Crlf2 −/− mice, recipient mice (WT or Crlf2 −/− mice) were lethally irradiated (9 Gy) and reconstituted by intravenous (i.v.) injection of BM cells from WT or Crlf2 −/− mice. To generate mixed BM chimeric mice, WT mice were lethally irradiated (9 Gy) and reconstituted by i.v. injection of mixed BM cells from Fcer1a mice and WT or Crlf2 −/− mice at a 1:1 ratio. The chimeric mice were provided with drinking water containing neomycin (1mg ml–1) and Polymyxin B (1000U ml–1) for 4 weeks. The mice were used from 8 weeks after reconstitution.
In vitro cervical lymph node cell culture to measure cytokine production
Cervical lymph node (cLN) cells were isolated from mice and cultured for 5 days in 96-well plates at 2×105 per 0.2ml per well with IL-2 (100 pmol l–1) and ragweed extract (5mg ml–1) in the presence of 1×105 irradiated conventional antigen-presenting cells (T-cell-depleted BALB/c splenocytes) in complete medium [RPMI-1640 (Sigma, St Louis, MO, USA) supplemented with 10% (vol/vol) fetal calf serum, penicillin (100U ml–1) streptomycin (100mg ml–1), l-glutamine (2mM) and 2-mercaptoethanol (50mM)]. The culture supernatants were collected and cytokine concentrations were assessed by ELISA using the DuoSet kit for mouse IL-4, IL-5 and IL-13 (R&D Systems).
Evaluation of nasal eosinophilic infiltration
Noses were removed from mice after facial skin was stripped. Hematoxylin and eosin staining was previously described (30). Briefly, samples were immediately fixed in 4% paraformaldehyde for 3 days and decalcified in 0.12mol l–1 EDTA solution (pH 6.5) for 7 days at room temperature. The EDTA solution was changed daily. After decalcification, tissues were embedded in paraffin, cut into 4 μm coronal sections. The numbers of eosinophils were counted by microscopy. In some experiments, noses were minced and digested for 50min at 37°C with collagenase (150U ml–1) and DNase I (10mg ml–1). Cell suspensions were filtered using a cell strainer and red blood cells were lysed. The frequency of eosinophils in nasal cells was evaluated by flow cytometry.
Flow cytometry
Nasal cells were washed in ice-cold staining buffer (1% BSA in PBS), incubated with each antibody for 20min, then washed twice with staining buffer. Data were acquired by a FACSCanto II flow cytometer (BD Biosciences) and analyzed using FlowJo software (version 7.6.1, Tree Star Inc., Ashland, OR, USA). Siglec-F+CCR3+ cells gated as side scatter (SSC)high CD45+ cells were defined as eosinophils.
ELISA for serum total and ragweed-specific IgE
Peripheral blood was collected from the inferior vena cava or heart 24h after the final nasal challenge. The serum IgE levels were measured by ELISA. Rat anti-mouse-IgE mAbs (23G3) (Southern Biotechnology Associates, Inc.) were used as capture antibodies. Rat anti-mouse-IgE mAb (R35-118) biotin-conjugated (BD Biosciences) or homemade biotinylated ragweed extracts (26, 31) were used as secondary reagents for total or ragweed-specific IgE measurements, respectively.
β-Hexosaminidase release assay
BM-derived mast cells (BMMCs) and BM-derived basophils (BMBAs) were prepared from WT or Crlf2 −/− mouse BM cells. BM cells were cultured with IL-3 (10U ml–1) in complete medium for 2 weeks (for BMBAs) or 4–6 weeks (for BMMCs) with medium change twice a week. BMBAs were sorted by a FACSAria II (BD Bioscience) as FceRI+c-Kit− cells. The cells were sensitized with mouse monoclonal IgE against OVA (100ng ml–1) for 18h at 37°C. Following sensitization, the cells were washed with MT buffer (137 mmol l–1 NaCl, 2.7 mmol l–1 KCL, 1.8 mmol l–1 CaCl2, 1 mmol l–1 MgCl2, 5.6 mmol l–1 glucose, 20 mmol l–1 HEPES, 0.1% BSA). The cells were incubated in a 96-well plate (1×105 per 200 μl per well) in MT buffer with or without OVA (1 or 10 μg ml–1) for 30min at 37°C. After incubation, supernatants were collected (150 μl), and 150 μl of 0.1% Triton X-100 was added to the remaining supernatant (50 μl) with cell pellets to make a cell lysate. Supernatants (50 μl) and cell lysates (50 μl) were incubated with 100 μl of PNAG/citrate buffer (3.5mg ml–1) for 90min at 37°C. Overall, 50 μl of 0.4M glycine buffer was added to stop the reaction. Absorbance was measured at 405nm. β-Hexosaminidase release (%) = 100×4 × Abs (supernatant)/[4 × Abs (lysate) + 3 × Abs (supernatant)].
Quantitative PCR analysis
Nasal mRNA expression levels were examined as previously described (30). Total RNAs from nasal mucosa were isolated using the RNeasy Mini Kit (Qiagen, Venlo, The Netherlands) or Sepasol (Nakarai, Kyoto, Japan) and cDNA was synthesized using ReverTra Ace (Toyobo, Osaka, Japan). Gene expression levels were quantified with TaqMan Gene Expression Assays (Applied Biosystems) and the Thermal cycler dice RT–PCR system (Takara Bio Inc., Otsu, Japan). Results were shown as relative to eukaryotic 18S rRNA levels. The specific primers and probes used for quantitative RT–PCR were TaqMan probes for Mcpt1 (Mm00656886_g1), Mcpt2 (Mm00484932_m1), Mcpt8 (Mm00484933_m1) and 18S rRNA (Applied Biosystems).
