Abstract
A 15-year-old boy presented to emergency services with accidental naphthalene ball ingestion. Following consumption he developed methaemoglobinaemia, massive intravascular haemolysis and acute kidney injury. He had no history suggestive of congenital haemoglobin M disease. Development of severe methaemoglobinaemia and intravascular haemolysis is quite unusual after consumption of a single ball of naphthalene. The patient was managed with ascorbic acid and intravenous N-acetyl cysteine. He also required haemodialysis for acute kidney injury that developed secondary to pigment nephropathy.
Background
Naphthalene balls contain a potent aromatic hydrocarbon and are commonly used in Indian households as a deodoriser and moth repellent. Accidental consumption of naphthalene even in minimal amounts can be highly toxic and can even be fatal. We present an unusual case of accidental moth ball consumption by a young boy leading to severe intravascular haemolysis, acquired methaemoglobinaemia, anaemia and acute kidney injury.
Case presentation
A 15-year-old boy accidentally consumed a single naphthalene ball mistaking it to be candy. After 3–4 initial episodes of non-bilious, non-projectile vomiting, his urine developed a blackish-brown discolouration (figure 1A). Subsequently, over 24 hours, there was a further decline in urine output to <100 mL/day with generalised swelling. On presentation to our hospital, he was in an irritable and confused state. On examination, he had tachycardia (heart rate 118/min), tachypnoea (RR 34/min), anasarca and deep central cyanosis. Other systemic examination was within normal limits. On catheterisation, the boy's urine was dark brown like cola colour and venipuncture blood sample displayed chocolate brown hue.
Figure 1.
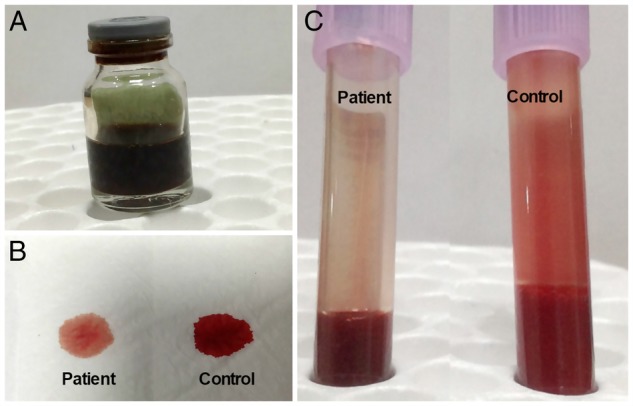
(A) Glass container showing cola coloured urine confirming haemoglobinuria. Bedside tests: (B) spot blot test and (C) tube test showing chocolate brown colour of the patient's blood compared with fresh red colour of a control sample.
Investigations
There was low haemoglobin oxygen saturation on pulse oximetry (70–75%), with normal partial pressure of oxygen on arterial blood gas analysis (ABG). Table 1 summarises the laboratory parameters, which showed that the patient suffered massive intravascular haemolysis and acute kidney injury. He was found to be glucose-6-phosphate dehydrogenase (G6PD)-deficient.
Table 1.
Sequential value of laboratory parameters during hospital stay
| Day-1 | Day-3 | Day-8 | Day-12 | |
|---|---|---|---|---|
| Hb (gm/L) | 36 | 85 | 113 | 119 |
| Urea (mg/dL) | 234 | 165 | 105 | 72 |
| Creatinine (mg/dL) | 6.9 | 7.99 | 6.7 | 4.8 |
| Urine output (mL/day) | <30 mL | 10 mL | 100 mL | 270 mL |
| MetHb (%) | 25.3% | 2.1% | 1.1% | – |
| T bilirubin (mg/dL) | 6.4 | 2.4 | 1.1 | 0.5 |
| LDH (IU/L) | 4485 | – | 410 | 220 |
Hb. Haemoglobin; MetHb, methaemoglobin; LDH, lactate dehydrogenase.
Differential diagnosis
A provisional diagnosis of acquired methaemoglobinaemia was considered in view of the swallowed moth ball as the inciting agent and disparity in the report of haemoglobin (Hb) oxygen saturation by pulse oximetry and ABG analysis. Co-oximetry of ABG confirmed the diagnosis with methaemoglobin (MetHb) of 25.3% (bedside test 1.B and C).
