Abstract
A fast and sensitive method was developed for in vivo determination of histamine in the brain microdialysate by reverse ion pair chromatography with electrochemical detection. The microdialysates were derivatized with o-phthalaldehyde and sodium sulfite, and separation was achieved using isocratic elution within 10 min. The separation was performed in an Agilent Eclipse Plus C18 column (3.0 × 150 mm, particle size 3.5 μm), and the mobile phase consisted of 100 mM monosodium phosphate (pH 6.0), 500 mg L−1 OSA and 20% methanol (v/v). The linearity (R2) was found to be >0.999, with a range from 2 to 50 nM and excellent repeatability (relative standard deviation, 2.29–6.04%), and the limit of detection was 0.4 nM. This method was successfully applied to analyze the extracellular concentration of histamine in the hypothalamus of rats, with probe recovery calculated in vivo.
Introduction
Histamine (HA) is widely distributed and acts as a neurotransmitter in the mammalian brain. The cell bodies of the histaminergic neuron are mainly located in the tuberomammillary nucleus, which is a small region of the hypothalamus (HYP), and fibers are distributed widely throughout the brain (1). HA is responsible for many physiological functions including food intake, state of arousal, learning and reinforcement, temperature regulation, cardiovascular control, etc. (2). As a result, it is essential for us to learn the functions of HA in the brain. It is also useful for us to learn ways to prevent as well as treat the related diseases. Neurotransmitters are mainly effective in the extracellular space. The traditional tissue homogenate method can only analyze the whole content of neurotransmitter from both intracellular and extracellular space. However, the microdialysis method is used to obtain the neurotransmitters directly from the extracellular space in animals that are awake or under anesthetics for further analysis (3–5), and thus, it has been widely adopted.
Currently, there are many techniques for the determination of HA such as capillary electrophoresis (6), biosensors (7), gas chromatography–mass spectrometry (8), liquid chromatography–mass spectrometry (9–11), ion chromatography (12) and high-performance liquid chromatography (HPLC) (13). Among these approaches, HPLC is the most widely used because of its operational advantages (cost and repeatability). However, HA in the microdialysate is difficult to determine due to the low concentration and complex matrix in the samples (14). Furthermore, HA cannot be directly detected by the fluorescence detector (FLD) or electrochemical detector (ECD) without derivatization. In the previously research, HA was major detected with FLD after pre- and postcolumn derivatization. During the postcolumn derivatization phase, o-phthalaldehyde (OPA) was always used after the microdialysate separated in cation-exchange chromatography (15) or ion pair chromatography (16). The other method utilized was precolumn derivatization with 4-(1-pyrene) butyric acid N-hydroxysuccinimide ester (17) or 4-(1-pyrene)butanoyl chloride (18). The sample was then separated in a reverse phase column and detected with FLD. However, both of the two types of method have their limitations, the former method needs an additional pump while the latter method needs a harsh reaction condition (100°C for 90 min).
ECD is also a sensitive detector, and limit of detection (LOD) of which was several fmol, even amol (19). Currently, it has been extensively used in neuroscience field because of its extreme sensitivity, especially for the routine determination of monoamines (20). Thus, ECD could be an alternative method for determining HA, especially for much lower concentrations. After derivatization with OPA and β-mercaptoethanol, determination of HA with HPLC, coupled with ECD, had been previously established. This method was applied to determine HA in rat mast cells (21). To circumvent the limitations of this method (i.e., unpleasant odor and unstable derived products), an improved method was established and HA reacted with OPA-sulfite. This approach was successfully utilized to determine HA and Nτ-methylhistamine in brain homogenate (22). However, this method (LOD of 0.125 pmol with 100 μL injection) was not sufficient for determining of HA in the microdialysate. Determination of HA in the microdialysate is similar to homogenate, because the components are similar. However, the much lower concentration of HA in the microdialysate requires high resolution to avoid the interference of amino acids, which can also react with OPA-sulfite. Additionally, the volume of microdialysate is limited.
Once the concentration of HA and the probe recovery was determined, the concentration of HA in the brain extracellular can be calculated. Unfortunately, because the probe recovery was influenced by many factors (e.g., diameter, length, geometry and material of the membrane, the perfusion flow rate, the perfusate characteristics, the sample matrix characteristics, etc.) and thus the probe recovery determined in vitro was not accurate because the environment of the brain is difficult to imitate (23, 24). The zero-net flux method is a widely used in vivo calibration method. The analyte of interest is perfused at various concentrations through the probe (Cin), and the concentration of this analyte is then measured at the outlet of the probe (Cout). A plot of Cin − Cout versus Cin yields a straight line and the intercept of the x axon is the extracellular concentration of the chemical in the analyte (24, 25).