Statistics
Continuous variables are presented as means and their standard errors (SEMs) and were compared between two groups using Student’s t-test. The time course of sneezing was compared using a linear mixed-effects model. The model included fixed effects for group, day and first-order interaction effect between group and day, with a compound symmetry covariance matrix as a covariance structure. Assessment days were treated as categorical factors. All P values were two sided, and P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS statistical software (version 22).
Results
TSLPR signaling is essential for early phase responses of AR but is dispensable for late-phase responses of AR
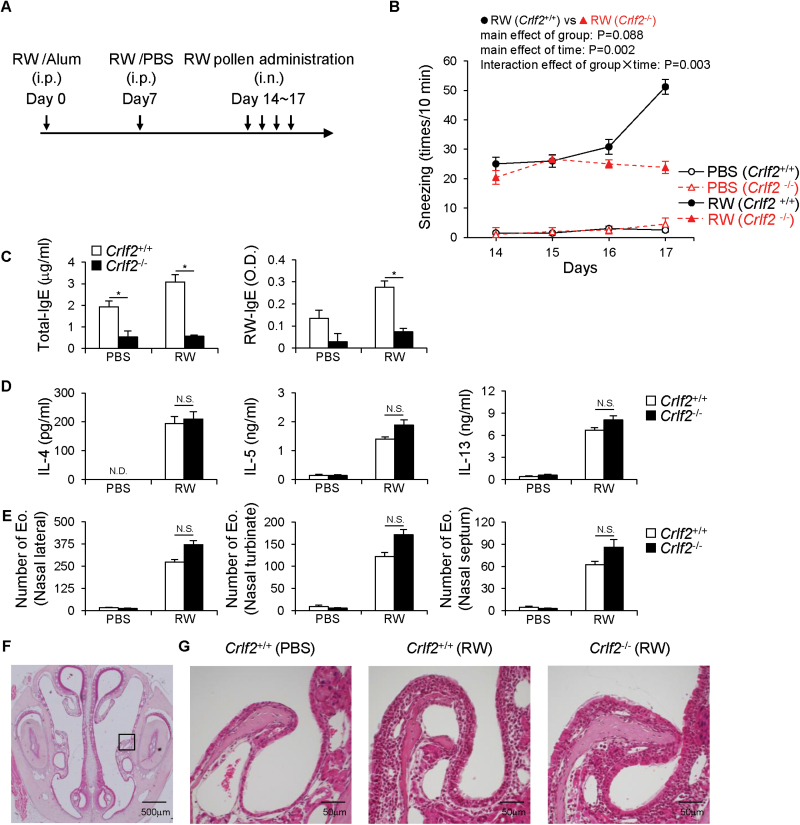
First, we investigated the role of TSLPR signaling in an acute AR model (26). WT and Crlf2 −/− mice were immunized with ragweed, then i.n. challenged with ragweed pollen for four consecutive days (days 14–17) (Fig. 1A). As previously reported (26), ragweed challenge increased the frequency of sneezing, a characteristic early phase response of AR, at day 17 in WT mice (Fig. 1B). However, Crlf2 −/− mice did not show an increase in sneezing, and sneezing counts at day 17 were the same as the first ragweed challenge (day 14) (Fig. 1B). The up-regulation of serum total- and ragweed-specific-IgE levels at day 18 was severely abrogated in Crlf2 −/− mice compared with WT mice (Fig. 1C). However, the production of type-2 cytokines (IL-4, IL-5 and IL-13) from draining cLN cells after in vitro re-stimulation with ragweed antigens (Fig. 1D) and nasal eosinophilia, which represent late-phase response of AR and nasal Th2 activation in experimental models of AR, at day 18 (Fig. 1E–G) were comparable between WT and Crlf2 −/− mice.
Fig. 1.
TSLPR signaling is required for early phase responses in murine acute allergic rhinitis. WT (Crlf2 +/+) and TSLPR-deficient (Crlf2 −/−) mice were i.p. immunized with ragweed (RW), then i.n. challenged with PBS or RW pollen for four consecutive days. (A) Experimental schema. (B) The number of sneezes was counted immediately after each nasal challenge for 10min. (C) Mouse sera were collected 1 day after the final nasal challenge (day 18). Total- and RW-specific IgE levels in the sera were determined by ELISA. (D) cLN cells were obtained from mice 1 day after the final nasal challenge (day 18). cLN cells were incubated with T-cell-depleted and irradiated splenocytes in the presence of IL-2 and RW extract for 5 days. Culture supernatants were harvested and the levels of IL-4, IL-5 and IL-13 were determined by ELISA. (E–G) Mouse nasal specimens were generated and subjected to hematoxylin and eosin staining. (E) Eosinophils (Eo) in the nasal lateral, nasal turbinate and nasal septum were counted. (F) A representative image of nasal cavity of a normal mouse is shown. (G) Magnified images of nasal lateral mucosa (black square in F) from acute allergic rhinitis mice are shown. Bars indicate 500 μm (F) and 50 μm (G). Data are representative of three independent experiments [mean, SEM, n = 2–3 (PBS), n = 5 (RW)]. *P < 0.05, N.D., not detected, N.S., not significant.
