IL-28B reduces Treg numbers but does not improve immunity induced by TB vaccines
Keywords: cytokine, IL-28B, immune regulation, regulatory T cells, tuberculosis vaccine
Abstract
Regulatory T cells (Tregs), which could be down-regulated by IL-28B, were reported to suppress T-cell-mediated immunity. The aim of this study was to investigate the role of IL-28B on the immune responses and protective efficacy of a tuberculosis (TB) subunit vaccine. First, a recombinant adenoviral vector expressing mouse IL-28B (rAd-mIL-28B) was constructed; then C57BL/6 mice were immunized with subunit vaccine ESAT6-Ag85B-Mpt64(190–198)-Mtb8.4-HspX (EAMMH) and rAd-mIL-28B together thrice or primed with Mycobacterium bovis bacillus Calmette–Gue′rin (BCG) and boosted by EAMMH and rAd-mIL-28B twice. At last the immune responses were evaluated, and the mice primed with BCG and boosted by subunit vaccines were challenged with virulent Mycobacterium tuberculosis H37Rv to evaluate the protective efficacy. The results showed that rAd-mIL-28B treatment significantly down-regulated the frequency of Tregs at 4 weeks after the last immunization but did not increase the Th1-type immune responses. Moreover, in the regimen of BCG priming and EAMMH boosting, rAd-mIL-28B treatment did not increase the antigen-specific cellular and humoral immune responses, and consequently did not reduce the bacteria load following H37Rv challenge. Instead, it induced more serious pathology reaction. In conclusion, IL-28B down-regulates Tregs following EAMMH vaccination but does not improve the protective immune responses.
Introduction
Tuberculosis (TB) remains a threat to humans’ health in the world. The current vaccine, attenuated Mycobacterium bovis bacillus Calmette–Gue′rin (BCG) does not limit the spread of the disease (1, 2), although it has a proven capacity to prevent severe and disseminated forms of TB in children (3, 4). Subunit vaccines were widely investigated due to their good efficiency against TB (5–7). Moreover, the strategy of BCG prime and subunit vaccine boost is regarded as a practical way to provide protection in adults (8). The vaccine-induced immune responses are being investigated to find a way to improve the protective efficacy of TB vaccines. Th1-type cellular immune responses are the key host protective responses against TB (9). It was reported that Th1-type immunity could be down-regulated by a distinct subset of CD4+ T cells highly expressing CD4, CD25 and Foxp3, referred to as regulatory T cells (Tregs) (10, 11). Tregs suppressed both T cells and antigen-presenting cells (12, 13), thus they might dampen the immune responses against Mycobacterium tuberculosis (14) and benefit the persistent infection.
The effects of Tregs on the immunization of TB vaccines received great attention in the past several years. It was reported that BCG-mediated protection against M. tuberculosis was diminished concomitantly with the emergence of Tregs (15). Fletcher et al. (16) found that a recombinant MVA expressing antigen 85A vaccination reduced TGF-β1 and Tregs, and enhanced IFN-γ responses to the recall antigen in BCG-primed subjects. Fedatto et al. (17) observed that a stronger protection conferred by heterologous vaccination against TB correlated with lower numbers of CD4+ Foxp3+ cells in lung tissue. However, it was also reported that depletion of Tregs via injection of anti-CD25 antibody during TB vaccine immunization did not enhance the protective effect (18). Therefore, the role of Tregs during TB vaccine immunization is still controversial and needs further investigation.
IL-28B, a cytokine recently discovered, is a member of the family of type-III IFNs (19). Having a similar signal transduction cascade with type-I IFNs (IFN-α and IFN-β), IL-28B induces antiviral and anti-tumor effects (20). Morrow et al. reported that IL-28B down-regulated Tregs, drove strong Th1-biased cellular immune responses and induced protection in some vaccine immunizations (21, 22).
In our previous study, we found that immunizing mice with TB fusion protein ESAT6-Ag85B-MPT64(190–198)-Mtb8.4 (EAMM) and Mtb10.4-HspX (MH) exhibited significant protection against M. tuberculosis (23). Therefore, we linked EAMM and HspX to construct a new fusion protein EAMMH which included ESAT6, Ag85B, the peptide 190–198 of Mpt64, Mtb8.4 and HspX. EAMMH was mixed with an adjuvant composed of dimo-thylidioctyl ammonium bromide (DDA) and polyriboinosinic-polyribocytidilic acid (Poly I:C) to construct a novel subunit vaccine candidate. As expected, the vaccine EAMMH induced stronger Th1 responses and higher protection against M. tuberculosis infection than BCG and EAMM plus MH in mice (24). In this study, we constructed an adenoviral vector encoding mouse IL-28B (rAd-mIL-28B). Mice were immunized with TB subunit vaccine EAMMH with or without additional treatment of rAd-mIL-28B. Then, the percentage of Tregs, antigen-specific immune responses and protective efficacy were examined. The results showed that rAd-mIL-28B reduced the frequency of Tregs in mice vaccinated with EAMMH, but did not enhance the vaccine-induced immunity.
Methods
Animals
Special pathogen-free female C57BL/6 mice were purchased from Sibeifu Experimental Animal Technology Co., Ltd (Beijing, China) and maintained in the Animal Center of Gansu University of Chinese Medicine (Lanzhou, China). All animals were female and 6–8 weeks old at the start of the experiments. For M. tuberculosis H37Rv challenge, animals were maintained in ABSL-3 laboratory at Wuhan University, China. Mice received free access to food and water throughout the study. All experiments were carried out according to the protocols approved by the Institutional Animal Caring and Using Committee.