The goal of this research study is to develop a sensitive and fast method for determining HA in the microdialysate from HYP and to calculate the extracellular concentration of HA in the HYP with the zero-net flux method.
Experiment
Chemicals and solutions
In this research study, ultrapure water was prepared with a Millipore Elix system (Millipore, Bedford, MA, USA). HA, sodium chloride, potassium chloride, calcium chloride, magnesium chloride, sodium dihydrogen phosphate, dibasic sodium phosphate, OPA, sodium sulfite, borax, phosphorous acid (85%), sodium hydroxide, 1-octanesulfonic acid, and sodium salt (OSA) were purchased from Sigma-Aldrich (Sigma-Aldrich Corp., St. Louis, MO, USA). Methanol (HPLC grade) was purchased from Tedia (Tedia Chemical, USA). Tryptophan (Trp), phenylalanine (Phe), lysine (Lys), leucine (Leu), isoleucine (Ile) and pentobarbital sodium were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
The stock solution of HA (0.01 M) was prepared in an artificial cerebrospinal fluid (aCSF) and stored at −20°C. The stock solution was stable in more than 1 month. Before the experiment, the solution was diluted with aCSF to the desired concentrations. The aCSF consisted of 145 mM NaCl, 2.70 mM KCl, 1.00 mM MgCl2, 1.20 mM CaCl2, 0.45 mM NaH2PO4 and 2.33 mM Na2HPO4, and pH was adjusted to 7.4 (26).
Microdialysis procedures
All animal experiments were carried out in accordance with protocols approved by the Animal Ethics Committee, Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences (SYXK(E)2009-0051, No. 00006626).
On the day of the experiment, male Sprague-Dawley rats (250–350 g, Slac Laboratory Animal Co. Ltd, Changsha, China) were anesthetized with 1% pentobarbital sodium (intraperitoneal injection, 0.7 mL/100 g) and laid on a stereotaxic frame (Stoelting, Wood Dale, IL, USA). Their skulls were exposed after their scalps were removed. The guide cannula (CMA/11; Harvard Apparatus, Holliston, MA, USA) was implanted into HYP (2.0 mm posterior to bregma, 0.5 mm lateral from the midline and 7.2 mm below the superior surface of the skull). The guide cannula was fixed with screws and dental cement. After the surgery, the rats were housed individually in plastic cages (60 × 50 × 45 cm) for a week to allow the animals to recover.
On the day of the microdialysis experiment, a probe (CMA/11; Harvard Apparatus) was inserted into the cannula. The molecular cutoff for the probe was 6,000 Da, and the length of the membrane was 2 mm and made of cuprophane. The rats were then placed into the microdialysis bowl. The aCSF was delivered at a rate of 1.5 μL min−1 using a 1 mL syringe (Hamilton 1000series; Harvard Apparatus) and a microinjection pump (KDS 101; KD Scientific, Holliston, MA, USA). Following a 2-h equilibration period, microdialysates were collected every 15 min (22.5 μL) and the samples were stored at −80°C for further analysis. All animals were sacrificed at the end of the experiment, and the placement of the guide cannula site was confirmed with histology methods.
Probe recovery of HA in vivo (zero-net flux method)
The zero-net flux method was utilized to determine the extracellular concentration of HA in HYP of rats. During the microdialysis procedure as previously described, the aCSF spiked with known concentrations of HA (Cin, 0, 5, 10, 15 and 20 nM) was perfused via a liquid switch (CMA; Harvard Apparatus). At each level, the HA flux across the probe's membrane was allowed to equilibrate for 15 min. Two dialysate samples were collected (each 15 min, 22.5 μL). The net HA flux (out-in) was measured at every perfusate concentration and the regression analysis was used to calculate the actual concentration of extracellular HA in the HYP.