To investigate the role of nose-specific TSLPR signaling, we induced a chronic (nasally sensitized) AR model (30). WT and Crlf2 −/− mice were i.n. administered ragweed pollen for 3 weeks (Fig. 2A; days 0–5, 7–12 and 14–17). As previously reported (30), WT mice showed an increase in the frequency of sneezing from day 5 (Fig. 2B). The frequencies of sneezing in Crlf2 −/− mice were continuously lower than that of WT mice throughout the experiment (Fig. 2B). Serum total- and ragweed-specific IgE levels at day 18 were lower in Crlf2 −/− mice compared with WT mice (Fig. 2C). The production of type-2 cytokines from cLN cells (Fig. 2D) and nasal eosinophilia at day 18 (Fig. 2E and F) were comparable between WT and Crlf2 −/− mice, similar to the results of the acute AR model (Fig. 1).
Fig. 2.
TSLPR signaling is required for early phase responses in murine chronic allergic rhinitis. WT (Crlf2 +/+) and TSLPR-deficient (Crlf2 −/−) mice were i.n. administered PBS or ragweed (RW) pollen for 3 weeks. (A) Experimental schema. (B) The number of sneezes was counted immediately after each nasal challenge for 10min. (C) Mouse sera were collected 1 day after the final nasal challenge (day 18). Total- and RW-specific IgE levels in the sera were determined by ELISA. (D) cLN cells were obtained from mice 1 day after the final nasal challenge (day 18). cLN cells were incubated with T-cell-depleted and irradiated splenocytes in the presence of IL-2 and RW extract for 5 days. Culture supernatants were harvested and the levels of IL-4, IL-5 and IL-13 were determined by ELISA. (E and F) Mouse nasal specimens were generated and subjected to hematoxylin and eosin staining. (E) Eosinophils (Eo) in the nasal lateral, nasal turbinate and nasal septum were counted. (F) Magnified images of nasal lateral mucosa (black square in Fig. 1F) from chronically allergic rhinitis mice are shown. Bars indicate 50 μm. Data are representative of three independent experiments [mean, SEM, n = 2–4 (PBS), n = 4–8 (RW)]. *P < 0.05; N.D., not detected; N.S., not significant.
Taken together, these findings indicate TSLPR signaling is essential for optimal allergen-specific IgE production and the induction of early phase responses of AR (sneezing). However, nasal TSLPR signaling is dispensable for Th2 activation and the induction of late-phase responses of AR.
TSLPR signaling in sensory neurons is dispensable for AR symptoms
A recent study demonstrated that TSLP directly stimulated sensory neurons and promoted itch in an atopic dermatitis model (24). However, i.n. administration of recombinant TSLP (up to 1 μg per 20 μl per dose) did not induce sneezing or itching behavior in WT mice (data not shown). Thus, we next examined whether TSLPR signaling enhanced the histamine responsiveness of nasal sensory neurons. WT and Crlf2 −/− mice had an equal frequency of sneezing in response to i.n. administered histamine (Supplementary Fig. 1A, available at International Immunology Online). In addition, recombinant TSLP pre-treatment did not enhance histamine-induced sneezing in WT mice (Supplementary Fig. 1B, available at International Immunology Online).
To examine the direct involvement of TSLPR signaling in non-hematopoietic cells (including sensory neurons) on AR symptoms, we generated BM chimeric mice and examined sneezing responses in acute AR (Fig. 1A). WT mice reconstituted with WT BM cells showed an increased frequency of sneezing, while WT mice reconstituted with Crlf2 −/− BM cells did not (Fig. 3A). In addition, mice reconstituted with Crlf2 −/− BM cells had lower serum total- and ragweed-specific IgE levels (Fig. 3B). In contrast, sneezing responses were equally induced in WT and Crlf2 −/− mice reconstituted with WT BM cells (Fig. 3C) with similar serum levels of total- and ragweed-specific IgE (Fig. 3D).
Fig. 3.
Hematopoietic TSLPR signaling is required for early phase responses of allergic rhinitis. BM chimeric mice (A and B; Crlf2 +/+←Crlf2 +/+ and Crlf2 +/+←Crlf2 −/−, C and D; Crlf2 +/+←Crlf2 +/+ and Crlf2 −/−←Crlf2 +/+) were sensitized with ragweed (RW) and i.n. administered RW pollen as in Fig. 1A. (A, C) The number of sneezes was counted immediately after each nasal challenge for 10min. (B, D) Mouse sera were collected 1 day after the final nasal challenge (day 18). Total- and RW-specific IgE levels in the sera were determined by ELISA. Data are representative of two independent experiments [mean, SEM, n = 8 (A, B), n = 5 (C, D)]. ***P < 0.005; N.S., not significant.
These results clearly demonstrated that hematopoietic cells were responsible for the low sneezing responses observed in Crlf2 −/− mice. Our study failed to link TSLPR signaling in sensory neurons to AR symptoms.
TSLPR-mediated mast cell/basophil activation is partially involved in AR
We next investigated the involvement of TSLPR signaling in mast cell and basophil function. BMMCs from Crlf2 −/− mice showed lower IgE cross-linking-induced degranulation, as assessed by β-hexosaminidase release, compared with WT BMMCs (Supplementary Fig. 2, available at International Immunology Online). On the other hand, Crlf2 −/− BMBAs showed normal IgE cross-linking-induced degranulation (Supplementary Fig. 2, available at International Immunology Online). In addition, nasal mRNA expression levels in naive mice indicated that the expression of mast-cell-specific genes, Mcpt1 and Mcpt2, was markedly lower (approximately 47-fold and 6.7-fold, respectively) in Crlf2 −/− mice compared with WT mice (Fig. 4A). However, in allergic nasal mucosa, induced by serial nasal ragweed administration (Fig. 2A), Mcpt1 and Mcpt2 mRNA levels were increased comparably in WT and Crlf2 −/− mice (Fig. 4B). A basophil-specific gene, Mcpt8, expression levels were also slightly lower (approximately 1.8-fold) in naive Crlf2 −/− mice but were not increased after chronic ragweed exposure (Fig. 4A and B).