Fusion protein and mycobacterial antigen preparation
The fusion protein EAMMH was constructed and purified as previously reported (24). Briefly, the plasmid encoding EAMMH was inserted into the multiple cloning sites of the expression vector pET30a (+). The plasmid was transformed into the Escherichia coli strain BL21 (DE3) and the fusion protein was obtained from supernatant. Single recombinant protein Ag85B, ESAT6 and HspX were purified by Ni-NTA His column (Novagen) as previously described (23).
Construction and preparation of rAd-mIL-28B and rAd-EGFP
The mouse IL-28B gene sequence was sub-cloned into shuttle plasmid pYr-adshuttle-1. Then the pYr-adshuttle-1-mIL-28B was constructed into the E3 region of pAd/PL-DEST adenovirus expression vector by homologous recombination in vitro. At last, the plasmid DNA was linearized with PacI, and rAd-mIL-28B was packaged in HEK293 cells. Adenoviral titer was determined by the method of Reed and Muench (25) following by verification of rAd-mIL-28B with PCR analysis. The recombined adenoviral vector encoding enhanced green fluorescent protein (rAd-EGFP), as control of rAd-mIL-28B, was prepared with the same method.
Vaccine immunization and rAd-mIL-28B treatment
The EAMMH protein 10 µg mixed in adjuvant of DDA (Sigma–Aldrich, Poole, UK) 250 µg and Poly I:C (Kaiping Qianniu Biochemical Pharmaceutical Company Limited, Guangdong, China) 50 µg, with PBS in a volume of 200 μl was injected subcutaneously on one side of the groin of mice. At 30min after protein vaccine immunization, 100 µl (containing 5×106 PFU of viruses) of rAd-mIL-28B or rAd-EGFP was injected at the same site. BCG (Danish 1331; 5×105 colony forming units, CFUs) was immunized subcutaneously.
Flow cytometry analysis of Tregs
Spleens from vaccine-immunized mice were aseptically removed and forced through a 200-mesh nylon strainer. Then single-cell suspensions were prepared with Lymphocyte-M (Dakewe Biotech Company Limited, Shenzhen, China) density gradient centrifugation. After washing with flow cytometry (FCM) staining buffer, the cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD4 (RM4-5) and allophycocyanin (APC)-conjugated anti-CD25 (PC61.5) for 30min at 4°C away from light. Then after fixation and permeabilization, intracellular molecules were stained with PE-conjugated anti-Foxp3 (FJK-16S). All of the antibodies and reagents for FCM staining were purchased from eBioscience (San Diego, CA, USA). FCM was performed by a BD FACS Calibur and the results were analyzed with FlowJo 7.6.1.
ELISPOT detecting the number of cells secreting IFN-γ
IFN-γ ELISPOT kits (Dakewe Biotech Company Limited, Shenzhen, China) were used according to the manufacturer’s protocol. In brief, freshly isolated spleen cells were seeded in duplicate at 5×105 cells per well in 100 µl of RPMI 1640 (HyClone, Logan, UT, USA) supplemented with penicillin, streptomycin, and 10% fetal calf serum (HyClone) and stimulated with ESAT-6 (10 µg ml−1), Ag85B (5 µg ml−1), and HspX (10 µg ml−1) respectively for 36h at 37°C, 5% CO2. PHA (1 µg ml−1) was used as a positive control. To assess background levels of IFN-γ secretion, the cells without any stimuli were used as a negative control. After the cells were removed, biotinylated antibody solution and streptavidin-HRP solution were added. At last, 3-amino-9-ethylcarbazole (AEC) substrate solution was added to each well, and the spots were counted by an ELISPOT reader (Bio-sys, GmbH, Karben, Germany).
ELISA assay for Ag85B-specific antibodies
After immunization, the Ag85B-specific IgG subclasses IgG1 and IgG2c were measured by ELISA. After being coated with 5 μg ml−1 of Ag85B (containing 0.05M buffer bicarbonate, pH 9.6) overnight at 4°C, the plates were washed five times with PBST (PBS containing 0.05% Tween 20). Then the plates were blocked with 1% BSA (MPBiomedicals, CA, USA) for 1h at 37°C. The BSA was removed and plates were then incubated with the mouse sera (diluted as 1:100, 1:200, 1:400, 1:800, 1:1600, 1:3200, 1:6400, 1:12800, 1:25600, respectively) for 1h at 37°C. Subsequently, the plates were incubated with the polyclonal HRP-conjugated second antibodies rabbit anti-mouse IgG1 (Rockland, PA, USA) or goat anti-mouse IgG2c (Bethyl, AL, USA) for 1h at 37°C. Color was developed in tetramethylbenzidine solution for 15min at 37°C away from light and then was terminated by 50 μl of 2M sulphuric acid and quantified at 450nm using a microplate reader (BioTek, VT, USA).
Protective efficacy assay
Mycobacterium tuberculosis H37Rv was used to challenge vaccine-immunized mice to analyze the protective efficacy. The mice were challenged with 50–100 CFUs of virulent strain H37Rv by aerosol. Ten weeks later, the animals were sacrificed and the right lobes of the lungs were removed and homogenized. Serial dilutions of lung homogenates were plated on 7H11 agar plates (BD, NJ, USA) enriched with 10% OADC (oleic acid- albumin-dextrose-catalase) (BD) and supplemented with ampicillin (10 µg ml−1), polymyxin B (41 µg ml−1) and amphotericin B (4.1 µg ml−1) to avoid contamination. 7H11 plates were incubated at 37°C and 5% CO2 for 3 weeks, and CFUs were counted. The left lobes of the lungs from infected mice were fixed in 10% neutral-buffered formalin and processed routinely for histopathology. Sections were prepared and stained with haematoxylin and eosin. The average percentage of granuloma area in a section was evaluated from five views distributed in its center and four corners under low magnification by a skilled pathologist. This work was performed at the ABSL-3 Laboratory of Wuhan University.