Apparatus and chromatography
Chromatography was performed with a Dionex Ultimate 3000 HPLC system which consisted of a biocompatible isocratic pump (ISO-3100BM), an autosampler (WPS 3000 TBSL) and an ECD (Coulochem III). The column was Agilent Eclipse Plus C18 (3.0 × 150 mm, particle size 3.5 μm) and the injection volume was 10 μL. The mobile phase consisted of 100 mM monosodium phosphate (pH 6.0), 500 mg L−1 OSA and 20% methanol (v/v), the flow rate was set to 0.35 mL min−1 and the temperature was set to 35°C for the column and 4°C for the autosampler. Potentials were set to 250/550 mV for ECD1/ECD2 of the analysis cell (Model 6011 coulometric, dual electrode cell).
Derivatization procedure
The derivatization reagent was prepared with the following procedure. First, the stock solution was prepared by disolving 17 mg of OPA into 0.5 mL methanol and then by adding 0.5 mL 1.25 M sodium sulfite and 4 mL 0.1 M of borax (pH 10.4). The stock solution (final concentration: OPA, 25 mM; sulfite, 125 mM) was kept at 4°C. The stock solution was prepared every 3 days. The working solution was prepared by adding 1 mL stock solution to 4 mL 0.1 M of borax (pH 10.4). During the analysis, 20 μL HA standard solutions or samples were mixed with 2 μL working solution.
Method validation
Linear equation was calibrated with standard solutions prepared at six different concentration levels (2.0, 5.0, 10.0, 15.0, 20.0 and 50.0 nM) in aCSF. To investigate the matrix effect, the linear equation was also calibrated in solutions prepared in the microdialysate. Calibration curves were constructed by plotting the peak areas of HA-OPA-sulfite (y, nA min) against the concentration (x, nM).
The standard solutions with HA (5.0 nM and 10.0 nM) were added to the microdialysates from the HYP for calculation of precision and recovery. The intra- and interday variability for the HA was assayed in triplicate on the same day as well as on three consecutive days, respectively.
Results
Optimization of derivatization conditions
The volume ratio between the microdialysate and the derivatization reagent was set to 10:1 during the derivatization procedure. The derivatization conditions for HA with OPA-sulfite were similar to that of the amino acids. The main factors affecting the derivatization yields were the pH value of the buffer, reaction time, temperature and concentration of OPA and sodium sulfite. The pH value was optimized to 10.4 according to the previously reports (27, 28). To obtain the best derivatization condition, various concentrations of OPA and sodium sulfite (0.05–25 mM, OPA: sulfite = 1:1, c/c) were tested with microdialysate (see Figure 1A). From Figure 1, one is able to see that the peak areas were stable at the concentration of OPA when ranging from 1 to 25 mM. Thus, 1 mM OPA was deemed sufficient for HA in the microdialysate to react completely. Considering the differences among the various samples, the final concentration of OPA was set to 5 mM.
Figure 1.
Optimization of derivatization conditions. (A) Effect of OPA concentrations on the responses of OPA-derivative of HA. Different concentrations of OPA and sodium sulfite (OPA: sulfite = 1:1, c/c) were tested with microdialysate. (B) Effect of OPA-reaction time under in vitro conditions on response of the OPA-derivatives of 10 nM HA. Derivation agents: 5 mM OPA and sulfite in 0.1 M borax, pH 10.4. (C) Stability of the derivative with different ratios between OPA and sulfite, concentration of OPA fixed at 5 mM. Peak areas were normalized at the reaction time of 1.5 min. During all of the analysis, the HA standard solutions or samples were mixed with the derivatization reagents at a ratio of 10:1 (20:2 µL). The derivatization reagents were in the buffer of 0.1 M borax (pH 10.4).
With the optimized concentration of the derivation agents (5 mM OPA and sulfite), the variation of derivatization time (1, 3, 5, 25 and 45 min) was further tested with the microdialysate (see Figure 1B). The results indicated that the reaction was completed in 1.5 min, and the peak area was stable between the reaction time 1.5 and 10 min. Thus, the reaction time was set to 1.5 min.
The reaction ratio of OPA, sodium sulfite, and HA was 1:1:1 (22), but HA can also react with OPA without thiol or sulfite as described previously (15, 16). To prevent the latter reaction and to get the major product of HA-OPA-sulfite, different ratios between sodium sulfite and OPA were investigated. The concentration of OPA was fixed at 5 mM, and concentrations of sodium sulfite were gradually changed to 1, 5, 25 and 50 mM. The result indicated that peak areas of HA in the microdialysate did not have significant differences between these groups under different derivatization conditions (data were not shown), which was also in accordance with previous reports (22). This was most likely because even when reacted with the least sodium sulfite, its level was much in excess of HA and other compounds with the amine group, such as amino acids.