Fig. 4.
FcεRI+-cell-specific TSLPR signaling partially contributes to sneezing. (A and B) Nasal mucosa was obtained from naive (A) or chronically allergic rhinitis (B) WT (Crlf2 +/+) and TSLPR-deficient (Crlf2 −/−) mice. Total RNAs were extracted and subjected to quantitative polymerase chain reaction analysis for the expression of Mcpt1, Mcpt2, Mcpt8 and 18S rRNA. (C and D) Mixed BM chimeric mice (WT←Fcer1a −/−+Crlf2 +/+ and WT←Fcer1a −/−+Crlf2 −/−) were i.n. administered ragweed (RW) pollen as in Fig. 2A. (C) The number of sneezes was counted immediately after each nasal challenge for 10min. (D) Mouse sera were collected 1 day after the final nasal challenge (day 18). Total- and RW-specific IgE levels in the sera were determined by ELISA. Data are representative of two independent experiments (mean, SEM, n = indicated in figure). **P < 0.01; ***P < 0.005; N.S., not significant.
To examine the role of FcεRI+-cell-specific TSLPR signaling in AR symptoms, we generated mixed BM chimeras, in which lethally irradiated WT mice were reconstituted with WT or Crlf2 −/− BM cells and Fcer1a −/− BM cells at a 1:1 ratio. Because ragweed-specific sneezing is completely abrogated in Fcer1a −/− mice (26, 30), only FcεRI+ cells (mast cells and basophils) originating from WT or Crlf2 −/− BM cells induce sneezing responses in chimeric mice. However, antigen-specific IgE is produced normally from Fcer1a −/− cells. The chimeric mice were i.n. administered ragweed pollen for 3 weeks (Fig. 2A). Both chimeric mice showed an increase in the frequency of sneezing by repeated nasal administration of ragweed pollen (Fig. 4C). However, Fcer1a −/−+Crlf2 −/− chimeric mice showed considerably lower sneezing levels compared with Fcer1a −/−+Crlf2 +/+ chimeric mice throughout the experiment (Fig. 4C). Serum total- and ragweed-specific IgE levels were comparable between the two chimeric mice strains (Fig. 4D). These results suggested that TSLPR signaling in mast cells and basophils was partially involved in AR symptoms.
The IL-33/ST2 pathway is essential for acute, but not chronic, ragweed-pollen-exposure-induced nasal Th2 responses
Considering the critical role of TSLP in Th2 development in pulmonary allergy (13, 15), it was surprising that Crlf2 −/− mice had normal nasal Th2 responses (Figs 1 and 2). Because we previously demonstrated that IL-33 was critical for Th2 activation in an acute AR model (26), we hypothesized that the IL-33/ST2, but not TSLPR, pathway might play a central role in nasal Th2 activation. However, different from the acute model (26), the production of type-2 cytokines from cLN cells and nasal eosinophilia was normal in ST2-deficient Il1rl1 −/− (Fig. 5A and B) and IL-33-deficient Il33 −/− (Fig. 5C and D) mice. Sneezing responses in Il1rl1 −/− (Fig. 5E) and Il33 −/− (Fig. 5F) mice were abrogated at early time points (days 5–11), but were increased and reached levels comparable to that of WT mice at later time points (days 14–17). Consistent with the sneezing response, Th2 development in cLNs and nasal eosinophilia was severely abrogated in Il1rl1 −/− (Fig. 6A and B) and Il33 −/− (Fig. 6C and D) mice during short-term (3 days) ragweed pollen exposure. Th2 responses and nasal eosinophilia were still defective in Il1rl1 −/− (Fig. 6E and F) and Il33 −/− (Fig. 6G and H) mice during longer (7 days) ragweed pollen exposure, although the defect was milder compared with that at 3 days of exposure. These results suggested that IL-33 was critical for the induction of nasal type-2 immunity by acute allergen exposure, while chronic allergen exposure evoked nasal IL-33-independent pro-Th2 pathway(s) regardless of the presence or absence of systemic sensitization.
Fig. 5.
Targeting IL-33/ST2 signaling does not ameliorate symptoms in chronic murine allergic rhinitis. WT (Il1rl1 +/+) and ST2-deficient (Il1rl1 −/−) mice (A, B and E), or WT (Il33 +/+) and IL-33-deficient (Il33 −/−) mice (C, D and F) were i.n. administered ragweed (RW) pollen as in Fig. 2A. (A, C) cLN cells were obtained from mice 1 day after the final nasal challenge (day 18). cLN cells were incubated with T-cell-depleted and irradiated splenocytes in the presence of IL-2 and RW extract for 5 days. Culture supernatants were harvested and the levels of IL-4, IL-5 and IL-13 were determined by ELISA. (B) Cells in the nasal mucosa were isolated 1 day after the final nasal challenge (day 18). The frequency of Siglec-F+CCR3+ eosinophils in CD45+ cells was examined. (D) Mouse nasal specimens were generated and subjected to hematoxylin and eosin staining. Eosinophils (Eo) in the nasal mucosa were counted. (E, F) The number of sneezes was counted immediately after each nasal challenge for 10min. Data are representative of two independent experiments [mean, SEM, n = 2 (PBS), n = 4 (RW)]. N.S., not significant.
Fig. 6.