Statistical analysis
Statistical analysis was done with SPSS 11.5 software. One-way ANOVA was used. Data were showed as means ± SD of the mean. P-value < 0.05 was considered statistically significant.
Results
rAd-mIL-28B down-regulated Tregs when used with TB subunit vaccine EAMMH vaccination
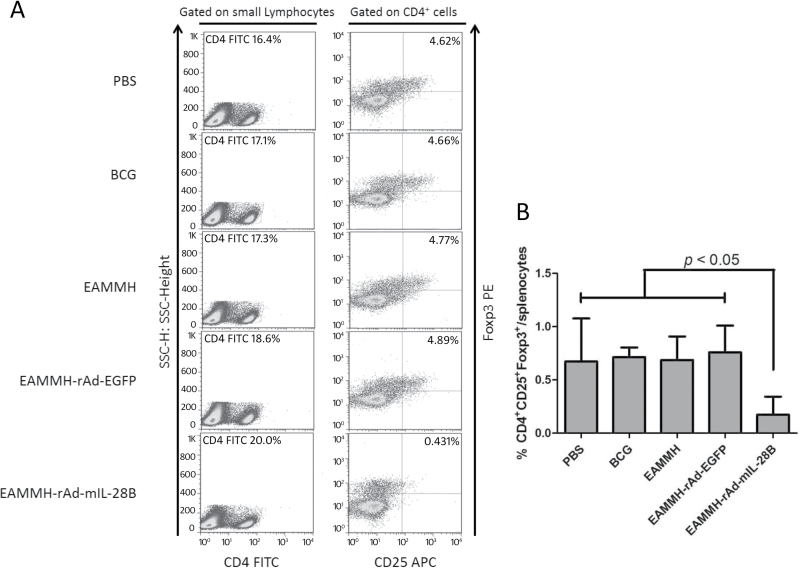
We detected the percentage of Tregs in mice immunized with TB subunit vaccine EAMMH with or without the treatment of rAd-mIL-28B at 4 weeks after the final immunization via FCM analysis (Fig. 1A). The results showed that EAMMH vaccination did not change the frequency of Tregs comparing with the group of PBS and BCG immunized mice. However, with the additional treatment of rAd-mIL-28B, the frequency of CD4+CD25+Foxp3+ Tregs significantly decreased from 0.69±0.22% to 0.17±0.17% (P < 0.05; Fig. 2A and B). These results demonstrated that rAd-mIL-28B treatment down-regulated Tregs in the spleen after TB vaccine immunization.
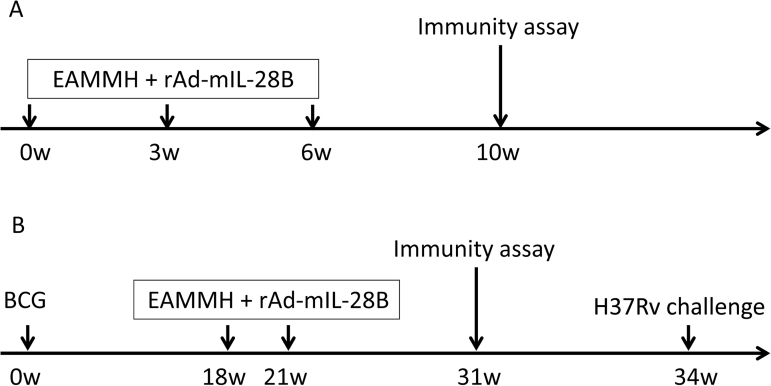
Fig. 1.
Experimental procedures. As the subunit vaccine is supposed to be a booster of BCG, two immunization strategies were used in the experiments. (A) The schedule of EAMMH immunization. C57BL/6 mice were immunized subcutaneously with the EAMMH vaccine with or without rAd-mIL-28B thrice at week 0, 3 and 6. Control mice were just injected with PBS or BCG at week 0. rAd-EGFP was used as a control for rAd-mIL-28B. Four weeks after last immunization, the percentage of CD4+CD25+Foxp3+ Tregs, levels of specific antibodies and the number of spleen lymph cells secreting IFN-γ were analyzed. (B) The strategy of BCG-prime and EAMMH-boost. Mice were primed with BCG at week 0 and boosted by the EAMMH vaccine with or without rAd-mIL-28B twice at week18 and 21. Ten weeks after the last vaccination when immune memory developed, all mice underwent immune assays as above. At week 34, mice were challenged with M. tuberculosis H37Rv by aerosol.
Fig. 2.
rAd-mIL-28B treatment reduced the percentage of CD4+CD25+Foxp3+ cells within splenocytes in the mice vaccinated with EAMMH. Mice were immunized as the procedure in Fig. 1A. At week 10, isolated spleen cells from immunized mice were stained with FITC-conjugated anti-CD4, APC-conjugated anti-CD25 and PE-conjugated anti-Foxp3. CD4+CD25+Foxp3+ Tregs were detected via FCM. (A) Three mice per group were analyzed and the representative results out of three were depicted. (B) Data collected from three mice per group were expressed as means ± SD.
