Abstract
A 50-year-old man was admitted in midwinter with fever, altered mental status and new onset generalised tonic-clonic seizure with urinary incontinence. Cerebrospinal fluid (CSF) analysis revealed an opening pressure of 14.5 cm of water, normal glucose and protein 82 mg/dL (reference range: 15–45 mg/dL). Cell count showed: red cells 11 (reference range: <5 mm3), white cell count 1 (reference range: <5 mm3). The patient's blood and CSF cultures had no growth. MRI of the brain with and without gadolinium contrast showed abnormal T2 and fluid-attenuated inversion recovery signals within bilateral ventricular nuclei, hippocampi, left frontal and parietal regions. Eastern equine encephalitis (EEE) antibody, IgG titre was 1:64 and IgM titre was <1:16. Three weeks later, his repeat/convalescent titres increased to 1:1024 and 1:32, respectively. Hence, a diagnosis of EEE was established. The patient was treated with supportive care. He recovered well with mildly impaired memory but no other cognitive deficits.
Background
On an average, about six cases of eastern equine encephalitis (EEE) are diagnosed in the USA every year.1 Transmission to humans usually occurs during May–October via an infected mosquito bite.2 Our patient presented in midwinter, which, although unusual, is not surprising during years with a warmer or late winter leading to a higher mosquito population. With climate change, other mosquito borne illnesses could similarly have prolonged or extended transmission periods. Depending on the patient's geographic location, season and clinical suspicion, there may be a higher yield in testing for endemic arboviral infections.
Case presentation
A 50-year-old man with a medical history of hyperlipidaemia, hypertension, sarcoidosis (not on steroids for 9 months) and atrial fibrillation (on anticoagulation), was admitted in midwinter as a transfer from an outside hospital after presenting with altered mental status and seizures. A week prior to presentation, he had experienced headache, shortness of breath and ‘cold-like’ symptoms. He then suddenly developed a generalised tonic-clonic seizure with urinary incontinence prompting admission. He was intubated for airway protection, administered intravenous phenytoin, intravenous ceftriaxone, ampicillin, vancomycin and acyclovir, and then transferred to our hospital. After his anticoagulation was reversed, on day 2 of hospitalisation, he underwent a lumbar puncture for cerebrospinal fluid (CSF) analysis.
The patient lived with his wife, in Florida, USA, in a wooded area. He had no history of recent travel, fishing or swimming in a lake and no tick or other insect bite. The patient did trap raccoons and consume them (after cooking). He had no pets other than a parakeet in a cage.
On examination, the patient was sedated and intubated, and on mechanical ventilation. He was feverish with a temperature of 40°C, but otherwise haemodynamically stable. His pupils were 2 mm, equal and reactive to light. His neck was supple but examination was limited due to the patient being intubated. The rest of his systemic examination was normal with no skin rash and no palpable lymphadenopathy.
EEG showed moderate generalised slowing and suppression with evidence of neither definitive epileptiform features nor subclinical seizure activity. There was slight asymmetry appreciated between the two hemispheres with the left hemispheric channels revealing more slowing than the right.
Investigations
A complete blood count showed leukocytosis with white cell count: 14 500 (normal: 4000–10 000/mm3) with a slight neutrophilic predominance of 82.6%. The haematocrit and platelet counts were normal. Electrolytes, renal and hepatic functions were also normal. International normalised ratio was 3.5. Sedimentation rate was 20 mm/h (normal: 0–15 mm/h).
CSF analysis revealed an opening pressure of 14.5 cm of water, normal glucose and protein 82 mg/dL (reference range: 15–45 mg/dL). Cell count showed: red cells 11 (reference range: <5 mm3), white cell count 1 (reference range: <5 mm3). Blood and CSF cultures had no growth. HIV antigen/antibody test, serology for west nile IgG and IgM, CSF enterovirus PCR and CSF herpes simplex virus (HSV) DNA PCR were negative.
MRI of the brain with and without gadolinium contrast showed abnormal T2 and fluid-attenuated inversion recovery (FLAIR) signals within bilateral ventricular nuclei, hippocampi, left frontal and parietal regions. There was no abnormal pachymeningeal enhancement (figures 1 and 2).
Figure 1.