IL-33/ST2 signaling is essential for acute nasal Th2 development. WT (Il1rl1 +/+) and ST2-deficient (Il1rl1 −/−) mice (A, B, E and F), or WT (Il33 +/+) and IL-33-deficient (Il33 −/−) mice (C, D, G and H) were i.n. administered ragweed (RW) pollen for three (A–D) or seven (E–H) consecutive days. (A, C, E, G) cLN cells were obtained from mice 1 day after the final nasal challenge (day 4: A and C; day 8: E and G). cLN cells were incubated with T-cell-depleted and irradiated splenocytes in the presence of IL-2 and RW extract for 5 days. Culture supernatants were harvested and the levels of IL-4, IL-5 and IL-13 were determined by ELISA. (B, D, F, H) Cells in the nasal mucosa were isolated 1 day after the final nasal challenge (day 4: B and D; day 8: F and H). The frequency of Siglec-F+CCR3+ eosinophils in CD45+ cells was examined. Data are representative of two independent experiments [mean, SEM, n = 2–4 (PBS), n = 4 (RW)]. *P < 0.05, **P < 0.01, ***P < 0.005.
TSLP and IL-33 contribute to nasal Th2 responses
The combination of epithelium-derived proallergic cytokines, TSLP and IL-33, might control nasal Th2 responses in the chronic AR model. We generated TSLPR and ST2 (Crlf2 −/− Il1rl1 −/−) double-deficient mice. Although Crlf2 −/− (Fig. 2E and F) and Il1rl1 −/− (Fig. 5A and B) mice had normal nasal Th2 responses, Crlf2 −/− Il1rl1 −/− mice had a significantly decreased production of type-2 cytokines from cLN cells (Fig. 7A) and nasal eosinophilia (Fig. 7B) compared with WT mice after 3 weeks of nasal ragweed exposure. Sneezing responses in Crlf2 −/− Il1rl1 −/− mice were completely abrogated throughout the experiment (data not shown) as observed in Crlf2 −/− mice (Fig. 2B). In the acute model, Crlf2 −/− Il1rl1 −/− mice showed a further decrease in type-2 cytokine production from cLN cells compared with Il1rl1 −/− mice, although the difference was not statistically significant (Supplementary Fig. 3, available at International Immunology Online).
Fig. 7.
Both TSLPR and ST2 signaling contribute to Th2 responses in chronically allergen exposed noses. WT (Crlf2 +/+ Il1rl1 +/+) and TSLPR/ST2 double-deficient (Crlf2 −/− Il1rl1 −/−) mice were i.n. administered ragweed (RW) pollen as in Fig. 2A. (A) cLN cells were obtained from mice 1 day after the final nasal challenge (day 18). cLN cells were incubated with T-cell-depleted and irradiated splenocytes in the presence of IL-2 and RW extract for 5 days. Culture supernatants were harvested and the levels of IL-4, IL-5 and IL-13 were determined by ELISA. (B) Cells in the nasal mucosa were isolated 1 day after the final nasal challenge (day 18). The frequency of Siglec-F+CCR3+ eosinophils in CD45+ cells was examined. Data are representative of two independent experiments [mean, SEM, n = 2 (PBS), n = 4 (RW)]. **P < 0.01, ***P < 0.005.
Taken together, the findings indicated that nose-mediated Th2 development and late-phase responses of AR were controlled by a combination of cytokines including TSLP and IL-33, especially in chronic nasal allergy.
Discussion
In this study, we showed that TSLPR signaling was essential for inducing early phase responses of AR. However, late-phase responses of AR were not controlled solely by TSLPR signaling but by multiple factors including TSLPR- and ST2-signaling pathways. IL-33 has a central role in inducing Th2 responses in the acute AR model while both TSLPR and ST2 contribute to Th2 responses in chronic allergen-exposed mice (Table 1).
Table 1.
Epithelium-derived proallergic cytokines (TSLP and IL-33) involved in nasal symptoms in acute and chronic models of allergic rhinitis
| Early phase responses (sneezing) | Late-phase responses (eosinophilia) | Th2 responses | |
|---|---|---|---|
| Acute allergen exposure | TSLP and IL-33 (non-redundant) | Mainly IL-33 | Mainly IL-33 |
| Chronic allergen exposure | TSLP | TSLP and IL-33 (redundant) | TSLP and IL-33 (redundant) |
In acute AR models, both TSLPR- and ST2-deficient mice showed ameliorated early phase responses (sneezing), thus both cytokines non-redundantly participate in inducing sneezing. However, IL-33 is critical to nasal Th2 responses and late-phase responses (eosinophilia) by acute allergen exposure, as ST2-deficient, but not TSLPR-deficient, mice had defective Th2 responses and nasal eosinophilia. After chronic allergen exposure, TSLPR-deficient, but not ST2-deficient, mice showed ameliorated sneezing responses. Both TSLPR-deficient and ST2-deficient mice had normal nasal Th2 responses and eosinophilia in chronic AR. However, nasal Th2 responses and eosinophilia were ameliorated in TSLPR/ST2-double-deficient mice; thus, both TSLP and IL-33 were redundant in Th2 activation and late-phase responses of AR in chronic allergen-exposed noses.
The TSLPR-mediated control of AR early phase responses might be attributable to the control of antigen-specific IgE production. In line with previous studies of OVA-induced pulmonary allergy models (13–15), TSLPR was essential for optimal antigen-specific IgE production in our AR models. Both the acute and chronic models of Crlf2 −/− mice had decreased ragweed-specific IgE production. Thus, TSLPR signaling participates in nasally and systemically evoked IgE production. Because the frequency of sneezing at early time points in the chronic AR model (~day 10) depended on local IgE production (30), TSLPR signaling might be essential for local IgE production in the nose. As the local application of corticosteroids can suppress nasal symptoms of AR with minimum systemic effects (1, 2, 30), the inhibition of TSLPR signaling locally in the nose might be a therapeutic method to treat the early phase responses of AR.
