Abstract
A rapid and sensitive ultra-high-performance liquid chromatography–quadrupole-time-of-flight mass spectrometric (UHPLC–Q-TOF-MS) method was developed for quantification of imipramine, one of the most widely used tricyclic antidepressants, and desipramine, an active metabolite of imipramine, in mouse serum. The developed method included a simple protein precipitation with acetonitrile in 50 μL of serum and analyte separation on an Acquity UPLC BEH C18 column using a gradient elution of acetonitrile with 0.1% formic acid and 20 mM ammonium formate. As a result, the entire analysis time was <20 min including the sample preparation and the LC–MS analysis. The limit of quantification was 5.0 ng mL−1 for both imipramine and desipramine, and calibration curves were linear over the concentration range of 5.0–1,000.0 and 5.0–250.0 ng mL−1 for imipramine and desipramine, respectively. Intraday precisions at three levels were 2.2–3.6 and 1.7–4.2% for imipramine and desipramine, respectively, whereas interday precisions were 2.6–5.0 and 2.0–8.4% for imipramine and desipramine, respectively. Accuracy ranged between 93.6 and 106.6% for imipramine and 94.1 and 106.4% for desipramine. Absolute recovery was 96.0–97.6% for imipramine and 87.0–99.5% for desipramine. Finally, the described method was applied to mice administered with imipramine, demonstrating the suitability for quantification of imipramine and desipramine for therapeutic drug monitoring or bioequivalence studies.
Introduction
Tricyclic antidepressants (TCAs) have been widely used for the treatment of adults and children with major depressive disorders as well as anxiety, eating disorders, neuropathic pain, enuresis and attention deficit hyperactivity disorder (1–4). Therapeutic drug monitoring (TDM), which is required to maintain plasma, serum or blood drug concentrations within a targeted therapeutic range (5), is very important for individualization of drug dosage. Because of the relatively narrow therapeutic/toxic index of TCAs, patients are usually monitored to avoid unwanted side-effects, nonresponsiveness and noncompliance (6–8). TCAs are rapidly absorbed from the gastrointestinal tract and undergo first-pass metabolism (9). Since they are highly protein-bound and have a large volume of distribution, they exhibit a long half-life of elimination (3).
Imipramine remains one of the most popularly used TCA drugs (10). Imipramine is metabolized in the liver primarily to desipramine, which also has antidepressant activity (11) by inhibiting the reuptake of norepinephrine and serotonin (12). TDM of imipramine and desipramine is essential due to wide interindividual variability in pharmacokinetics, production of active metabolites and a high risk of drug–drug interactions (13).
A series of methods have been investigated for quantification of TCAs, including imipramine and desipramine, in various biological samples including serum, plasma, urine and oral fluids (Supplementary Table SI) (2, 11, 14–20). Although liquid chromatography coupled with ultraviolet detection (LC–UV) methods can offer an economic advantage, the majority of bioanalytical methods are based on LC–tandem mass spectrometry (LC–MS-MS), because the mass spectrometric detection enables more specific, sensitive, rapid and flexible quantification of analytes of interest in complex matrices compared with the UV detection. LC coupled with MS detection (LC–MS)-based bioanalytical methods involve sample clean-up and/or extraction procedures that are performed primarily using protein precipitation, liquid–liquid extraction (LLE) or solid-phase extraction. In particular, a triple quadrupole (QQQ)–MS operated in selective reaction monitoring mode has been employed as the gold standard for quantitative bioanalytical assays (21, 22). However, recent years have witnessed a significant shift in the bioanalysis field toward employing high-resolution MS systems, such as time-of-flight (TOF) detectors, especially quadrupole-TOF (Q-TOF) systems (23, 24). Q-TOF provides high mass accuracy for both precursor and productions as well as speed and resolving power with full scan sensitivity. In the meanwhile, ultra-high-performance liquid chromatography (UHPLC) allows for increased resolution and sensitivity as well as excellent reproducibility compared with HPLC. As a result, the hyphenation between UHPLC and Q-TOF-MS has been introduced as an excellent analytical platform for qualitative and quantitative tasks (25–27). Indeed, UHPLC–Q-TOF-MS is now considered as a good alternative to HPLC–MS-MS for quantitative assays (28). Although UHPLC–Q-TOF-MS is prevalently used for targeted and nontargeted analysis of a variety of compounds in foods (27, 29), plants (30, 31), biological fluids (32, 33) and tissues (31, 34), there are still limited applications of UHPLC–Q-TOF-MS for bioanalysis of drug molecules (35, 36). The aim of the present study was to develop a novel, reliable and efficient UHPLC–Q-TOF-MS method for quantification of imipramine and desipramine in biological fluids while making the method as simple as possible so that the method could be readily applied.
Experimental
Chemicals
Imipramine, desipramine, amitriptyline, ammonium formate, hexane, ethyl acetate (EtOAc) and tert-butyl methyl ether (TBME) were of analytical grade and purchased from Sigma-Aldrich (St Louis, MO, USA). LC-grade formic acid (FA) was obtained from Sigma-Aldrich and LC-grade acetonitrile (ACN) and water were from J.T. Baker (Center Valley, PA, USA). Doubly distilled water was obtained using a Milli-Q water purification system from Millipore (Bedford, MA, USA).
Animals
Eight-week-old male ICR mice were obtained from Koatech Co., Ltd (Seoul, Korea). All animal care procedures were conducted in accordance with the US National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of School of Pharmacy, Sungkyunkwan University (Approval No. SKKUP-2014-06).
