Abstract
A stereoselective high performance liquid chromatography method has been developed for the chiral separation of the enantiomers of six antihistamines, doxylamine, carbinoxamine, dioxopromethazine, oxomemazine, cetirizine and hydroxyzine. The effects of mobile phase additive, column temperature and flow rate on the retention time and resolution were studied. Enantiomeric separation of cetirizine, doxylamine and hydroxyzine were achieved on cellulose tris-(3,5-dichlorophenylcarbamate) immobilized on silica gel chiral stationary phase known as Chiralpak IC (RS = 3.74, RS = 1.85 and RS = 1.74, respectively).
Introduction
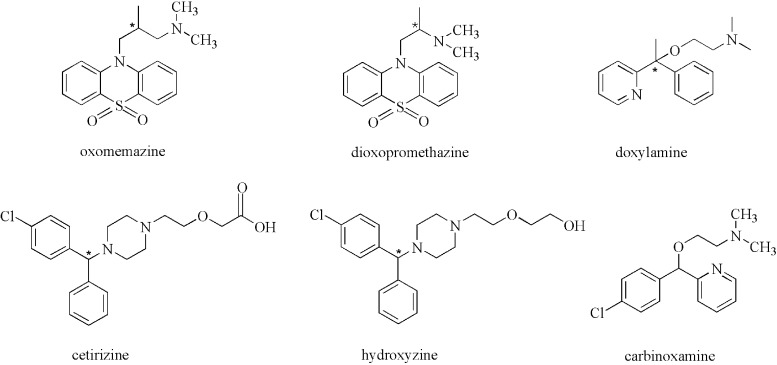
The antihistamine drugs, such as carbinoxamine, oxomemazine, dioxopromethazine, doxylamine, cetirizine and hydroxyzine, are used to relieve or prevent the symptoms of hay fever and other allergies (such as allergic rhinitis and atopic dermatitis) (1–3). It is well known that a pair of enantiomers can have different biological activities and toxicological profiles. For example, in the treatment of urticaria, pharmacological activity has been contributed mainly by R-cetirizine, while S-cetirizine is inactive, so it is necessary to have analytical methodologies for their separation.
Many methods have been reported for the separation of antihistamines, such as capillary electrophoresis (CE), supercritical fluid chromatogram (SFC) and high performance liquid chromatography (HPLC) (4–6). Enantioseparation of dioxopromethazine, cetirizine, hydroxyzine and doxylamine by CE have been widely studied (7–12). In HPLC, Hu et al. (13) resolved the enantiomers of cetirizine using HPLC on a chiral ovomucoid column. However, protein chiral stationary phases (CSPs) tend to be less stable. Kang et al. (14) separated cetirizine on a Chiralpak AD-H column by LC–MS. In this study, Chiralpak IC, where the chiral selectors is cellulose tris-(3,5-dichlorophenylcarbamate), was used to separate the six antihistamines. Relative to similar columns, it might possess advantages in terms of robustness and the range of mobile phase solvents that can be utilized (15–17).
In this study, the effects of organic modifiers, mobile phase additive, column temperature and flow rate on the retention time and resolution of the enantiomers of oxomemazine, doxylamine, dioxopromethazine, carbinoxamine, hydroxyzine and cetirizine (the structures are shown in Figure 1) were studied.
Figure 1.
The structures of six antihistamines.
Experimental
Apparatus
The HPLC instrument used in this study was an Agilent 1,100 series apparatus (Palo Alto, CA, USA) equipped with a quaternary pump, a vacuum degasser, a column oven, a multiple wavelength UV detector, an auto-sampler and HP Chemstation software.
Chemicals
The analytical Chiralpak IC column (250 mm × 4.6 mm, 5 µm) was supplied by Daicel Chemical Industries Ltd (Japan). HPLC-grade n-hexane, ethanol and isopropanol were obtained from T&J Kermel Reagent Company. Diethylamine (DEA) was analytically pure and was purchased from T&J Kermel Reagent Company. Doxylamine, cetirizine, carbinoxamine, oxomemazine, dioxopromethazine and hydroxyzine were obtained from New Drugs Research and Development Center of Zhengzhou University.