Treatment
The patient was managed with oxygen inhalation, forced alkaline diuresis and blood component support (4 units of packed red blood cells). Haemodialysis (5 sessions in total) was given in view of the anuria and severe metabolic acidosis. As the patient was G6PD deficient, methylene blue was not considered and he was started on ascorbic acid (1000 mg/day orally in divided doses) and N-acetyl cysteine (NAC) (1800 mg/day in divided doses).
Outcome and follow-up
The MetHb decreased from 25.3% (day 1 of therapy) to 1.1% (day 8 of therapy) and there was significant improvement in other laboratory parameters (table 1). Gradually, the patient's urine output improved and he became dialysis-free. However, his creatinine levels only normalised after 1.5 months of the insult. He was educated and counselled regarding avoidance of chemicals, toxins, certain drugs (eg, Sulfa drugs) and other precipitating factors.
Discussion
Methaemoglobinaemia results from formation of MetHb in the blood. It is an altered state of haemoglobin in which ferric (Fe+3) ions are formed in the Hb due to oxidisation of the native ferrous ions (Fe+2).1 MetHb cannot bind to oxygen, rendering the Hb molecule unable to deliver oxygen to the tissues. There are many aetiologies for the development of MetHb, both acquired and congenital. Common aetiologies are mentioned in table 2.2–5
Table 2.
Causes of methaemoglobinaemia2–5
|
|
|
|
Naphthalene is a whitish crystalline volatile polycyclic aromatic hydrocarbon commonly used as a deodoriser and moth repellent in household and industrial settings. Its molecular formula is C10H8, with a mass of 128 g/mol. Accidentally, it can be confused with edible substances like candy especially by children and may even prove to be fatal.6 It is readily absorbed by human skin especially if oily and by the intestines in combination with fatty meals, and gets concentrated in the adipose tissue in human beings. The acute and chronic effects of naphthalene poisoning involve multiple organ systems of the body.7 8
Usually, haemolysis is precipitated in susceptible individuals, for instance, G6PD-deficient individuals, as they have low tolerance to oxidative stress.9 10 Naphthalene being a potent oxidant oxidises Hb to MetHb. Formation of methaemoglobinaemia causes acute impairment of the oxygen delivery mechanism without allowing sufficient compensation to take place.11 Initial symptoms manifest as blue, pale or grey discolouration of the skin, nail beds and lips. Headache, palpitations, confusion, fatigue, lethargy and dyspnoea are also other common symptoms. Higher levels of MetHb and accompanying haemolysis can lead to altered sensorium, acute renal failure, respiratory depression, coma, seizures and death.12 Underlying comorbid conditions such as heart, lung or liver disease and anaemia can increase the toxicity of MetHb.
Cyanosis is usually central and is clinically detectable at MetHb levels >1.5 gm/dL. Clinical suspicion should be raised by subtle symptoms including:13
Development of brownish to bluish red coloured blood or plasma noticed during sampling.
Blackish to brown coloured urine.
Sudden onset of hypoxia with cyanosis after an alleged history of an oxidant.
- Disparity in pulse oximetry and ABG analysis:
- In pulse oximetry oxygen saturation (SpO2) is <90% and partial pressure of oxygen >70 mm Hg;
- SpO2 fails to rise despite rising fraction of inspired oxygen;
- Disparity in saturation of oxygen on pulse oximetry and ABG analysis.
For a definitive diagnosis of MetHb, co-oximetry is the gold standard, using the Evelyn-Malloy method.14 Other ancillary tests may be carried out for the diagnosis of the cause of MetHb once the diagnosis is established. Assay for G6PD activity, incubation of blood with methylene blue to differentiate haemoglobin M disease and cytochrome b5R deficiency are also available in tertiary centres, to pinpoint the aetiology.
Treatment of methaemoglobinaemia in an acute setting should be initiated as soon as possible. It includes stabilisation of the airway, oxygen supplementation and augmenting circulation by ionotropes or mechanical ventilation if needed. Blood transfusions are usually required to treat underlying shock and to compensate for the ongoing haemolysis. Haemodialysis may also be required in the setting of acute renal failure due to haemoglobinuria.