Stability of the four groups was also investigated. Peak areas of all groups were gradually decreased in 12 h in 4°C (see Figure 1C). The more sodium sulfite in the derivatization reagent, the more stable the derivatization product produced. Thus, the ratio of OPA and sulfite was set at 1:5 and the product was stable in 7 h (<2% was degradation).
Optimization of separation conditions
The retention time of HA–OPA-sulfite was similar to some hydrophilic amino acids' derivative products in the reverse chromatography. Additionally, some hydrophobic amino acids would increase the analysis time. An ion pair reagent could improve the separation, for example, OSA could increase the retention time of the amino compounds that contain positive charge and reduce the retention time of the compounds that contain negative charge or no charge. There are several key parameters affecting the retention time in ion pair chromatography including pH value, concentration of the ion pair reagent and the organic reagent (29). In this research study, different pH values (4.5–6.0) were tested for separation with 100 mM phosphate and 400 mg L−1 OSA in 15% methanol (see Figure 2A). To protect the column, the maximum pH value was set to 6.0 because the silica-based packing has some solubility in the pH > 6 aqueous mobile phase. To avoid the interference of the other metabolites, some hydrophobic amino acids with large capacity factors were simultaneously investigated with HA including Trp, Phe, Lys, Leu and Ile. The capacity factors of the amino acids were reduced as pH values increased, while the capacity factor of HA was slightly affected. This was most likely due to the absence of a carboxy group. To separate HA from the other amino acids and shorten the separation time, the pH value 6.0 was selected.
Figure 2.
Optimization of separation conditions. (A) Effect of pH on the capacity factor of the HA and some hydrophobic amino acids. Mobile phase: 100 mM phosphate and 400 mg L−1 OSA in 15% methanol. (B) Effect of OSA concentration on the capacity factor of the HA. Different concentrations of OSA were investigated with 100 mM phosphate (pH 6.0) in 20% of methanol.
Different concentrations of ion pair reagent OSA were investigated with 100 mM phosphate (pH 6.0) in 20% of methanol. The capacity factor of HA was 4.2, and the values were larger than 10.0 for the amino acids investigated (Trp, Phe, Lys, Ile and Leu) when no OSA was added. The results with OSA range from 300 to 600 mg L−1 and are shown in Figure 2B. During the concentration of OSA increased from 0 to 600 mg L−1, the capacity factor of HA was approximately stable, but the capacity factor of the amino acids was decreased. While OSA was added in the mobile phase, the sulfo group in OSA interacts with the solid phase C18, then the retention times of the amino acids were reduced. However, the imine group in the HA–OPA-sulfite contains positive charge, thus, the retention time of HA was stable while the OSA was added. In the end, 500 mg L−1 OSA was selected, as this concentration was enough to separate HA from the other amino acids.
The type of buffer is also important in the mobile phase. Two different buffer solutions (phosphate and citric acid) were investigated in this research study (pH 6.0). HA (100 mM) was completely separated from the other compounds in phosphate buffer; however, it was not well separated in citrate buffer. Although citrate has more buffer capacity than phosphate at the pH value 6.0, phosphate was chosen in the end.
The retention times of all the compounds decreased following the increase of methanol in the mobile phase. Then, 20% methanol was selected because HA can be well separated and the separation time was also acceptable.
Optimization of detection conditions
To optimize the detecting potential, the ECD-1 was set to 0 mV and ECD-2 was optimized from 0 to 750 mV with a step of 50 mV (see Figure 3A). From Figure 3, it is evident that HA did not oxidize under 250 mV and the responses increased slightly when the potential was higher than 550 mV. Thus, the detection potential was set to 250 mV for ECD-1 and 550 mV for ECD-2. The ECD-1 was used to decrease the noise from the other chemicals, and ECD-2 was used for the analysis.
Figure 3.
Optimization of detection conditions. (A) Hydrodynamic voltammograms of OPA-sulfite derivatives. Mobile phase: 0.1 M phosphate, 500 mg L−1 OSA, pH 6.0, containing 20% (v/v) methanol. (B) Chromatograms (HA standard) from coulometric cell and amperometric cell, signals 1 and 2 were from the amperometric cell and coulometric cell, respectively.