rAd-mIL-28B treatment did not enhance the Th1-type immune responses induced by EAMMH vaccination
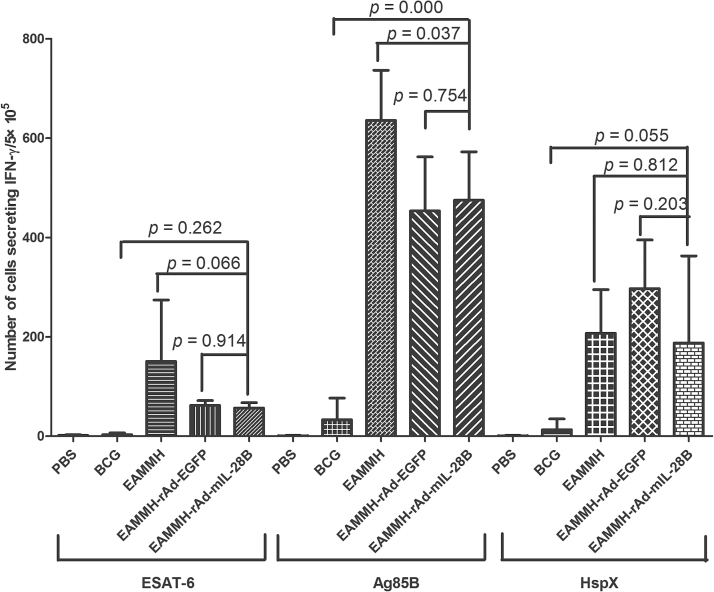
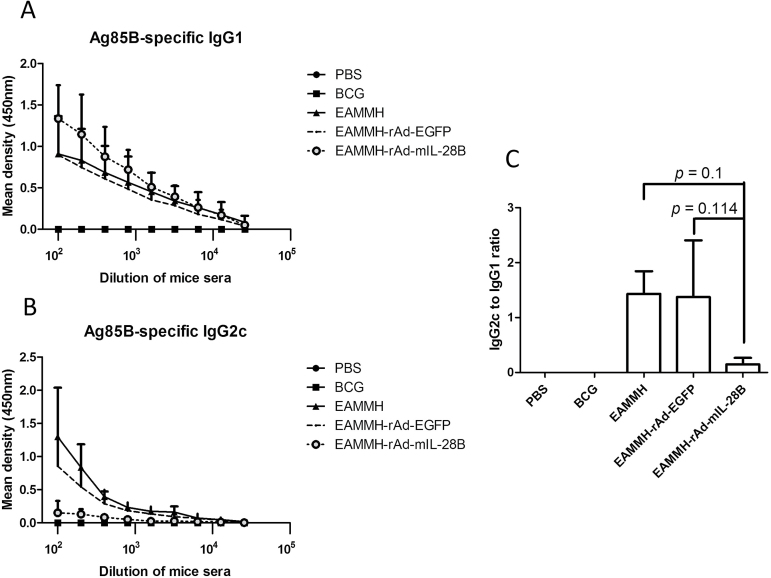
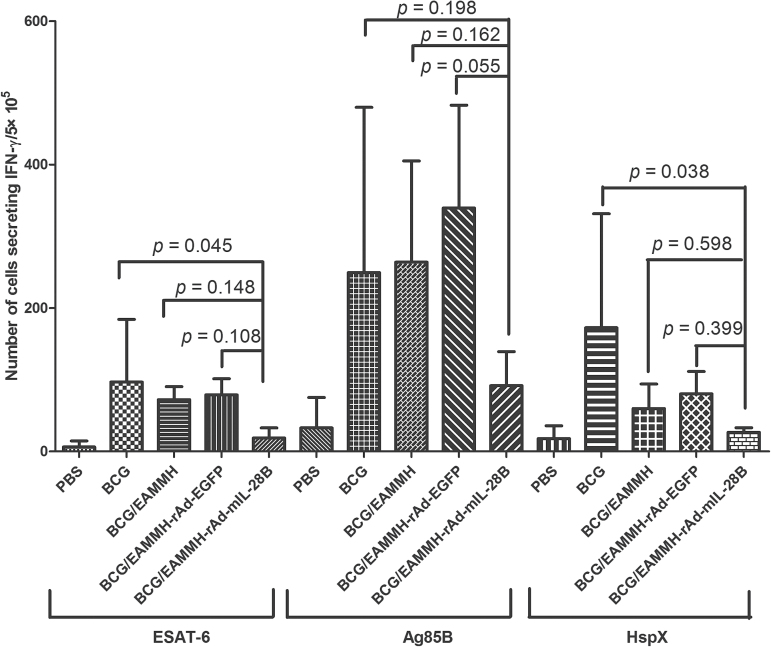
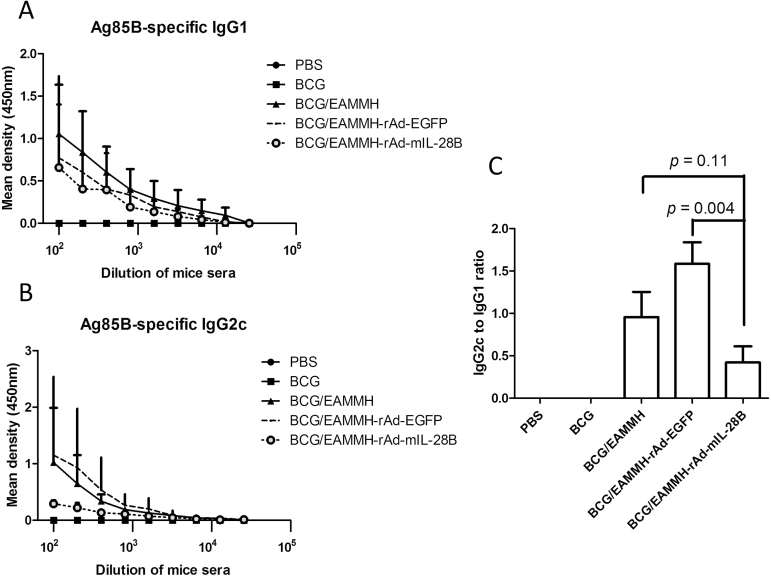
At the fourth week after the last immunization, with the stimulation of ESAT-6, Ag85B or HspX, the frequency of IFN-γ- producing T cells from spleens of the EAMMH/rAd-mIL-28B immunized mice decreased obviously (Fig. 3). As for the levels of Ag85B-specific antibody, the level of IgG1 in EAMMH/rAd-mIL-28B group showed a trend of increasing comparing with EAMMH/rAd-EGFP group (Fig. 4A). However, additional rAd-mIL-28B treatment induced significantly lower IgG2c than EAMMH alone (P < 0.05; Fig. 4B), and the ratio of IgG2c/IgG1 therefore tended to decrease, although there was no significant difference (Fig. 4C). These data indicated that rAd-mIL-28B did not enhance the Th1-type cell-mediated immune responses at 4 weeks after treatment.