Sample preparation
Doxylamine, carbinoxamine, oxomemazine, dioxopromethazine, hydroxyzine and cetirizine were dissolved in appropriate amounts of ethanol. The solutions were all filtered (0.22 µm) to prepare the sample solution.
Chromatographic conditions
The basic solvent of mobile phase was n-hexane, ethanol or isopropanol was chosen as a mobile phase modifier, and DEA was used as the mobile phase additive. The mobile phase was filtered with a 0.45-µm solvent filter and ultrasonically degassed. The detection wavelengths of cetirizine, carbinoxamine, oxomemazine, hydroxyzine and dioxopromethazine were all set at 227 nm, while doxylamine was set at 262 nm. The volume of sample injected was 10 µL.
Results
Effect of alcohol modifier
The effect that the content of mobile phase modifiers had on the six antihistamines was studied. The results are shown in Table I.
Table I.
Effect of Content of Alcohols on the Enantioseparation of Six Antihistamines
| Compound | Isopropanol (modifier) |
Ethanol (modifier) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| tR1 | tR2 | α | RS | % | tR1 | tR2 | α | RS | % | |
| Doxylamine | 43.242 | 48.635 | 1.13 | 0.36 | 5 | 22.684 | 25.795 | 1.16 | 0.41 | 5 |
| 23.199 | – | – | 10 | 14.547 | – | – | 10 | |||
| Carbinoxamine | 27.077 | – | – | 10 | 14.768 | – | – | 10 | ||
| Cetirizine | 46.722 | 56.422 | 1.22 | 1.43 | 5 | 18.987 | 21.357 | 1.15 | 2.02 | 5 |
| 25.037 | 30.295 | 1.24 | 1.76 | 10 | 12.157 | 13.383 | 1.14 | 1.87 | 10 | |
| 14.513 | 17.308 | 1.25 | 1.88 | 20 | 8.666 | 9.348 | 1.12 | 1.59 | 20 | |
| 11.167 | 13.159 | 1.25 | 1.66 | 30 | 7.128 | 7.637 | 1.13 | 1.47 | 30 | |
| 10.578 | 12.254 | 1.22 | 1.53 | 40 | 6.819 | 7.213 | 1.11 | 1.30 | 40 | |
| Hydroxyzine | 38.088 | 40.725 | 1.08 | 0.51 | 5 | 22.191 | – | – | 5 | |
| 21.465 | 22.507 | 1.06 | 0.41 | 10 | 12.592 | – | – | 10 | ||
| 13.274 | – | – | 20 | 8.220 | – | – | 20 | |||
| Oxomemazine | >100 | – | – | 10 | 38.864 | – | – | 10 | ||
| Dioxopromethazine | >100 | – | – | 10 | 41.684 | 42.809 | 1.03 | 0.37 | 10 | |
| 52.189 | – | – | 20 | 19.736 | 20.144 | 1.03 | 0.32 | 20 | ||
| 31.660 | – | – | 30 | 13.057 | 13.237 | 1.02 | 0.26 | 30 | ||
| 22.849 | – | – | 40 | 10.183 | – | – | 40 | |||
tR1, tR2: retention times (min); α: separation factor; RS: resolution factor; “–” means that separation was not seen. Chromatographic conditions. The basic solvent of mobile phase was n-hexane, the temperature was at 25°C and the flow rate was 0.8 mL min−1.
Effect of mobile phase additive DEA
The effect that DEA had on doxylamine, carbinoxamine, dioxopromethazine, cetirizine, oxomemazine and hydroxyzine was studied. The results are shown in Table II.
Table II.