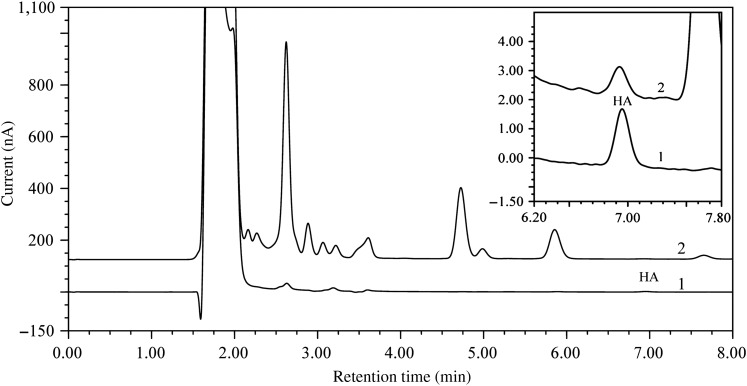
Common detect cells for ECD are coulometric and amperometric cells. In this study, both the coulometric (6011 cell) and amperometric cells (5041 cell) were investigated in this HPLC system for HA. Under the same column and sample, the signal of the coulometric cells was about ten times that of the amperometric cells, with a 0.002-inch gasket. The ratios between the signal and noise of these two cells were almost the same, which can be seen from the normalization of the HA peak (see Figure 3B). The coulometric cells were selected in the end, because it can easily reach equilibrium and need less maintenance. With the optimized parameters, HA was detected and completely separated with the other compounds. Typical chromatograms of the standard solution and the microdialysate are shown in Figure 4.
Figure 4.
Elution profiles of HA standard solution and the microdialysate after derivatization with OPA-sulfite. Chromatograph 1: standard amino acids solution (10 nM). Chromatograph 2: microdialysis sample from HYP.
Method validation
The linearity of the detector response to the standards was determined. During the analysis, a range of concentrations (2–50 nM) of HA was selected for the detection. The statistical results are presented in Table I. Linear correlation coefficients (R2) were found to be greater than 0.999 in both the aCSF and the microdialysate. The membrane of the microdialysis probe can block the large molecules, while the small molecules (e.g., inorganic salt, amine acids and neurotransmitters) could pass through the membrane due to the concentration gradient. Additionally, concentration of the inorganic salt in the microdialysate was much higher than other metabolites. Thus, the matrix effect was limited because of the similar environment of the aCSF and the microdialysate.
Table I.
The Statistical Parameters of the Assay
| Linear regression, correlation coefficient, LOD and LOQ (n = 3) | Linear equation | y = 0.0258x + 0.0226a |
| y = 0.0263x + 0.0753b | ||
| R2 | 0.9991a/0.9995b | |
| Linear range (nM) | 2–50 | |
| LOD (nM) | 0.4 | |
| LOQ (nM) | 1.3 | |
| Intra- and interday precisionc | Intraday (RSD, 5 nM/10 nM) | 3.92/2.29% |
| Interday (RSD, 5 nM/10 nM) | 4.56/6.04% | |
| Recovery of histamined | Basal (nM) | 2.08 ± 0.06 |
| Spiked (nM) | 5 | |
| Measured (nM) | 7.04 ± 0.37 | |
| Recovery (%) | 99.1 ± 8.1 | |
| Basal (nM) | 2.92 ± 0.18 | |
| Spiked (nM) | 10 | |
| Measured (nM) | 12.65 ± 0.47 | |
| Recovery (%) | 97.4 ± 5.4 |
ay, peak area (nA·min); x, concentration (nM), solutions were prepared in aCSF.
bSolutions were prepared in microdialysate.
cAssayed in sextuplicate on the same day and on three consecutive days.
dMean ± SD of six measurements.
The LOD at a signal-to-noise of 3:l was 0.4 nM, and the limit of quantification (LOQ) at a signal-to-noise of 10:l was 1.3 nM. The intraday and interday precision of the assay were between 2.29% and 6.04% (relative standard deviation, RSD), respectively. The recoveries of HA were between 97.4 and 98.9% (microdialysis samples spiked with 10 and 5 nM HA).