Even though Crlf2 −/− mice showed reduced IgE production and ameliorated early phase responses of AR, these mice developed normal Th2 and AR late-phase responses. This was surprising considering the well-characterized role of TSLP in Th2 development and the association of Th2 cells and IgE production. Although Th2 development and IgE production usually occur concurrently, studies demonstrated the differential control of these two immunological events (31–34). Currently, it is unclear how TSLPR signaling regulates IgE production without affecting Th2 development in the nose. Because Crlf2 −/− B cells normally produce IgE in response to IL-4 plus anti-CD40 antibody, and TSLP did not enhance IL-4 plus anti-CD40-antibody-induced IgE production from WT B cells in vitro (data not shown), controlling DC and/or T-cell activation is more likely in the TSLPR-mediated regulation of IgE production. Follicular helper T cells (Tfh) are a distinct T-cell subset that control antibody production (33–35). Because a subset of Tfh cells is specifically involved in IgE production by producing IL-4 (33–36), it is of interest to investigate the role of TSLP on Tfh cell function.
A previous study demonstrated that TSLP directly activated sensory neurons and promoted itch in an atopic dermatitis model (24). Sneezing responses are also a consequence of the immune-mediated activation of afferent neurons in the nose (37). However, our study did not observe a link between TSLPR signaling in non-hematopoietic cells and sneezing responses in AR. Although we did not completely exclude the involvement of neuronal TSLPR signaling in AR, TSLPR signaling in hematopoietic cells might have a more important role in the development of AR symptoms.
Mast cells are important effector cells in AR that produce and respond to TSLP (11, 12, 38). Previous studies demonstrated that TSLP promoted mast cell development, proliferation and activation (11, 12, 38). In the current study, we showed that the IgE-cross-linking-induced degranulation of Crlf2 −/− BMMCs, but not BMBAs, was less efficient than that of WT cells. Targeting TSLPR signaling in FcεRI+ cells slightly ameliorated AR symptoms. Although Crlf2 −/− mice had functional and developmental defects in mast cells, basophils in Crlf2 −/− mice showed a much milder phenotype. In addition, only mast-cell-, but not basophil-, specific genes were increased in chronically ragweed-exposed noses. These results suggest that TSLPR signaling in mast cells, rather than in basophils, might have major contribution to the reduced sneezing responses in FcεRI+-specific TSLPR-deficient mice. A previous study with TSLP-deficient mice also showed a reduced number of mast cells in several organs at steady state (11). The i.p. injection of compound 48/80, a specific inducer of mast cell degranulation, into naive WT and TSLP-deficient mice demonstrated a lower mortality ratio in TSLP-deficient mice (11). Therefore, TSLPR signaling in mast cells might contribute to allergic responses. However, because mast cells are activated by IgE and cytokines in a chronic allergic environment, the abrogation of TSLPR signaling in mast cells (and basophils) alone had relatively little effect on sneezing frequency in AR. Because ragweed-specific sneezing was completely abrogated, yet some IgE responses remained in Crlf2 −/− mice, both IgE- and TSLPR-mediated signals in mast cells (and perhaps basophils) might contribute to the defective sneezing response in Crlf2 −/− mice.
We showed that nasal Th2 responses were not controlled by TSLP alone, but rather by multiple factors including TSLP and IL-33. Previous studies of asthma models demonstrated blocking TSLPR signaling alone ameliorated allergic symptoms associated with reduced Th2 responses (13–15). Furthermore, a study of a murine OVA-specific AR model demonstrated that treatment with a neutralizing antibody against TSLP reduced OVA-challenge-induced nasal goblet cell hyperplasia, a marker for late-phase responses of murine AR (39). A possible explanation for the difference in findings is the use of a pure antigen, OVA, while we used crude ragweed pollen in this study. The local administration of a pure antigen elicited antigen-specific T-cell-dependent epithelial TSLP production (13, 40). The T-cell/epithelia/TSLP pathway generates a vicious circle (40), and thus targeting TSLP might improve type-2 responses in such conditions. Natural allergens, such as ragweed pollen, induce multiple pathways in addition to the Th2–TSLP pathway through Toll-like-receptor ligands (41), and their enzymatic (42) or particulate (31) properties. Indeed, ragweed pollen induces the production of a variety of cytokines including IL-33 both in the nose (26) and lung (31). In addition, a chronic airway exposure to a mixture of airborne allergens induces lung pathology that is mediated by TSLP and IL-33 pathways in mice (43). Nasal TSLP and IL33 levels were reported to be positively correlated with each other in human nasal allergy (27). Thus, the ragweed-pollen-induced AR models used in the current study might represent physiologically relevant disease models.
By comparing the different ragweed exposure models, we showed that the IL-33/ST2 pathway is critical for acute nasal Th2 activation. The IL-33/ST2 pathway is essential for sneezing responses in acute, but not chronic, allergen exposure. Thus, the IL-33/ST2 pathway might have a central role in inducing acute type-2 immunity including IgE production in the nose and is not redundant with the role of nasal TSLPR signaling. However, repeated allergen exposure also evokes Th2 responses in Il1rl1 −/− and Il33 −/− mice. We showed that a combination of targeting TSLPR and ST2 significantly improved Th2 responses and late-phase responses of AR in the chronic AR model. These results demonstrated that TSLPR and ST2 signaling have a redundant role in Th2 activation in chronic allergen-exposed noses (Table 1). However, as Crlf2 −/− Il1rl1 −/− mice still demonstrated substantial levels of nasal Th2 responses in the chronic AR model, other factor(s) also participate in nasal Th2 responses.