Effect of DEA on the Six Antihistamines
| Compound | Isopropanol (modifier) |
Ethanol (modifier) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| tR1 | tR2 | α | RS | % | tR1 | tR2 | α | RS | % | |
| Doxylamine | 11.944 | 12.362 | 1.05 | 0.70 | 5 | 11.172 | 12.341 | 1.15 | 2.65 | 5 |
| 9.449 | – | – | 10 | 7.465 | 7.840 | 1.09 | 1.85 | 10 | ||
| 6.702 | – | – | 20 | 6.702 | 7.011 | 1.09 | 1.12 | 20 | ||
| 6.008 | – | – | 30 | 6.009 | 6.220 | 1.07 | 0.83 | 30 | ||
| 5.673 | – | – | 40 | 5.418 | 5.533 | 1.05 | 0.56 | 40 | ||
| Carbinoxamine | 15.724 | 16.359 | 1.05 | 1.24 | 5 | 13.437 | 14.048 | 1.06 | 1.22 | 5 |
| 10.832 | 11.322 | 1.06 | 1.00 | 10 | 9.296 | 9.719 | 1.07 | 0.93 | 10 | |
| 7.289 | 7.534 | 1.06 | 0.66 | 20 | 7.024 | 7.219 | 1.05 | 0.63 | 20 | |
| 6.342 | 6.515 | 1.05 | 0.59 | 30 | 6.154 | 6.282 | 1.04 | 0.53 | 30 | |
| 5.910 | 6.051 | 1.05 | 0.56 | 40 | 5.554 | – | – | 40 | ||
| Oxomemazine | >100 | – | – | 5 | 61.677 | 63.221 | 1.03 | 0.63 | 5 | |
| >100 | – | – | 10 | 31.346 | 31.769 | 1.02 | 0.37 | 10 | ||
| 40.385 | – | – | 20 | 16.136 | – | – | 20 | |||
| Dioxopromethazine | >100 | – | – | 5 | 83.919 | 87.558 | 1.05 | 1.20 | 5 | |
| >100 | – | – | 10 | 38.365 | 39.704 | 1.04 | 0.92 | 10 | ||
| 43.851 | 44.670 | 1.02 | 0.47 | 20 | 18.183 | 18.668 | 1.03 | 0.62 | 20 | |
| 26.733 | – | – | 30 | 12.368 | 12.601 | 1.03 | 0.50 | 30 | ||
| 19.242 | – | – | 40 | 9.451 | – | – | 40 | |||
| Cetirizine | 32.867 | 43.704 | 1.36 | 5.75 | 5 | 18.725 | 21.306 | 1.17 | 2.82 | 5 |
| 21.355 | 28.026 | 1.37 | 5.08 | 10 | 11.985 | 13.304 | 1.15 | 2.59 | 10 | |
| 11.795 | 14.504 | 1.31 | 4.65 | 20 | 8.588 | 9.340 | 1.14 | 2.17 | 20 | |
| 9.669 | 11.570 | 1.29 | 4.07 | 30 | 7.364 | 7.910 | 1.13 | 1.87 | 30 | |
| 8.625 | 10.208 | 1.29 | 3.74 | 40 | 6.424 | 6.803 | 1.12 | 1.56 | 40 | |
| Hydroxyzine | 34.758 | 38.497 | 1.12 | 1.99 | 5 | 21.507 | 22.233 | 1.04 | 0.66 | 5 |
| 16.382 | 17.549 | 1.09 | 1.74 | 10 | 12.175 | 12.405 | 1.03 | 0.49 | 10 | |
| 9.953 | 10.471 | 1.08 | 1.13 | 20 | 8.029 | – | – | 20 | ||
| 7.853 | 8.163 | 1.07 | 0.82 | 30 | 6.672 | – | – | 30 | ||
| 6.942 | 7.164 | 1.06 | 0.64 | 40 | 5.796 | – | – | 40 | ||
Chromatographic conditions. The basic solvent of mobile phase was n-hexane with 0.1% DEA, and the column temperature was at 25°C with a flow rate of 0.8 mL min−1.
Effect of the content of DEA
Experiments were carried out to study the effect of content of additive DEA on the enantioseparation of doxylamine, cetirizine and hydroxyzine. The results are shown in Table III.
Table III.
Effect of Content of DEA on the Enantioselectivity of Three Antihistamines
| Compound | tR1 | tR2 | α | RS | DEA (%) |
|---|---|---|---|---|---|
| Doxylamine | 7.465 | 7.840 | 1.09 | 1.85 | 0.1 |
| 7.447 | 7.837 | 1.09 | 1.93 | 0.2 | |
| 7.302 | 7.669 | 1.09 | 1.88 | 0.3 | |
| Cetirizine | 8.625 | 10.208 | 1.29 | 3.74 | 0.1 |
| 8.401 | 9.979 | 1.28 | 3.66 | 0.2 | |
| 8.391 | 9.867 | 1.28 | 3.63 | 0.3 | |
| Hydroxyzine | 16.382 | 17.549 | 1.09 | 1.74 | 0.1 |
| 16.017 | 17.116 | 1.09 | 1.67 | 0.2 | |
| 15.562 | 16.575 | 1.08 | 1.60 | 0.3 |
Chromatographic conditions. Doxylamine: mobile phase was n-hexane–ethanol (90/10, v/v); cetirizine: mobile phase was n-hexane–isopropanol (60/40, v/v); hydroxyzine: mobile phase was n-hexane–isopropanol (90/10, v/v). The column temperature was at 25°C, and the flow rate was 0.8 mL min−1.
