Abstract
Inverse psoriasis is characterised by the involvement of flexural skin folds. This form of psoriasis has distinct clinical and therapeutic features. This report refers to the case of a 48-year-old Caucasian man who was observed in our department, with a clinically and biopsy proven diagnosis of inverse psoriasis. For 2 years, the patient was treated with different combinations of corticosteroids, vitamin D analogues and methotrexate, with no satisfactory response. Given the lack of a clinical response and comorbidities, latent tuberculosis was excluded, and we started treatment with ustekinumab. We chose this biological agent because the patient was a long-distance truck driver and refused the possibility of autoinjections. The patient underwent three ustekinumab injections, which resulted in significant improvement of pruritus, erythaematous lesions and quality of life.
Background
Inverse psoriasis is characterised by the involvement of flexural skin folds such as the inguinal region, gluteal cleft, axillae and external genitalia. A ∼3–7% of patients with psoriasis suffer from this type of disease.1 2 This form of psoriasis has distinct clinical and therapeutic features.3 Ustekinumab is a fully human IgG1 monoclonal antibody that binds to the shared p40 subunit of interleukin-12 (IL-12) and IL-23. It is approved for moderate-to-severe plaque psoriasis in patients who are candidates for phototherapy or systemic therapy.4 5
Case presentation
We report a case of a 48-year-old Caucasian man under observation by our dermatology department due to pruriginous, erythaematous plaques located in the groin, gluteal cleft and penis.
He had a personal history of arterial hypertension, diabetes mellitus and dyslipidaemia. These conditions had been treated with perindopril (10 mg/day) and metformin/vildagliptin (1000 mg/50 mg/day).
Physical examination revealed well circumscribed, macerated, shiny erythaematous plaques located in the right groin, gluteal cleft, shaft and glans penis. Psoriasis area and severity index score was 8 and Dermatology Life Quality Index (DLQI) scored 14. The remaining physical examination showed the presence of psoriasiform plaques located on the elbows, nail pitting and onycholysis in both hands.
Investigations
Upon this clinical presentation, a cutaneous biopsy was performed and the results confirmed the diagnosis of psoriasis.
Blood analysis revealed elevated glucose (280 mg/dL), cholesterol (221 mg/dL), triglycerides (408 mg/dL) and 7.8% of glycated haemoglobin. Serological and virological markers were negative.
Treatment
The patient was treated for 2 years with different combinations of topical corticosteroids, topical vitamin D analogues and methotrexate (20 mg/week), with no satisfactory response. Given the lack of a clinical response and his comorbidities, after the exclusion of latent tuberculosis, we began treatment with ustekinumab (45 mg initially and an additional 45 mg 4 weeks later, followed by 45 mg every 12 weeks). We chose this biological agent because the patient was a long-distance truck driver and refused the possibility of autoinjections.
Outcome and follow-up
After the first injection, the patient developed an external otitis with tympanic perforation that forced the interruption of this treatment. Three months later, once the infectious otitis was cured, we restarted ustekinumab. The patient completed three injections with significant improvements of pruritus, erythaematous lesions and quality of life (final DLQI of 2). The groin lesions cleared up almost entirely, and mild erythaema was present in the gluteal cleft and penis (figure 1). This is a case of inverse psoriasis successfully treated with ustekinumab. Intertiginous psoriasis is a treatment-resistant form of psoriasis and biological agents need to be considered in cases resistant to conventional therapy. Moreover, it is important to report similar cases of this condition and conduct appropriate follow-up appointments to confirm the long-term efficacy of this treatment.
Figure 1.
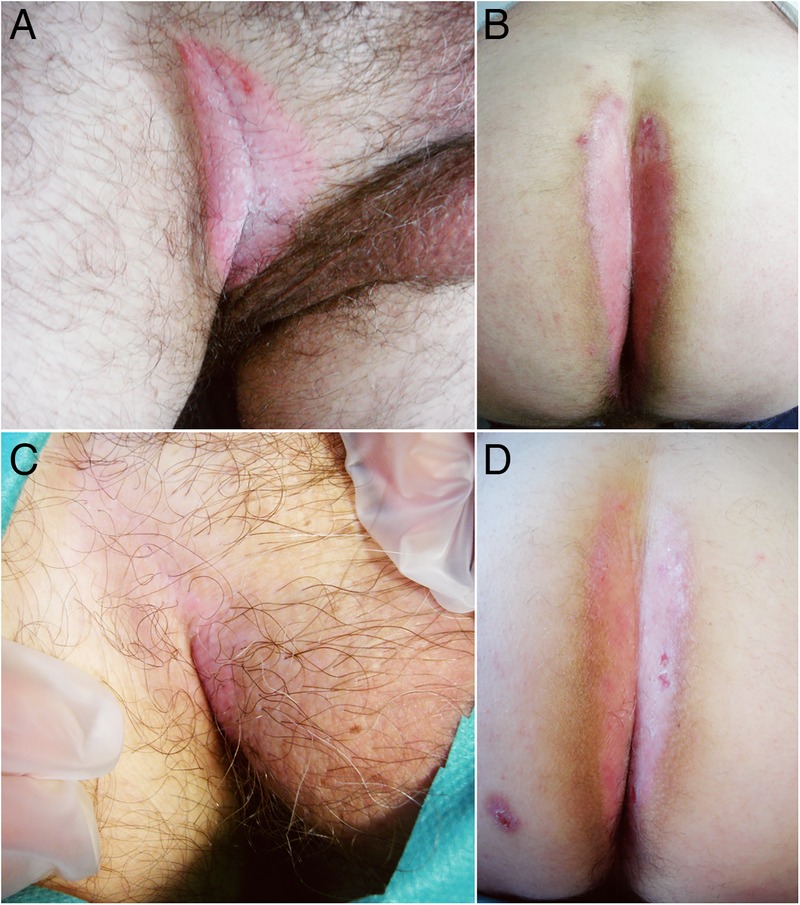
Well circumscribed, macerated, shiny erythaematous plaques located in the right groin and gluteal cleft. (A and B) Before treatment; (C and D) after treatment.
Learning points.
Inverse psoriasis is a treatment-resistant form of psoriasis.
First line therapies include topical corticosteroids, topical vitamin D analogues and topical calcineurin inhibitors.
We must consider comorbidities when choosing proper systemic medication.
Ustekinumab should be considered in cases resistant to conventional therapy.
Footnotes
Contributors: MAC was the first author. All the authors contributed substantially to the conception and design of this manuscript; the draft and article were revised by all the authors, who also approved the final version of this manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kundakci N, Türsen U, Babiker MO et al. The evaluation of the sociodemographic and clinical features of Turkish psoriasis patients. Int J Dermatol 2002;41:220–4. 10.1046/j.1365-4362.2002.01462.x [DOI] [PubMed] [Google Scholar]
- 2.Wang G, Li C, Gao T et al. Clinical analysis of 48 cases of inverse psoriasis: a hospital-based study. Eur J Dermatol 2005;15:176–8. [PubMed] [Google Scholar]
- 3.Omland SH, Gniadecki R. Psoriasis inversa: a separate identity or a variant of psoriasis vulgaris? Clin Dermatol 2015;33:456–61. 10.1016/j.clindermatol.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 4.Stelara (ustekinumab) [prescribing information] Beerse, Belgium: Janssen Biotech, 2009. [Google Scholar]
- 5.Papp KA, Langley RG, Lebwohl M et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675–84. 10.1016/S0140-6736(08)60726-6 [DOI] [PubMed] [Google Scholar]