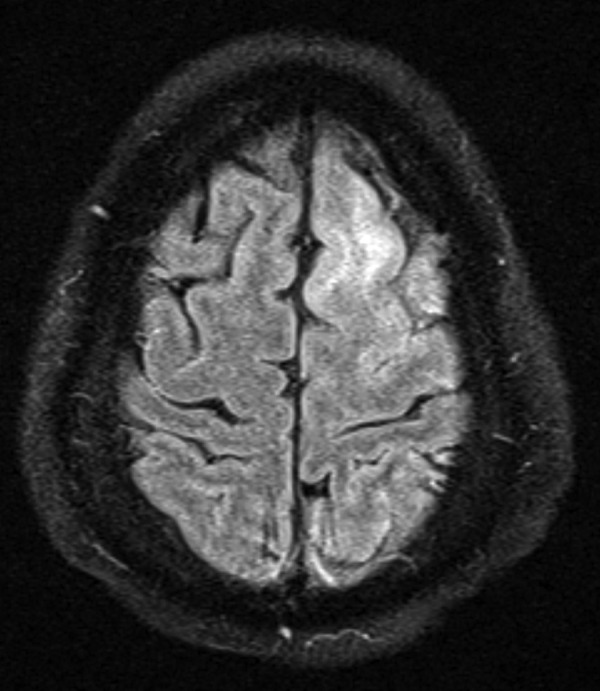
Brain MRI—Fluid-attenuated inversion recovery signal abnormality involving left frontal region.
Figure 2.
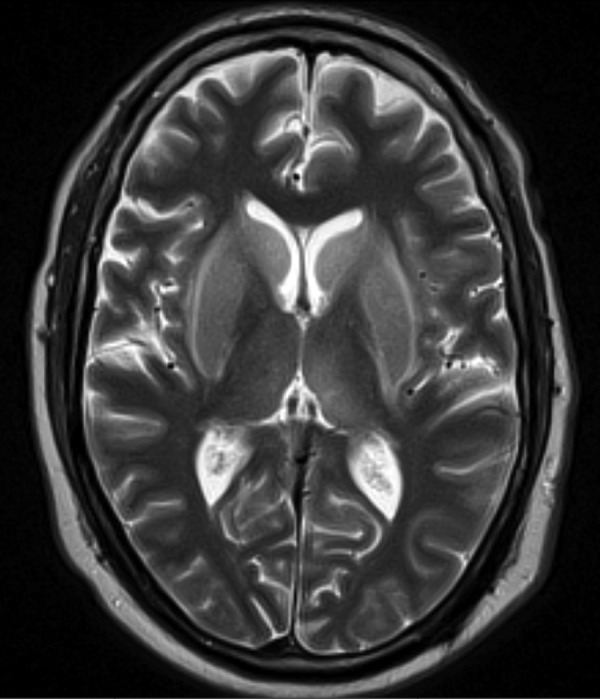
Brain MRI—T2 signal abnormality involving bilateral ventricular nuclei.
On serum arboviral panel, the patient's EEE Ab, IgG titre was 1:64 and IgM titre was <1:16. Three weeks later, repeat/convalescent IgG and IgM titres increased to 1:1024 and 1:32, respectively.
Differential diagnosis
Differential diagnoses in this case included various causes of encephalitis: West Nile encephalitis, rabies encephalitis (due to exposure to raccoons), equine encephalitides (EEE, St Louis, etc), HIV-related encephalitis, HSV encephalitis and neurosarcoidosis due to the patient's history of sarcoidosis.
His radiological findings were not consistent with neurosarcoidosis. His normal appearing CSF white cell count made the diagnosis of meningitis unlikely. With a clinical picture suggestive of encephalitis, negative HSV PCR on the CSF and the patient's endemicity, serologies for arboviruses were sent and helped establish a diagnosis.
Treatment
All antimicrobials were discontinued after the CSF analysis resulted. The patient remained hospitalised for about 6 weeks, underwent a tracheostomy and feeding gastrostomy placement, and received supportive care. A repeat brain MRI 3 weeks into the hospitalisation showed an overall improvement of the oedema and signal changes in the left frontal high convexity, mesial left thalamus and lenticular nuclei, with improving enhancement in the left frontal lobe and bilateral basal ganglia. He was discharged to a subacute rehabilitation facility and, following a 10-day stay, was discharged home.
The case was reported to the Florida Department of Health and through them to the Centers for Disease Control and Prevention (CDC).
Outcome and follow-up
During a follow-up clinic visit 2 weeks after being discharged home, the patient appeared to have regained much of his strength and function, and continued to improve overall. Two months following discharge, his wife noted mildly impaired memory in the patient, but no other cognitive deficits and no difficulties with language; he was ambulating well with no falls and remained on antiseizure medication, lacosamide 100 mg, twice daily as recommended by the neurologist.