Effect of column temperature
The effect that the column temperature had on doxylamine, hydroxyzine and cetirizine was studied. The results are shown in Table IV.
Table IV.
Effect of Column Temperature on the Three Antihistamines
| Compound | tR1 | tR2 | α | RS | Temperature (°C) |
|---|---|---|---|---|---|
| Doxylamine | 9.019 | 9.837 | 1.14 | 2.44 | 15 |
| 8.301 | 8.881 | 1.11 | 2.32 | 20 | |
| 7.465 | 7.840 | 1.09 | 1.85 | 25 | |
| 7.156 | 7.456 | 1.07 | 1.65 | 30 | |
| 6.793 | 7.021 | 1.05 | 1.37 | 35 | |
| Cetirizine | 10.345 | 12.519 | 1.31 | 3.93 | 15 |
| 9.388 | 11.314 | 1.30 | 3.85 | 20 | |
| 8.625 | 10.208 | 1.29 | 3.74 | 25 | |
| 7.875 | 9.095 | 1.28 | 3.51 | 30 | |
| 7.353 | 8.368 | 1.27 | 3.20 | 35 | |
| Hydroxyzine | 21.379 | 23.435 | 1.11 | 2.10 | 15 |
| 18.969 | 20.561 | 1.10 | 1.93 | 20 | |
| 16.382 | 17.549 | 1.09 | 1.74 | 25 | |
| 14.925 | 15.840 | 1.08 | 1.56 | 30 | |
| 13.806 | 14.547 | 1.07 | 1.38 | 35 |
Chromatographic conditions. Doxylamine: mobile phase was n-hexane–ethanol (90/10, v/v, 0.1% DEA); cetirizine: mobile phase was n-hexane–isopropanol (60/40, v/v, 0.1% DEA); hydroxyzine: mobile phase was n-hexane–isopropanol (90/10, v/v/, 0.1% DEA). The flow rate was 0.8 mL min−1.
Effect of flow rate
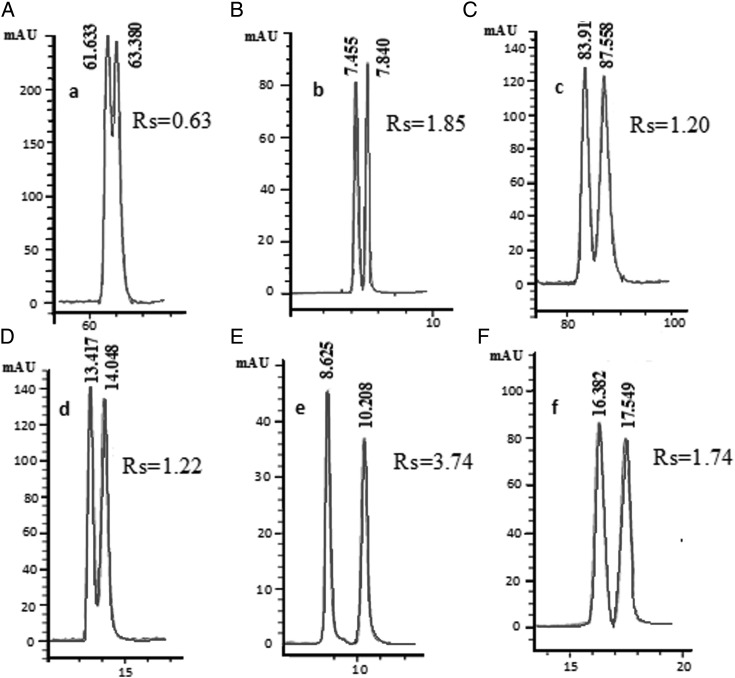
The effect that the flow rate had on doxylamine, cetirizine and hydroxyzine was investigated. The results are shown in Table V. The chromatogram of oxomemazine, doxylamine, dioxopromethazine, carbinoxamine, cetirizine and hydroxyzine is shown in Figure 2.