Fig. 3.
rAd-mIL-28B treatment did not induce more IFN-γ-producing spleen lymphocytes. The procedure of immunization and immunity assay was shown in Fig. 1A. Isolated spleen cells were plated in duplicate at 5×105 cells per well and stimulated with ESAT-6 (10 µg ml−1), Ag85B (5 µg ml−1) or HspX (10 µg ml−1), respectively. IFN-γ-secreting cells were determined by ELISPOT per 5×105 cells. Data were expressed as means ± SD. n = 3.
Fig. 4.
rAd-mIL-28B treatment increased Ag85B-specific IgG1 and decreased Ag85B-specific IgG2c following EAMMH vaccination. The procedure of immunization was performed according to Fig. 1A. At week 10, mice were bled to detect serum antibodies with indirect ELISA. (A) The level of the Ag85B-specific IgG1. (B) The level of the Ag85B-specific IgG2c. (C) The ratio of IgG2c/IgG1 when serum was diluted at 1:100. Data were expressed as means ± SD. n = 3.
rAd-mIL-28B impaired vaccine-induced immune memory responses in the BCG-prime and subunit vaccine-boost strategy
Most people in the world have been vaccinated with BCG, and the BCG-prime and subunit vaccine-boosting strategy is believed to be a potential practical way to improve the protection against TB in adults. Thus, we investigated the effect of IL-28B in the BCG-prime and subunit vaccine-boost strategy. Mice were primed with BCG and boosted with the EAMMH with or without rAd-mIL-28B at the 18th and 21st weeks after BCG priming (Fig. 1B). To observe the long-term immune memory induced by vaccination, antigen-specific immune responses including IFN-γ production and serum antibodies were analyzed 10 weeks after the last immunization. The results showed that there were lower numbers of IFN-γ-producing cells in BCG/EAMMH/rAd-mIL-28B immunized mice compared to BCG/EAMMH immunized mice, although the difference was not significant (Fig. 5). Meanwhile, the level of Ag85B-specific IgG1 did not increase following rAd-mIL-28B treatment (Fig. 6A). The level of Ag85B-specific IgG2c in the BCG/EAMMH-rAd-mIL-28B group was lower than that of the BCG/EAMMH-rAd-EGFP group (P = 0.194 at the dilution of 1:100; Fig. 6B), which led to the decrease of the ratio of IgG2c/IgG1 in mice treated with rAd-mIL-28B (P = 0.004; Fig. 6C). Taken together, the results showed that application of rAd-mIL-28B during vaccination might impair the TB vaccine-induced immune memory.
Fig. 5.
The capability to produce IFN-γ decreased following the treatment of rAd-mIL-28B in mice primed with BCG and boosted with subunit vaccine. Mice were immunized according to Fig. 1B. At week 31, 10 weeks after the final immunization, isolated spleen cells from immunized mice were plated in duplicate at 5×105 cells per well and stimulated with ESAT-6 (10 µg ml−1), Ag85B (5 µg ml−1) or HspX (10 µg ml−1), respectively. IFN-γ-secreting cells were determined by ELISPOT per 5×105 cells. Data were expressed as means ± SD. n = 3.
Fig. 6.
rAd-mIL-28B induced the reduction of anti-Ag85B antibodies in mice primed with BCG and boosted with subunit vaccine. Mice were immunized according to Fig. 1B. At week 31, the mice were bled to confirm levels of the anti-Ag85B antibodies IgG2c and IgG1 by ELISA. (A) The level of the Ag85B-specific IgG1. (B) The level of the Ag85B-specific IgG2c. (C) The ratio of IgG2c/IgG1 when serum was diluted at 1:100. Data were expressed as means ± SD. n = 3.