Determination of the basal level of HA with probe recovery calibrated in vivo
The recovery was interference by several parameters including diameter, length, geometry and material of the membrane, the perfusion flow rate, the perfusate characteristics and the sample matrix characteristics, etc. (23). Recovery in vitro cannot be used for in vivo calibration with accuracy because of the effect of the brain environment (24), which was impossible to imitate accurately. Probe recovery calibrated in vivo could avoid the limitations, and thus, the zero-net flux method was one method which has been widely used. Figure 5 presents this method of four rats. The slope of the line was the recovery of the probe calculated in vivo, which was higher than calculated in vitro. The average recovery of the probe determined in vivo is 49.2 ± 11.4% (n = 4). The average concentration of extracellular HA of rat HYP was 8.2 ± 1.3 nM (n = 4).
Figure 5.
Zero-net flux of four rats. HYP HA levels corrected for in vivo probe recovery are represented by the x-intercepts of the plotted linear regression lines.
Discussion
Fast scan cyclic voltammetry can be used for the determination of HA (30); however, it was difficult to be oxygenated on the glassy carbon electrode or the metal electrode in DC mode. Thus, pre-column derivatization was used to detect HA by ECD, e.g., OPA-sulfite derivatization was used for determination of HA in the brain homogenate (22). The procedure needs to be optimized for the detection of HA in the microdialysate because concentrations of HA and amino acids are different from the brain homogenate. The concentration of HA in the microdialysate is very low, and to avoid the dilution of the sample, the volume ratio between the microdialysate and the derivatization reagent was set to 10:1 during the derivatization procedure. The main factors affecting the derivatization yields including the pH value of the buffer, reaction time and concentration of OPA and sodium sulfite were optimized in this study.
Because of the amino acids can also react with OPA, an ion pair reagent was utilized to separate HA from the amino acids, whose concentrations were much higher than HA. Thus, the much lower concentration of HA in the microdialysate requires high resolution to avoid the interference of amino acids. Key parameters affecting the retention time including pH value, concentration of the ion pair reagent and the organic reagent were optimized.
Common detect cells for ECD are coulometric and amperometric cells. The compounds can completely react in the coulometric cells, and the signal is also enhanced. In this study, the coulometric and amperometric cell provide the same sensitivity. However, in the coulometric cell, the signal stability is considerably greater and the detector response is much more stable owing to a large electrode surface; only a small fraction is needed for the completion of the redox reaction, in which case partial electrode surface deactivation does not affect the overall response significantly (31). It is easy to operate, without routine maintenance which was important for the amperometric cell. Thus, the coulometric cell was chosen.
This established approach was successfully applied to determinate HA in the HYP precisely with probe recovery calibrated in vivo. It can also be used for the routine determination of HA in vivo of the other animals or in different brain states.
Conclusion
Determination of HA in the brain microdialysate is still a challenging task, because of the low concentration, complex matrix, and small sample volume. In the current research, a simple and rapid HPLC method with an electrochemical determination approach was developed for the determination of HA in the microdialysates. Under the optimized parameters, HA reacted with OPA-sulfite completely in 1.5 min. The derivatization product of HA was well separated, and the total separation time was <10 min. The LOD was below 0.4 nM. The proposed method was successfully applied to in vivo study of HA in HYP. The concentration of extracellular HA in the rat HYP was 8.2 ± 1.3 nM. This fast and sensitive approach can be used for the routine determination of HA in vivo of other animals or in different brain states of the rat/mice.
Acknowledgments
The authors express their gratitude to Mrs Erin Beatson for proof reading and specially thank the anonymous reviewers and the editor for their professional and intensive comments. The work was supported by National Natural Science Foundation of China [91132307/H09 and 31171061/C090208], the Ministry of Science and Technology of China [2012BAI23B02] to F.X. and China Postdoctoral Science Foundation Funded Project (2013M542094).