Table V.
Effect of Flow Rate on the Enantioselectivity of Three Antihistamines
| Compound | tR1 | tR2 | α | RS | Flow rate (mL min−1) |
|---|---|---|---|---|---|
| Doxylamine | 14.942 | 15.699 | 1.09 | 1.92 | 0.4 |
| 9.972 | 10.482 | 1.09 | 1.88 | 0.6 | |
| 7.465 | 7.840 | 1.09 | 1.85 | 0.8 | |
| 5.962 | 6.260 | 1.09 | 1.79 | 1.0 | |
| 4.985 | 5.238 | 1.09 | 1.76 | 1.2 | |
| Cetirizine | 17.157 | 20.268 | 1.29 | 4.46 | 0.4 |
| 11.574 | 13.701 | 1.29 | 4.07 | 0.6 | |
| 8.625 | 10.208 | 1.29 | 3.74 | 0.8 | |
| 6.876 | 6.137 | 1.28 | 3.39 | 1.0 | |
| 5.759 | 6.819 | 1.29 | 3.23 | 1.2 | |
| Hydroxyzine | 31.543 | 33.714 | 1.09 | 1.91 | 0.4 |
| 21.566 | 23.091 | 1.09 | 1.83 | 0.6 | |
| 16.382 | 17.549 | 1.09 | 1.74 | 0.8 | |
| 13.042 | 13.966 | 1.09 | 1.66 | 1.0 | |
| 10.952 | 11.739 | 1.09 | 1.59 | 1.2 |
Chromatographic conditions. Doxylamine: mobile phase was n-hexane–ethanol (90/10, v/v, 0.1% DEA); cetirizine: mobile phase was n-hexane–isopropanol (60/40, v/v, 0.1% DEA); hydroxyzine: mobile phase was n-hexane–isopropanol (90/10, v/v, 0.1% DEA). The column temperature was at 25°C.
Figure 2.
HPLC chromatograms of six antihistamines. (A) oxomemazine, (B) doxylamine, (C) dioxopromethazine, (D) carbinoxamine, (E) cetirizine and (F) hydroxyzine. Chromatographic conditions. Oxomemazine, dioxopromethazine and carbinoxamine: n-hexane-ethanol (95/5, V/V, 0.1% DEA); doxylamine: n-hexane-ethanol (90/10, V/V, 0.1% DEA); cetirizine: n-hexane-isopropanol (60/40, V/V, 0.1% DEA); hydroxyzine: n-hexane-isopropanol (90/10, V/V, 0.1% DEA). The column temperature was at 25̊C, the flow rate was 0.8 mL min-1.
Discussion
Effect of alcohol modifier
The results showed that the enantiomers were not separated well except cetirizine. It was reported that basic or acidic additives in the mobile phase often contribute to improve resolution of chiral compounds (18). Therefore, DEA was used as a mobile phase additive for the six compounds.
Effect of mobile phase additive DEA
The results showed that the resolution increased significantly, and the retention was decreased with the basic additive DEA, which was due to the interaction between DEA and CSP, which decreased the interaction between the compounds and CSP.
The results also showed that with the increase in the proportion of alcohols, the elution capacity of mobile phase was increased, and the interaction between enantiomers and CSP was decreased, hence the retention time and resolution were decreased.
Effect of the content of DEA
The results indicated that the retention and resolution change little over the DEA concentration range of 0.1–0.3%. Therefore, 0.1% DEA was used.
Effect of column temperature
The parameters are calculated as follows (19):
Here, α = k′2/k′1, α is separation factor, R is the gas constant and T is the temperature in K, ΔR,SΔH° and ΔR,SΔS° are enthalpy change and entropy change of two enantiomers from the mobile phase to the stationary phase during the distribution process, respectively. Van't Hoff plots were drawn for ln α vs 1/T for two isomers.