Methylene blue is highly effective in symptomatic, non-G6PD deficient individuals as it increases NADPH by G6PD mediated mechanism.3 Ascorbic acid in high doses is also moderately effective as it acts as an antioxidant especially in G6PD-deficient cases.15 Other modalities such as hyperbaric oxygen, NAC and exchange transfusion can also be tried, although their efficacy is doubtful.
Learning points.
Systemic exposure (oral, topical or inhaled) of oxidants may may cause fatal toxic hazards such as methemoglobinaemia, especially in glucose-6-phosphate dehydrogenase-deficient individuals.
Timely recognition of subtle signs and symptoms of underlying methemoglobinaemia and acute intravascular haemolysis along with prompt supportive measures can improve survival outcomes.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed
References
- 1.Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med 1999;34:646–56. 10.1016/S0196-0644(99)70167-8 [DOI] [PubMed] [Google Scholar]
- 2.Hegesh E, Hegesh J, Kaftory A. Congenital methemoglobinemia with a deficiency of cytochrome b5. N Engl J Med 1986;314:757–61. 10.1056/NEJM198603203141206 [DOI] [PubMed] [Google Scholar]
- 3.Coleman MD, Coleman NA. Drug-induced methaemoglobinaemia. Treatment issues. Drug Saf 1996;14:394–405. [DOI] [PubMed] [Google Scholar]
- 4.Tomar LR, Aggarwal A, Jain P et al. Acute viral hepatitis E presenting with haemolytic anaemia and acute renal failure in a patient with glucose-6-phosphate dehydrogenase deficiency. Trop Doct 2015;45:245–6. 10.1177/0049475514559959 [DOI] [PubMed] [Google Scholar]
- 5.Hoffman C, Abubakar H, Kalikiri P et al. Nosocomial methemoglobinemia resulting from self-administration of benzocaine spray. Case Rep Anesthesiol 2015;2015:685304 10.1155/2015/685304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gidron E, Leurer J. Naphthalene poisoning. Lancet 1956;270:228–31. 10.1016/S0140-6736(56)91152-7 [DOI] [PubMed] [Google Scholar]
- 7.Lim HC. Mothballs: bringing safety issues out from the closet. Singapore Med J 2006;47:1003. [PubMed] [Google Scholar]
- 8.Stohs SJ, Ohia S, Bagchi D. Naphthalene toxicity and antioxidant nutrients. Toxicology 2002;180:97–105. 10.1016/S0300-483X(02)00384-0 [DOI] [PubMed] [Google Scholar]
- 9.Rahman MM, Mogni Mowla SG, Rahim A et al. Severe haemolytic anaemia due to ingestion of naphthalene (mothball) containing coconut oil. J Coll Physicians Surg Pak 2012;22:740–1. doi:11.2012/JCPSP.740741 [PubMed] [Google Scholar]
- 10.Kundra TS, Bhutatani V, Gupta R et al. Naphthalene poisoning following ingestion of mothballs: a Case Report. J Clin Diagn Res 2015;9:UD01–2. 10.7860/JCDR/2015/15503.6274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macgregor RR. Naphthalene poisoning from the ingestion of moth balls. Can Med Assoc J 1954;70:313–14. [PMC free article] [PubMed] [Google Scholar]
- 12.Lim HC, Poulose V, Tan HH. Acute naphthalene poisoning following the non-accidental ingestion of mothballs. Singapore Med J 2009;50:e298–301. [PubMed] [Google Scholar]
- 13.Cortazzo JA, Lichtman AD. Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth 2014;28:1043–7. 10.1053/j.jvca.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 14.Molthrop DC Jr, Wheeler RH, Hall KM et al. Evaluation of the methemoglobinemia associated with sulofenur. Invest New Drugs 1994;12:99–102. 10.1007/BF00874438 [DOI] [PubMed] [Google Scholar]
- 15.Rino PB, Scolnik D, Fustiñana A et al. Ascorbic acid for the treatment of methemoglobinemia: the experience of a large tertiary care pediatric hospital. Am J Ther 2014;21:240–3. 10.1097/MJT.0000000000000028 [DOI] [PubMed] [Google Scholar]