References
- 1.Haas H., Panula P.; The role of histamine and the tuberomamillary nucleus in the nervous system; Nature Reviews Neuroscience, (2003); 4(2): 121–130. [DOI] [PubMed] [Google Scholar]
- 2.Brown R.E., Stevens D.R., Haas H.L.; The physiology of brain histamine; Progress in Neurobiology, (2001); 63(6): 637–672. [DOI] [PubMed] [Google Scholar]
- 3.Drew K.L., Pehek E.A., Rasley B.T., Ma Y.L., Green T.K.; Sampling glutamate and GABA with microdialysis: suggestions on how to get the dialysis membrane closer to the synapse; Journal of Neuroscience Methods, (2004); 140(1–2): 127–131. [DOI] [PubMed] [Google Scholar]
- 4.Yao T., Okano G.; Simultaneous determination of l-glutamate, acetylcholine and dopamine in rat brain by a flow-injection biosensor system with microdialysis sampling; Analytical Sciences, (2008); 24(11): 1469–1473. [DOI] [PubMed] [Google Scholar]
- 5.Cheng G.W., Hsu K.C., Lee C.F., Wu H.L., Huang Y.L.; On-line microdialysis coupled with liquid chromatography for biomedical analysis; Journal of Chromatographic Science, (2009); 47(8): 624–630. [DOI] [PubMed] [Google Scholar]
- 6.Vitali L., Valese A.C., Azevedo M.S., Gonzaga L.V., Costa A.C.O., Piovezan M. et al. ; Development of a fast and selective separation method to determine histamine in tuna fish samples using capillary zone electrophoresis; Talanta, (2013); 106: 181–185. [DOI] [PubMed] [Google Scholar]
- 7.Niculescu M., Frebort I., Pec P., Galuszka P., Mattiasson B., Csoregi E.; Amine oxidase based amperometric biosensors for histamine detection; Electroanalysis, (2000); 12(5): 369–375. [Google Scholar]
- 8.Fernandes J.O., Judas I.C., Oliveira M.B., Ferreira I.M.P.L.V.O., Ferreira M.A.; A GC–MS method for quantitation of histamine and other biogenic amines in beer; Chromatographia, (2001); 53: S327–S331. [Google Scholar]
- 9.Hogan A.M., Crean C., Barrett U.M., Guihen E., Glennon J.D.; Histamine determination in human urine using sub-2 mu m C18 column with fluorescence and mass spectrometric detection; Journal of Separation Science, (2012); 35(9): 1087–1093. [DOI] [PubMed] [Google Scholar]
- 10.Bourgogne E., Mathy F.X., Boucaut D., Boekens H., Laprevote O.; Simultaneous quantitation of histamine and its major metabolite 1-methylhistamine in brain dialysates by using precolumn derivatization prior to HILIC-MS/MS analysis; Analytical and Bioanalytical Chemistry, (2012); 402(1): 449–459. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida T., Hamada H., Murakawa H., Yoshimoto H., Tobino T., Toda K.; Determination of histamine in seafood by hydrophilic interaction chromatography/tandem mass spectrometry; Analytical sciences, (2012); 28(2): 179–182. [DOI] [PubMed] [Google Scholar]
- 12.Yang X.R., Yu B.S., Nie L.H., Yao S.Z.; Ion chromatographic determination of histamine in fish with series bulk acoustic wave detection; Journal of Chromatographic Science, (1998); 36(1): 29–32. [Google Scholar]
- 13.Molnar-Perl I.; Quantitation of amino acids and amines in the same matrix by high-performance liquid chromatography, either simultaneously or separately; Journal of Chromatography A, (2003); 987(1–2): 291–309. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z.P., Wu J.L., Wu S.H., Bao A.M.; High-performance liquid chromatographic determination of histamine in biological samples: the cerebrospinal fluid challenge—a review; Analytica Chimica Acta, (2013); 774: 1–10. [DOI] [PubMed] [Google Scholar]
- 15.Yamatodani A., Fukuda H., Wada H., Iwaeda T., Watanabe T.; High-performance liquid-chromatographic determination of plasma and brain histamine without previous purification of biological samples—cation-exchange chromatography coupled with post-column derivatization fluorometry; Journal of Chromatography, (1985); 344(Nov): 115–123. [DOI] [PubMed] [Google Scholar]
- 16.Yoshitake S., Ijiri S., Kehr J., Yoshitake T.; Inhibition of histamine release by local and intracerebroventricular infusion of galanin in hypothalamus, hippocampus and prefrontal cortex of awake rat: a microdialysis study; Neuroscience Letters, (2013); 534: 58–63. [DOI] [PubMed] [Google Scholar]