rAd-mIL-28B treatment did not improve the protective efficacy of BCG-prime and EAMMH-boost strategy
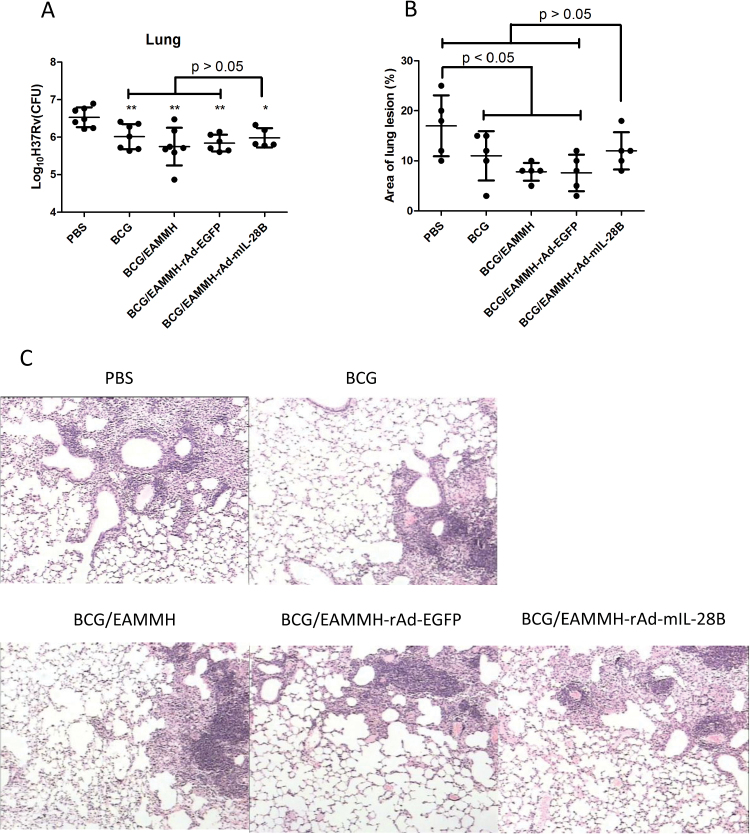
In the strategy of BCG-prime and EAMMH-boosting, the mice were challenged with M. tuberculosis H37Rv 13 weeks after the last EAMMH immunization. The results showed that there were no differences in the bacteria loads at lung tissues among all groups immunized with the subunit vaccine when compared with the BCG group, although BCG-prime and EAMMH-boost with or without rAd-mIL-28B obviously reduced the bacteria loads compared to the PBS group (P < 0.05; Fig. 7A). Additional rAd-mIL-28B treatment did not induce lower bacteria loads than the BCG/EAMMH-rAd-EGFP group or other controls (P > 0.05; Fig. 7A). Moreover, the pathological analysis showed that following M. tuberculosis challenge the granuloma area in the lungs of BCG, BCG/EAMMH or BCG/EAMMH-rAd-EGFP groups decreased significantly compared with PBS control (P < 0.05), but there was no obvious decrease in the BCG/EAMMH-rAd-mIL-28B group compared with the PBS group (P > 0.05; Fig. 7B and C), which indicated that the application of rAd-mIL-28B was detrimental to the protection induced by the BCG-prime and EAMMH-boost strategy.
Fig. 7.
rAd-mIL-28B treatment did not improve the protective efficacy of BCG-prime and EAMMH-boost strategy following H37Rv challenge. Mice were primed with BCG and boosted with subunit vaccine, then challenged with H37Rv. The procedure of experiments was shown in Fig. 1B. (A) At 10 weeks after challenge, the right lobe of the lungs was homogenized and the serial dilutions of the homogenates were plated on 7H11 agar. The CFUs were counted after incubation at 37°C for 3 weeks. CFUs in the lungs were expressed as means ± SD. n ≥ 5. *P < 0.05, **P < 0.01 versus PBS group. (B) At 10 weeks after challenge, the left lobe of the lungs was processed routinely for histopathology to detect pathologic lesions induced by M. tuberculosis H37Rv challenge. The area ratio of granuloma in sections was expressed as means ± SD. n = 5. (C) The representative pathology lesions of the left lobe of the lungs.
Discussion
To evaluate the impacts of IL-28B and Tregs on TB vaccine-induced immunization, we constructed the recombinant adenovirus rAd-mIL-28B and investigated the immune responses and protective efficacy induced by TB subunit vaccine EAMMH with or without additional rAd-mIL-28B treatment. The results indicated that rAd-mIL-28B treatment significantly down-regulated the frequency of Tregs as expected. However, following rAd-mIL-28B treatment, Th1-type immune responses such as antigen-specific IFN-γ producing lymphocytes and IgG2c antibodies did not increase, but decreased to some degree. Moreover, in BCG-prime and subunit vaccine-boost strategy, rAd-mIL-28B treatment induced more severe pathologic lesions following the virulent strain H37Rv challenge rather than increase the protective efficacy.
IL-28B, a member of type-III IFNs, has received wide attention in recent years because of its type-I IFN-like anti-viral activity (26–29) and immune regulation role in some diseases (30, 31). IL-28B could reduce the number of Tregs and augment antigen-specific immune response to a multi-clade HIV Gag antigen in a Th1-biased fashion and showed the increased protective effect when used as an adjuvant in DNA vaccination (21). Therefore, we wondered if IL-28B could down-regulate Tregs and enhance the protective efficacy of TB subunit vaccine. Our experiments showed that rAd-mIL-28B down-regulated the frequency of Tregs but did not enhance vaccine-induced Th1-type memory immunity. Moreover, rAd-mIL-28B treatment did not show any benefits to the protection against M. tuberculosis in the BCG-prime and EAMMH-boost strategy.
IgG1 and IgG2c class switch are dependent on Th2-produced IL-4 and Th1-produced IFN-γ in C57BL/6 mice respectively (32, 33). Therefore, vaccine-induced antigen-specific IgG1 and IgG2c isotypes could reflect characteristics of immune responses (34). In this study, the IgG subclass was determined to evaluate the Th1/Th2 immune balance regulated by rAd-mIL-28B. Compared to the control group, additional IL-28B treatment led to a lower ratio of IgG2c/IgG1, which suggested that mice treated with rAd-mIL-28B tended to induce a Th2-type rather than Th1-type immune response.
Tregs could strongly suppress the antigen-specific immune responses induced by other T-cell subsets. Attenuation of Tregs was reported to be a way to improve vaccine induced cell-mediated immunity (35). It was reported that depletion of Tregs with the treatment of denileukindiftitox was associated with enhanced immune responses to tumor antigen vaccination (36). Down-regulation of Tregs could enhance the viral vaccine’s efficacy (37). Jaron et al. administrated anti-CD25 mAb thrice with an interval of 2 weeks to BCG-vaccinated mice and then challenged the mice with M. tuberculosis. They found that the mycobacterial load in the lung tissue of the mice was reduced, compared to that in the control mice (35). However, the inhibition of Tregs was also reported to induce variable effects during vaccination. For example, the similar strategy of Treg attenuation via injection of anti-CD25 mAb thrice did not enhance the protection of hsp 65 DNA vaccine against M. tuberculosis (17). Quinn et al. (18) also found that anti-CD25 mAb treatment once prior to vaccination with BCG increased the production of Th1 cytokines but did not reduce the mycobacterial load in the lungs. In this study, we used rAd-mIL-28B to reduce Tregs in mice vaccinated with EAMMH and found that the down-regulation of Tregs did not enhance the vaccine-induced protective immune responses.