A follow-up MRI 6 months later showed resolution of the FLAIR signal abnormality involving the bilateral lenticular nuclei, mesial left thalamus and frontal high convexity. Basal ganglia and thalamic enhancement remained unchanged.
Discussion
EEE virus (EEEV) is a member of the genus Alphavirus, family Togaviridae. It is transmitted to humans through the bite of an infected mosquito. Birds are the amplifying host while humans and horses are considered ‘dead-end’ hosts. Overall, only about 4–5% of human EEEV infections result in EEE. EEEV infection is thought to confer life-long immunity against reinfection. Case fatality rate is around 35%.3
In the USA, an average of six human cases of EEE are reported annually.1 Transmission to humans usually occurs during May–October.2 Most cases of EEE have been reported from Florida, Georgia, Massachusetts and New Jersey.
CDC guidelines for diagnosis of an arbovirus infection require the presence of an acute febrile illness with encephalitis during a time when viral transmission is likely and at least one of the following criteria is met:
Greater than fourfold increase in the viral antibody titre between acute and convalescent sera (often 10 weeks apart)
Isolation of the virus from CSF, blood or tissue
IgM positive to the organism in the CSF; presumptive positives can be made from the remaining biochemical assays (eg, haemoagglutinin inhibition, immunofluorescence, neutralisation, complement fixation).4
Treatment is mainly supportive. A multifaceted approach including aggressive management of cerebral oedema and cessation of seizures may prevent brain herniation and secondary neurological injury. Case reports have described using high-dose methylprednisolone and intravenous immunoglobulin, with some success.5
No human vaccine is available for routine use.6 An investigational new drug vaccine is available for high-risk environmental and laboratory workers.7 8 A killed vaccine is available for use in horses. It is recommended to vaccinate all horses at least twice a year, and up to four times in areas where the vector season is prolonged, such as Florida, USA.9
Avoiding exposure to mosquitoes is the best prevention strategy in humans. Use of repellents, protective clothing and screens is recommended.
Learning points.
A normal cerebrospinal fluid white cell count may be seen in encephalitis, especially early in the presentation and if there is no meningeal involvement.
There may be a higher yield in testing for endemic arboviral infections depending on the geographic location and clinical suspicion.
Although eastern equine encephalitis is known to occur in the summer months, in case of a warmer or late winter with a higher mosquito population, it should still be suspected. This would apply to other mosquito borne illnesses as well.
Early diagnosis, supportive care and rehabilitation are mainstays in this disease with high fatality and long-term sequelae in survivors.
Footnotes
Contributors: KJS and KC were involved in direct medical care of the patient and clinical management. The manuscript preparation and literature review were performed by KJS with input from KC.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.CDC. Epidemiology and geographic distribution—Eastern equine encephalitis: arbonet, arboviral diseases branch, centers for disease control and prevention. http://www.cdc.gov/EasternEquineEncephalitis/tech/epi.html
- 2.Morris C. Eastern equine encephalomyelitis. In: Monath TP, ed. The arboviruses: epidemiology and ecology. Vol 3 Boca Raton, FL: CRC Press, 1988:1–20. [Google Scholar]
- 3.Deresiewicz RL, Thaler SJ, Hsu L et al. . Clinical and neuroradiographic manifestations of eastern equine encephalitis. N Engl J Med 1997;336:1867–74. doi:10.1056/NEJM199706263362604 [DOI] [PubMed] [Google Scholar]
- 4.[No authors listed] Case definitions for infectious conditions under public health surveillance. Centers for disease control and prevention. MMWR Recomm Rep 1997;46:1–55. [PubMed] [Google Scholar]
- 5.Wendell LC, Potter NS, Roth JL et al. . Successful management of severe neuroinvasive eastern equine encephalitis. Neurocrit Care 2013;19:111–15. doi:10.1007/s12028-013-9822-5 [DOI] [PubMed] [Google Scholar]
- 6.CDC. Symptoms—Eastern equine encephalitis. http://www.cdc.gov/EasternEquineEncephalitis/tech/symptoms.html
- 7.Ashford RW. Encyclopedia of arthropod-transmitted infections of man and domesticated animals. CAB International, 2001:158. [Google Scholar]
- 8.Committee on special immunizations program for laboratory personnel engaged in research on countermeasures for select agents. Protecting the frontline in biodefense research. The National Academies Press, 2011:40. [PubMed] [Google Scholar]
- 9.Vaccinations for adult horses developed by the American Association of Equine Practitioners Infectious Disease Committee, 2008 and updated by the AAEP biological and therapeutic agents committee in 2012. http://www.aaep.org/info/guidelines-50