- 17.Yoshitake T., Yamaguchi M., Nohta H., Ichinose F., Yoshida H., Yoshitake S. et al. ; Determination of histamine in microdialysis samples from rat brain by microbore column liquid chromatography following intramolecular excimer-forming derivatization with pyrene-labeling reagent; Journal of Neuroscience Methods, (2003); 127(1): 11–17. [DOI] [PubMed] [Google Scholar]
- 18.Yoshitake T., Ijiri S., Yoshitake S., Todoroki K., Yoshida H., Kehr J. et al. ; Determination of histamine in microdialysis samples from guinea pig skin by high-performance liquid chromatography with fluorescence detection; Skin Pharmacology and Physiology, (2012); 25(2): 65–72. [DOI] [PubMed] [Google Scholar]
- 19.Ferry B., Gifu E.P., Sandu I., Denoroy L., Parrot S.; Analysis of microdialysate monoamines, including noradrenaline, dopamine and serotonin, using capillary ultra-high performance liquid chromatography and electrochemical detection; Journal of chromatography B, (2014); 951: 52–57. [DOI] [PubMed] [Google Scholar]
- 20.Bi S.Y., Wang W., Lian K.Q., Xu X.D., Niu L.M., Shi H.M. et al. ; Determination of monoamine neurotransmitters in brains of paraquat-induced mouse by HPLC with electrochemical detection; Asian Journal of Chemistry, (2013); 25(15): 8593–8596. [Google Scholar]
- 21.Jensen T.B., Marley P.D.; Development of an assay for histamine using automated high-performance liquid-chromatography with electrochemical detection; Journal of Chromatography B, (1995); 670(2): 199–207. [DOI] [PubMed] [Google Scholar]
- 22.Maldonado M., Maeyama K.; Simultaneous electrochemical measurement method of histamine and N-tau-methylhistamine by high-performance liquid chromatography-amperometry with o-phthalaldehyde-sodium sulfite derivatization; Analytical Biochemistry, (2013); 432(1): 1–7. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y.P., Liang X.Z., Lunte C.E.; Comparison of recovery and delivery in-vitro for calibration of microdialysis probes; Analytica Chimica Acta, (1995); 316(3): 403–410. [Google Scholar]
- 24.Watson C.J., Venton B.J., Kennedy R.T.; In vivo measurements of neurotransmitters by microdialysis sampling; Analytical Chemistry, (2006); 78(5): 1391–1399. [DOI] [PubMed] [Google Scholar]
- 25.Krebs-Kraft D.L., Rauw G., Baker G.B., Parent M.B.; Zero net flux estimates of septal extracellular glucose levels and the effects of glucose on septal extracellular GABA levels; European Journal of Pharmacology, (2009); 611(1–3): 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sauvinet V., Parrot S., Benturquia N., Bravo-Moraton E., Renaud B., Denoroy L.; In vivo simultaneous monitoring of gamma-aminobutyric acid, glutamate, and l-aspartate using brain microdialysis and capillary electrophoresis with laser-induced fluorescence detection: analytical developments and in vitro/in vivo validations; Electrophoresis, (2003); 24(18): 3187–3196. [DOI] [PubMed] [Google Scholar]
- 27.Rowley H.L., Martin K.F., Marsden C.A.; Determination of in vivo amino acid neurotransmitters by high-performance liquid chromatography with o-phthalaldehyde-sulphite derivatisation; Journal of Neuroscience Methods, (1995); 57(1): 93–99. [DOI] [PubMed] [Google Scholar]
- 28.Monge-Acuna A.A., Fornaguera-Trias J.; A high performance liquid chromatography method with electrochemical detection of gamma-aminobutyric acid, glutamate and glutamine in rat brain homogenates; Journal of Neuroscience Methods, (2009); 183(2): 176–181. [DOI] [PubMed] [Google Scholar]
- 29.Cecchi T.; Ion pairing chromatography; Critical Reviews in Analytical Chemistry, (2008); 38(3): 161–213. [DOI] [PubMed] [Google Scholar]
- 30.Hashemi P., Dankoski E.C., Wood K.M., Ambrose R.E., Wightman R.M.; In vivo electrochemical evidence for simultaneous 5-HT and histamine release in the rat substantia nigra pars reticulata following medial forebrain bundle stimulation; Journal of Neurochemistry, (2011); 118(5): 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jirovsky D., Kosina P., Myslinova M., Styskala J., Ulrichova J., Simanek V.; HPLC analysis of rosmarinic acid in feed enriched with aerial parts of Prunella vulgaris and its metabolites in pig plasma using dual-channel coulometric detection; Journal of Agricultural and Food Chemistry, (2007); 55(19): 7631–7637. [DOI] [PubMed] [Google Scholar]