Their regression equations are shown in Table VI. The results showed that the linearity of the regression equations was good. From the above equation, the value of ΔR,SΔH° and ΔR,SΔS° was calculated (the results are shown in Table VI). Over the temperature range of 288–308 K, they all conform to ∣ΔR,SΔH°∣ > ∣TΔR,SΔS°∣.
Table VI.
The Value of ΔR,SΔH° and ΔR,SΔS° of Three Antihistamines
| Compound | Doxylamine | Cetirizine | Hydroxyzine |
|---|---|---|---|
| ΔR,SΔH° (kJ mol−1) | −0.365 | −0.141 | −0.166 |
| ΔR,SΔS° (J mol−1) | −1.138 | −0.218 | −0.473 |
| The regression equation | ln α = 365.79/T – 1.1407 (r = 0.9978) | ln α = 140.75/T – 0.2180 (r = 0.9993) | ln α = 166.55/T– 0.4732 (r = 0.9994) |
Therefore, the chiral separation process of the three compounds was all controlled by enthalpy. As the column temperature increased, a corresponding decrease in retention was observed, the resolution was also decreased. This would be explained by the fact that a thermodynamic process was faster at higher temperatures, which resulted in lower enantiomeric retention.
The results indicated that the column temperature should be carefully controlled for optimum chiral separation of enantiomers. Furthermore, 25°C was closer to room temperature, so the other parameters were optimized at 25°C.
Effect of flow rate
The results showed that the change in separation factor was not significant over the flow rate range of 0.4–1.2 mL min−1. The resolution of doxylamine, hydroxyzine and cetirizine decreased as the flow rate increased over the range of 0.4–1.2 mL min−1. To reduce the analytical time, the flow rate of 0.8 mL min−1 was used.
Conclusion
The enantiomers of doxylamine, oxomemazine, carbinoxamine, dioxopromethazine, cetirizine and hydroxyzine were first separated with Chiralpak IC. The optimum chromatographic conditions of six compounds are as follows: cetirizine: n-hexane–isopropanol (60/40, v/v, 0.1% DEA), RS = 3.74; hydroxyzine: n-hexane–isopropanol (90/10, v/v, 0.1% DEA), RS = 1.74; doxylamine: n-hexane–ethanol (90/10, v/v, 0.1% DEA), RS = 1.85; oxomemazine, dioxopromethazine and carbinoxamine: n-hexane–ethanol (95/5, v/v, 0.1 DEA), RS = 0.63, RS = 1.20 and RS = 1.22, respectively. The column temperature was at 25°C, and the flow rate was 0.8 mL min−1.
References
- 1.Amr M.M., Nigel R.W.; Histamine and antihistamines; Anaesthesia & Intensive Care Medicine, (2014); 15(5): 250–255. [Google Scholar]
- 2.Flavia C.L.H., Rohit K.K.; Antihistamine therapy in allergic rhinitis; Immunology and Allergy Clinics of North America, (2011); 31(3): 509–543. [DOI] [PubMed] [Google Scholar]
- 3.Akiko I., Tamihiro K., Fumiko M., Yoshinao S., Masako M.; Effective treatment of pruritus in atopic dermatitis using H1 antihistamines (second-generation antihistamines): changes in blood histamine and tryptase levels; Journal of Dermatological Science, (2003); 33(1): 23–29. [DOI] [PubMed] [Google Scholar]
- 4.Zhu D.R., Li X., Sun J.Y., You T.Y.; Chemometrics optimization of six antihistamines separations by capillary electrophoresis with electrochemiluminescence detection; Talanta, (2012); 88(15): 265–271. [DOI] [PubMed] [Google Scholar]
- 5.Toribio L., del Nozal M.J., Bernal J.L., Cristofol C., Alonso C.; Study of the enantiomeric separation of oxfendazole and cetirizine using subcritical fluid chromatography on an amylose-based column; Journal of Chromatography A, (2006); 1121(2): 268–273. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z.F., Yang G.L., Liang G.J., Zhou Y., Chen Y.; Study on the enantiomer separation of cetirizine dihydrochloride using proteinate- and amylose-based chiral stationary phase; Acta Pharmaceutica Sinica, (2004); 39(3): 204–207. [PubMed] [Google Scholar]