Our work suggested that IL-28B could impair immune memory by down-regulating Tregs. It is well-known that the ability of vaccine to confer protection depends on numerous memory cells. In response to antigen stimulation, naive T cells will differentiate into a large number of short-lived effector T cells and a small number of long-lived memory T (Tm) cells which mediate secondary immune responses (38). Developing of excessive antigen-specific effector T cells might damage the generation of CD8+ Tm cells (39, 40). Removing antigens completely by strong primary responses was detrimental to the production of CD4+ Tm cells (41). De Goer de Herve et al. reported that Tregs were required for the generation of functional CD8+ Tm cells. They found that in the absence of Tregs the memory cells proliferated poorly (40). Accumulated data showed that lower levels of inflammatory stimulation and a lower amount of antigens favored the generation of long-lived memory cells (42). These understandings indicated that inhibition of Tregs did not always enhance the long-term immune protection. In present research, we observed that rAd-mIL-28B decreased the frequency of Tregs at 4 weeks after the last vaccination. Morrow et al. (21) also observed that IL-28B down-regulated the Tregs at 2 weeks after vaccination. We found that the reduction of Tregs by rAd-mIL-28B did not enhance the long-term protective efficacy of EAMMH vaccination. The reason may be that additional IL-28B treatment made EAMMH immunization to induce more effector T cells rather than long-lived memory T cells.
In conclusion, rAd-mIL-28B reduced the frequency of Tregs during EAMMH vaccination, but this did not improve the protective immune responses. The reason might be that the down-regulated Tregs impaired the vaccine induced Th1-type immune memory.
Funding
Chinese National Major Science and Technology Projects (2012ZX10003-008-006); National Science Foundation of China (81072499, 31470895).
Conflict of interest statement: The authors have applied for the patents on EAMMH vaccine and DDA plus Poly I:C adjuvant (Publication No. 2014101871546 and No. 201110199072.x) that relate to this study. These patents belong to Lanzhou University (China). The authors have no conflict of interests.
References
- 1. Fine P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339. [DOI] [PubMed] [Google Scholar]
- 2. Schön T. Lerm M. and Stendahl O. 2013. Shortening the ‘short-course’ therapy- insights into host immunity may contribute to new treatment strategies for tuberculosis. J. Intern. Med. 273:368. [DOI] [PubMed] [Google Scholar]
- 3. Rouanet C. Debrie A. S. Lecher S. and Locht C. 2009. Subcutaneous boosting with heparin binding haemagglutinin increases BCG-induced protection against tuberculosis. Microbes Infect. 11:995. [DOI] [PubMed] [Google Scholar]
- 4. Trunz B. B. Fine P. and Dye C. 2006. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 367:1173. [DOI] [PubMed] [Google Scholar]
- 5. Abel B., Tameris M., Mansoor N., et al. 2010. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. Am. J. Respir. Crit. Care Med. 181:1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Billeskov R. Elvang T. T. Andersen P. L. and Dietrich J. 2012. The HyVac4 subunit vaccine efficiently boosts BCG-primed anti-mycobacterial protective immunity. PLoS One 7:e39909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Day C. L., Tameris M., Mansoor N., et al. 2013. Induction and regulation of T-cell immunity by the novel tuberculosis vaccine M72/AS01 in South African adults. Am. J. Respir. Crit. Care Med. 188:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luo Y., Wang B., Hu L., et al. 2009. Fusion protein Ag85B-MPT64(190–198)-Mtb8.4 has higher immunogenicity than Ag85B with capacity to boost BCG-primed immunity against Mycobacterium tuberculosis in mice. Vaccine 27:6179. [DOI] [PubMed] [Google Scholar]
- 9. Cooper A. M. 2009. Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 27:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sakaguchi S. Sakaguchi N. Asano M. Itoh M. and Toda M. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151. [PubMed] [Google Scholar]
- 11. Sakaguchi S. 2004. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531. [DOI] [PubMed] [Google Scholar]
- 12. O’Garra A. and Vieira P. 2004. Regulatory T cells and mechanisms of immune system control. Nat. Med. 10:801. [DOI] [PubMed] [Google Scholar]
- 13. Joosten S. A. and Ottenhoff T. H. 2008. Human CD4 and CD8 regulatory T cells in infectious diseases and vaccination. Hum. Immunol. 69:760. [DOI] [PubMed] [Google Scholar]
- 14. Ordway D., Henao-Tamayo M., Harton M., et al. 2007. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J. Immunol. 179:522. [DOI] [PubMed] [Google Scholar]
- 15. Ordway D. J., Shang S., Henao-Tamayo M., et al. 2011. Mycobacterium bovis BCG-mediated protection against W-Beijing strains of Mycobacterium tuberculosis is diminished concomitant with the emergence of regulatory T cells. Clin. Vaccine Immunol. 18:1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fletcher H. A., Pathan A. A., Berthoud T. K., et al. 2008. Boosting BCG vaccination with MVA85A down-regulates the immunoregulatory cytokine TGF-beta1. Vaccine 26:5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fedatto P. F., Sergio C. A., Paula M. O., et al. 2012. Protection conferred by heterologous vaccination against tuberculosis is dependent on the ratio of CD4(+) /CD4(+) Foxp3(+) cells. Immunology 137:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quinn K. M. Rich F. J. Goldsack L. M. de Lisle G. W. Buddle B. M. Delahunt B. and Kirman J. R. 2008. Accelerating the secondary immune response by inactivating CD4(+)CD25(+) T regulatory cells prior to BCG vaccination does not enhance protection against tuberculosis. Eur. J. Immunol. 38:695. [DOI] [PubMed] [Google Scholar]