In summary, we demonstrated that TSLPR signaling plays a critical role in the early phase response of AR by controlling the IgE-mast-cell/basophil pathway. Nose-mediated activation of Th2 responses and late-phase responses of AR are controlled by a combination of factors including TSLP and IL-33. Although TSLPR and ST2 had an important contribution to nasal Th2 responses, targeting the two cytokines did not fully prevent nasal Th2 development. Nasal allergen sensitization can precede the onset of other allergic diseases, such as asthma, and the treatment of AR can improve asthma symptoms (3, 30). Thus, further study is required to fully elucidate the mechanisms underlying nasal Th2 activation. A full understanding of nose-specific immunological events will reduce disease burden not only from AR but also from other allergic disorders.
Supplementary data
Supplementary data are available at International Immunology Online.
Funding
This work was supported by MEXT Grant-in-Aid for Scientific Research, the Strategic Research Foundation at Private Universities (S1001055); JSPS KAKENHI, a Grant-in-Aid for Scientific Research B (24390253); JSPS KAKENHI, a Grant-in-Aid for Young Scientists B (26860340); the Takeda Science Foundation; and Grant-in-Aid for Graduate Students, Hyogo College of Medicine.
Supplementary Material
Acknowledgements
We thank all members of the Yoshimoto laboratory for their support and discussions, K. Kumasako and C. Minemoto for secretarial assistance, and M. Nagata, Y. Kanazawa and M. Hitomi for technical assistance.
Conflict of interest statement: The authors have no financial conflicts of interest.
References
- 1. Brozek J. L., Bousquet J., Baena-Cagnani C. E, et al. 2010. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J. Allergy Clin. Immunol. 126:466. [DOI] [PubMed] [Google Scholar]
- 2. Wallace D. V., Dykewicz M. S., Bernstein D. I., et al. 2008. The diagnosis and management of rhinitis: an updated practice parameter. J. Allergy Clin. Immunol. 122:S1. [DOI] [PubMed] [Google Scholar]
- 3. Khan D. A. 2014. Allergic rhinitis and asthma: epidemiology and common pathophysiology. Allergy Asthma Proc. 35:357. [DOI] [PubMed] [Google Scholar]
- 4. Ryu J. H., Yoo J. Y., Kim M. J., et al. 2013. Distinct TLR-mediated pathways regulate house dust mite-induced allergic disease in the upper and lower airways. J. Allergy Clin. Immunol. 131:549. [DOI] [PubMed] [Google Scholar]
- 5. Bartemes K. R. Kephart G. M. Fox S. J. and Kita H. 2014. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J. Allergy Clin. Immunol. 134:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim D. Y., Fukuyama S., Nagatake T., et al. 2012. Implications of nasopharynx-associated lymphoid tissue (NALT) in the development of allergic responses in an allergic rhinitis mouse model. Allergy 67:502. [DOI] [PubMed] [Google Scholar]
- 7. Chanez P. Vignola A. M. Vic P. Guddo F. Bonsignore G. Godard P. and Bousquet J. 1999. Comparison between nasal and bronchial inflammation in asthmatic and control subjects. Am. J. Respir. Crit. Care Med. 159:588. [DOI] [PubMed] [Google Scholar]
- 8. Soumelis V., Reche P. A., Kanzler H., et al. 2002. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 3:673. [DOI] [PubMed] [Google Scholar]
- 9. Ito T., Wang Y. H., Duramad O., et al. 2005. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 202:1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Omori M. and Ziegler S. 2007. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J. Immunol. 178:1396. [DOI] [PubMed] [Google Scholar]
- 11. Han N. R. Oh H. A. Nam S. Y. Moon P. D. Kim D. W. Kim H. M. and Jeong H. J. 2014. TSLP induces mast cell development and aggravates allergic reactions through the activation of MDM2 and STAT6. J. Invest. Dermatol. 134:2521. [DOI] [PubMed] [Google Scholar]
- 12. Nagarkar D. R. Poposki J. A. Comeau M. R. Biyasheva A. Avila P. C. Schleimer R. P. and Kato A. 2012. Airway epithelial cells activate TH2 cytokine production in mast cells through IL-1 and thymic stromal lymphopoietin. J. Allergy Clin. Immunol. 130:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou B., Comeau M. R., De Smedt T., et al. 2005. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 6:1047. [DOI] [PubMed] [Google Scholar]
- 14. Seshasayee D., Lee W. P., Zhou M., et al. 2007. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J. Clin. Invest. 117:3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Al-Shami A. Spolski R. Kelly J. Keane-Myers A. and Leonard W. J. 2005. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 202:829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gauvreau G. M., O’Byrne P. M., Boulet L. P., et al. 2014. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N. Engl. J. Med. 370:2102. [DOI] [PubMed] [Google Scholar]
- 17. Bunyavanich S., Melen E., Wilk J. B., et al. 2011. Thymic stromal lymphopoietin (TSLP) is associated with allergic rhinitis in children with asthma. Clin. Mol. Allergy 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramasamy A., Curjuric I., Coin L. J., et al. 2011. A genome-wide meta-analysis of genetic variants associated with allergic rhinitis and grass sensitization and their interaction with birth order. J. Allergy Clin. Immunol. 128:996. [DOI] [PubMed] [Google Scholar]