- 7.Tao Q.F., Zhu X.P., Zhao Y.; Chiral resolution of cetirizine by capillary electrochromatography using norvancomycin hydrochloride as chiral selector; Chinese Journal of Pharmaceutical Analysis, (2007); 27(5): 686–688. [Google Scholar]
- 8.Chou Y.W., Huang W.S., Ko C.C., Chen S.H.; Enantioseparation of cetirizine by sulfated-beta- cyclodextrin-mediated capillary electrophoresis; Journal of Separation Science, (2008); 31(5): 845–852. [DOI] [PubMed] [Google Scholar]
- 9.Nojavan S., Fakhari A.R.; Chiral separation and quantitation of cetirizine and hydroxyzine by maltodextrin-mediated CE in human plasma: effect of zwitterionic property of cetirizine on enantioseparation; Electrophoresis, (2011); 32(6–7): 764–771. [DOI] [PubMed] [Google Scholar]
- 10.Yu H.H., Hsin L.W., Wu S.; Quantitative enantiomeric analysis of chloreyelizine, hydroxyzine and meclizine by capillary electrophoresis; Analytical and Bioanalytical Chemistry, (2003); 376(6): 859–863. [DOI] [PubMed] [Google Scholar]
- 11.Peter M., Iva V., Emil H.; Chiral separation of dioxopromethazine in eye drops by CZE with charged cyclodextrin; Journal of Pharmaceutical and Biomedical Analysis, (2003); 33(2): 157–164. [DOI] [PubMed] [Google Scholar]
- 12.Arkadiusz K., Kazimiera D., Anna B.; Comparison of chiral separation of basic drugs in capillary electrophoresis and liquid chromatography using neutral and negatively charged cyclodextrins; Analytica Chimica Acta, (2009); 645(1–2): 98–104. [DOI] [PubMed] [Google Scholar]
- 13.Hu Y.P., Song Y.R., Wang D.F., Yang Y.P., Hou D.Y., Ou Y.J.; Study on enantiomeric separation of cetirizine on HPLC with a chiral ovomucoid column; Chinese Journal of Pharmaceutical Analysis, (2004); 24(3): 289–293. [Google Scholar]
- 14.Kang S.W., Jang H.J., Moore V.S., Park J.Y., Kim K.A., Youm J.R. et al. ; Enantioselective determination of cetirizine in human plasma by normal-phase liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry; Journal of Chromatography B, (2010); 878(32): 3351–3357. [DOI] [PubMed] [Google Scholar]
- 15.Zhang T., Franco P., Nguyen D., Hamasaki R., Miyamoto S., Ohnishi A. et al. ; Complementary enantiorecognition patterns and specific method optimization aspects on immobilized polysaccharide-derived chiral stationary phases; Journal of Chromatography A, (2012); 1269: 178–188. [DOI] [PubMed] [Google Scholar]
- 16.Ferretti R., Gallinella B., Torre F.L., Zanitti L., Turchetto L., Mosca A. et al. ; Direct high-performance liquid chromatography enantioseparation of terazosin on an immobilised polysaccharide-based chiral stationary phase under polar organic and reversed-phase conditions; Journal of Chromatography A, (2009); 1216(28): 5385–5390. [DOI] [PubMed] [Google Scholar]
- 17.Qu H.T., Li J.Q., Wu G.S., Shen J., Shen X.D., Yoshio O.; Preparation and chiral recognition in HPLC of cellulose 3,5-dichlorophenylcarbamates immobilized onto silica gel; Journal of Separation Science, (2011); 34(5): 536–541. [DOI] [PubMed] [Google Scholar]
- 18.Shamsipur M., Abdollahpour A., Heydari R.; Development and validation of a new high performance liquid chromatographic method for enantioseparation of dorzolamide hydrochloride on a coated cellulose phenylcarbamate chiral stationary phase; Journal of Liquid Chromatography & Related Technologies, (2011); 34(14): 1367–1380. [Google Scholar]
- 19.Zhou J., Liu Q., Su N., Fu G.J., Pei W.J., Zhang Z.Z.; Separation of betaxolol hydrochloride with new bonded cellulose chiral stationary phase and determination of the enantiomers in plasma by HPLC; Journal of Liquid Chromatography & Related Technologies, (2012); 35(13): 1767–1778. [Google Scholar]