- 19. Witte K. Witte E. Sabat R. and Wolk K. 2010. IL-28A, IL-28B, and IL-29: promising cytokines with type I interferon-like properties. Cytokine Growth Factor Rev. 21:237. [DOI] [PubMed] [Google Scholar]
- 20. Donnelly R. P. and Kotenko S. V. 2010. Interferon-lambda: a new addition to an old family. J. Interferon Cytokine Res. 30:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morrow M. P. Pankhong P. Laddy D. J. Schoenly K. A. Yan J. Cisper N. and Weiner D. B. 2009. Comparative ability of IL-12 and IL-28B to regulate Treg populations and enhance adaptive cellular immunity. Blood 113:5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morrow M. P., Yan J., Pankhong P., et al. 2010. Unique Th1/Th2 phenotypes induced during priming and memory phases by use of interleukin-12 (IL-12) or IL-28B vaccine adjuvants in rhesus macaques. Clin. Vaccine Immunol. 17:1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xin Q., Niu H., Li Z., et al. 2013 Subunit vaccine consisting of multi-stage antigens has high protective efficacy against Mycobacterium tuberculosis infection in mice. PLoS One 8:e72745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Niu H., Peng J., Bai C., et al. 2015. Multi-stage tuberculosis subunit vaccine candidate LT69 provides high protection against Mycobacterium tuberculosis infection in mice. PLoS One 10:e0130641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reed L. J. and Muench H. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493. [Google Scholar]
- 26. Clausen L. N., Weis N., Astvad K., et al. 2011. Interleukin-28B polymorphisms are associated with hepatitis C virus clearance and viral load in a HIV-1-infected cohort. J .Viral Hepat. 18:e66. [DOI] [PubMed] [Google Scholar]
- 27. Balagopal A. Thomas D. L. and Thio C. L. 2010. IL28B and the control of hepatitis C virus infection. Gastroenterology 139:1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shindo H., Maekawa S., Komase K., et al. 2013. IL-28B (IFN-lambda3) and IFN-alpha synergistically inhibit HCV replication. J. Viral Hepat. 20:281. [DOI] [PubMed] [Google Scholar]
- 29. Charlton M. R., Thompson A., Veldt B. J., et al. 2011. Interleukin-28B polymorphisms are associated with histological recurrence and treatment response following liver transplantation in patients with hepatitis C virus infection. Hepatology 53:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen C., Tan B., Zhou Y., et al. 2012. IL-28B genetic variant is associated with the risk of schizophrenia in the Chinese Han population. DNA Cell Biol. 31:988. [DOI] [PubMed] [Google Scholar]
- 31. Yoshimoto K., Kishida T., Nakano H., et al. 2011. Interleukin-28B acts synergistically with cisplatin to suppress the growth of head and neck squamous cell carcinoma. J. Immunother. 34:139. [DOI] [PubMed] [Google Scholar]
- 32. Wang N. S. McHeyzer-Williams L. J. Okitsu S. L. Burris T. P. Reiner S. L. and McHeyzer-Williams M. G. 2012. Divergent transcriptional programming of class-specific B cell memory by T-bet and RORα. Nat. Immunol. 13:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Neubert K., Lehmann C. H., Heger L., et al. 2014. Antigen delivery to CD11c+CD8- dendritic cells induces protective immune responses against experimental melanoma in mice in vivo . J. Immunol. 192:5830. [DOI] [PubMed] [Google Scholar]
- 34. Matsuo K., Okamoto H., Kawai Y., et al. 2014. Vaccine efficacy of transcutaneous immunization with amyloid beta using a dissolving microneedle array in a mouse model of Alzheimer’s disease. J. Neuroimmunol. 266:1. [DOI] [PubMed] [Google Scholar]
- 35. Jaron B. Maranghi E. Leclerc C. and Majlessi L. 2008. Effect of attenuation of Treg during BCG immunization on anti-mycobacterial Th1 responses and protection against Mycobacterium tuberculosis . PLoS One 3:e2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morse M. A., Hobeika A. C., Osada T., Serra D., Niedzwiecki D., Lyerly H. K., Clay T. M. 2008. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood 112:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bayry J. 2014. Regulatory T cells as adjuvant target for enhancing the viral disease vaccine efficacy. Virusdisease 25:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Masopust D. Kaech S. M. Wherry E. J. and Ahmed R. 2004. The role of programming in memory T-cell development. Curr. Opin. Immunol. 16:217. [DOI] [PubMed] [Google Scholar]
- 39. Shen C. H. Talay O. Mahajan V. S. Leskov I. B. Eisen H. N. and Chen J. 2010. Antigen-bearing dendritic cells regulate the diverse pattern of memory CD8 T-cell development in different tissues. Proc. Natl Acad. Sci. USA 107:22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Goer de Herve M. G., Jaafoura S., Vallee M., Taoufik Y. 2012. FoxP3(+) regulatory CD4 T cells control the generation of functional CD8 memory. Nat. Commun. 3:986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim C. Wilson T. Fischer K. F. and Williams M. A. 2013. Sustained interactions between T cell receptors and antigens promote the differentiation of CD4+ memory T cells. Immunity 39:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mueller S. N. Gebhardt T. Carbone F. R. and Heath W. R. 2013. Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 31:137. [DOI] [PubMed] [Google Scholar]