- 19. Birben E. Sahiner U. M. Karaaslan C. Yavuz T. S. Cosgun E. Kalayci O. and Sackesen C. 2014. The genetic variants of thymic stromal lymphopoietin protein in children with asthma and allergic rhinitis. Int. Arch. Allergy Immunol. 163:185. [DOI] [PubMed] [Google Scholar]
- 20. Nilsson D., Henmyr V., Hallden C., et al. 2014. Replication of genomewide associations with allergic sensitization and allergic rhinitis. Allergy 69:1506. [DOI] [PubMed] [Google Scholar]
- 21. Kamekura R., Kojima T., Koizumi J., et al. 2009. Thymic stromal lymphopoietin enhances tight-junction barrier function of human nasal epithelial cells. Cell Tissue Res. 338:283. [DOI] [PubMed] [Google Scholar]
- 22. Mou Z., Xia J., Tan Y., et al. 2009. Overexpression of thymic stromal lymphopoietin in allergic rhinitis. Acta Otolaryngol 129:297. [DOI] [PubMed] [Google Scholar]
- 23. Zhu D. D. Zhu X. W. Jiang X. D. and Dong Z. 2009. Thymic stromal lymphopoietin expression is increased in nasal epithelial cells of patients with mugwort pollen sensitive-seasonal allergic rhinitis. Chin. Med. J. (Engl.) 122:2303. [PubMed] [Google Scholar]
- 24. Wilson S. R., The L., Batia L. M., et al. 2013. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 155:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsushita K. Kato Y. Akasaki S. and Yoshimoto T. 2015. Proallergic cytokines and group 2 innate lymphoid cells in allergic nasal diseases. Allergol. Int. 64:235. [DOI] [PubMed] [Google Scholar]
- 26. Haenuki Y., Matsushita K., Futatsugi-Yumikura S., et al. 2012. A critical role of IL-33 in experimental allergic rhinitis. J. Allergy Clin. Immunol. 130:184. [DOI] [PubMed] [Google Scholar]
- 27. Miljkovic D. Bassiouni A. Cooksley C. Ou J. Hauben E. Wormald P. J. and Vreugde S. 2014. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy 69:1154. [DOI] [PubMed] [Google Scholar]
- 28. Muto T. Fukuoka A. Kabashima K. Ziegler S. F. Nakanishi K. Matsushita K. and Yoshimoto T. 2014. The role of basophils and proallergic cytokines, TSLP and IL-33, in cutaneously sensitized food allergy. Int. Immunol. 26:539. [DOI] [PubMed] [Google Scholar]
- 29. Hoshino K., Kashiwamura S., Kuribayashi K., et al. 1999. The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J. Exp. Med. 190:1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kato Y. Akasaki S. Muto-Haenuki Y. Fujieda S. Matsushita K. and Yoshimoto T. 2014. Nasal sensitization with ragweed pollen induces local-allergic-rhinitis-like symptoms in mice. PLoS One 9:e103540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsushita K. and Yoshimoto T. 2014. B cell-intrinsic MyD88 signaling is essential for IgE responses in lungs exposed to pollen allergens. J. Immunol. 193:5791. [DOI] [PubMed] [Google Scholar]
- 32. Kumamoto Y. Linehan M. Weinstein J. S. Laidlaw B. J. Craft J. E. and Iwasaki A. 2013. CD301b+ dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity 39:733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harada Y. Tanaka S. Motomura Y. Ohno S. Yanagi Y. Inoue H. and Kubo M. 2012. The 3’ enhancer CNS2 is a critical regulator of interleukin-4-mediated humoral immunity in follicular helper T cells. Immunity 36:188. [DOI] [PubMed] [Google Scholar]
- 34. Vijayanand P., Seumois G., Simpson L. J., et al. 2012. Interleukin-4 production by follicular helper T cells requires the conserved Il4 enhancer hypersensitivity site V. Immunity 36:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morita R., Schmitt N., Bentebibel S. E., et al. 2011. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Futatsugi-Yumikura S., Matsushita K., Fukuoka A., et al. 2014. Pathogenic Th2-type follicular helper T cells contribute to the development of lupus in Fas-deficient mice. Int. Immunol. 26:221. [DOI] [PubMed] [Google Scholar]
- 37. Undem B. J. and Taylor-Clark T. 2014. Mechanisms underlying the neuronal-based symptoms of allergy. J. Allergy Clin. Immunol. 133:1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ying S., O’Connor B., Ratoff J., et al. 2005. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J. Immunol. 174:8183. [DOI] [PubMed] [Google Scholar]
- 39. Miyata M., Hatsushika K., Ando T., et al. 2008. Mast cell regulation of epithelial TSLP expression plays an important role in the development of allergic rhinitis. Eur. J. Immunol. 38:1487. [DOI] [PubMed] [Google Scholar]
- 40. Dewas C., Chen X., Honda T., et al. 2015. TSLP Expression: analysis with a ZsGreen TSLP Reporter Mouse. J. Immunol. 194:1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kamijo S., Takai T., Kuhara T., et al. 2009. Cupressaceae pollen grains modulate dendritic cell response and exhibit IgE-inducing adjuvant activity in vivo. J. Immunol. 183:6087. [DOI] [PubMed] [Google Scholar]
- 42. Boldogh I., Bacsi A., Choudhury B. K., et al. 2005. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J. Clin. Invest. 115:2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Iijima K. Kobayashi T. Hara K. Kephart G. M. Ziegler S. F. McKenzie A. N. and Kita H. 2014. IL-33 and thymic stromal lymphopoietin mediate immune pathology in response to chronic airborne allergen exposure. J. Immunol. 193:1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.