Standard solutions
Separate stock solutions (1.0 mg mL−1) of imipramine, desipramine and amitriptyline (internal standard, IS) were prepared by dissolving accurately weighed amounts of each reference compound in methanol. All stock solutions were stored at −20°C and were stable for at least 3 months. Working solutions were prepared by serial dilution of the stock solutions in methanol.
Sample preparation
Simple protein precipitation in ACN was used for sample preparation. Briefly, 50 µL of working solution or methanol was added to 50 µL of mouse serum. Next, 50 µL of IS (100 ng mL−1) was added to the mixture. A total of 850 µL of ACN as an extraction solvent was added to the mixture and vortexed for 3 min. After centrifugation at 12,300 g for 5 min using a centrifuge from Gyrozen (Incheon, Korea), the supernatant was removed and dried under a stream of pure nitrogen. The extract was reconstituted in 100 µL of an ACN–H2O mixture (1 : 1) and passed through a 0.2-µm filter (Whatman, Piscataway, NJ, USA) prior to injection.
Instruments and conditions
Chromatography was performed on an Acquity UPLC system (Waters Co., Milford, MA, USA) equipped with a binary solvent delivery system, a cooling autosampler and a thermostatically controlled column compartment. Separation was achieved on an Acquity UPLC BEH C18 column (50 × 2.1 mm, 1.7 µm) from Waters (Milford, MA, USA) maintained at 45°C. The mobile phase consisted of 0.1% FA in 20 mM ammonium formate buffer (A) and 0.1% FA in ACN (B). The gradient program was as follows: 0–5 min, 20–60% B; 5–6 min, 60–90% B, 6–7 min, 90% B, followed by allowing the system to equilibrate for an additional 3 min at the initial conditions. The flow rate was set at 0.3 mL min−1. The autosampler was maintained at 4°C and the injection volume was 5 µL using a partial loop mode.
Mass spectrometry was conducted with a Waters Acquity Xevo G2 Q-TOF tandem mass spectrometer (Waters Corp., Manchester, UK) equipped with an electrospray ionization interface in positive ion mode. The optimized parameters were set as follows: capillary voltage, 3.0 kV; sample cone, 30 V; extraction cone, 4.0 V; source temperature, 120°C; desolvation temperature, 300°C; desolvation gas (nitrogen), 600 L h−1 and cone gas, 0 L h−1. The instrument was controlled by MassLynx software (version 4.1, Waters Co., Milford, MA, USA) and calibrated by direct infusion of a sodium formate solution (5 mM). Data were acquired from m/z 100 to 1,500 Da and corrected during acquisition using an external reference (lock spray) composed of a solution of 2 μg mL−1 leucine enkephalin (m/z 556.2771) infused at a flow rate of 20 μL min−1 to guarantee accuracy and reproducibility of data acquisition. An MSE scan function was applied for simultaneous detection of precursor ions and fragment ions at high and low collision energies in a single injection run, i.e. the collision energy was switched between a low and high level in alternate scans. The high collision energy ramp ranged from 20 to 45 V. All the data for the validation study and the real sample analysis were obtained using the MSE scan function. Raw data were acquired and processed using the MassLynx software. TargetLynx software (version 4.1, Waters Co., Milford, MA, USA) calculates the peak area from each run. It was used for post-acquisition data processing. Extracted ion chromatograms (EICs) of narrow mass range (<0.05 Da) were employed for the quantification.
Method validation
Method validation was carried out as follows according to the United States Food and Drug Administration's bioanalytical method validation procedures.
Linearity and sensitivity
Calibration curves were established by plotting the peak area ratio of analyte to IS against the analyte concentration using mouse serum spiked at various concentrations. The spiked concentrations for imipramine were 5.0, 12.5, 25.0, 50.0, 125.0, 250.0, 500.0 and 1,000 ng mL−1 and 5.0, 12.5, 25.0, 50.0, 125.0 and 250.0 ng mL−1 for desipramine. The resulting ratios were fitted to a linear regression model using 1/χ as a weighting factor.
The limit of quantification (LOQ) was measured as the lowest concentration at which imipramine or desipramine could be quantified with acceptable accuracy (nominal ± 20%) and precision (<20% CV). The LOQ was included as the lowest concentration in the calibration curves.
Precision and accuracy
Precision and accuracy were determined using mouse sera spiked at three concentrations (low, intermediate and high levels). Precision was measured as the % RSD of peak areas from replicate analyses, and accuracy was determined as the ratio of back-calculated standard concentration to the nominal value. Intraday assay precision and accuracy were assessed from five replicates at each concentration level on the same day. Interday precision and accuracy were evaluated by analyzing three replicates at each concentration level on 3 separate days.
Recovery
Absolute recovery was evaluated at three concentration levels using the following equation: absolute recovery (% ) = (peak area of analyte in spiked serum extract/peak area of standard in neat solution) × 100.
Stability
Stability of the analytes in spiked serum extracts was monitored at three concentrations (5.0, 50.0 and 500.0 ng mL−1 for imipramine and 5.0, 50.0 and 250.0 ng mL−1 for desipramine) at different time intervals. Fifty microliters of spiked serum extract at each concentration was prepared (n = 9), and three serum samples for each concentration were analyzed immediately (t = 0 h), whereas the remaining samples were kept in an autosampler at 4°C and analyzed after 24 h (n = 3) and 48 h (n = 3). The relative difference of the back-calculated concentration after 24 or 48 h to t = 0 h was used as an estimate of stability.
Results
Optimization of sample preparation method
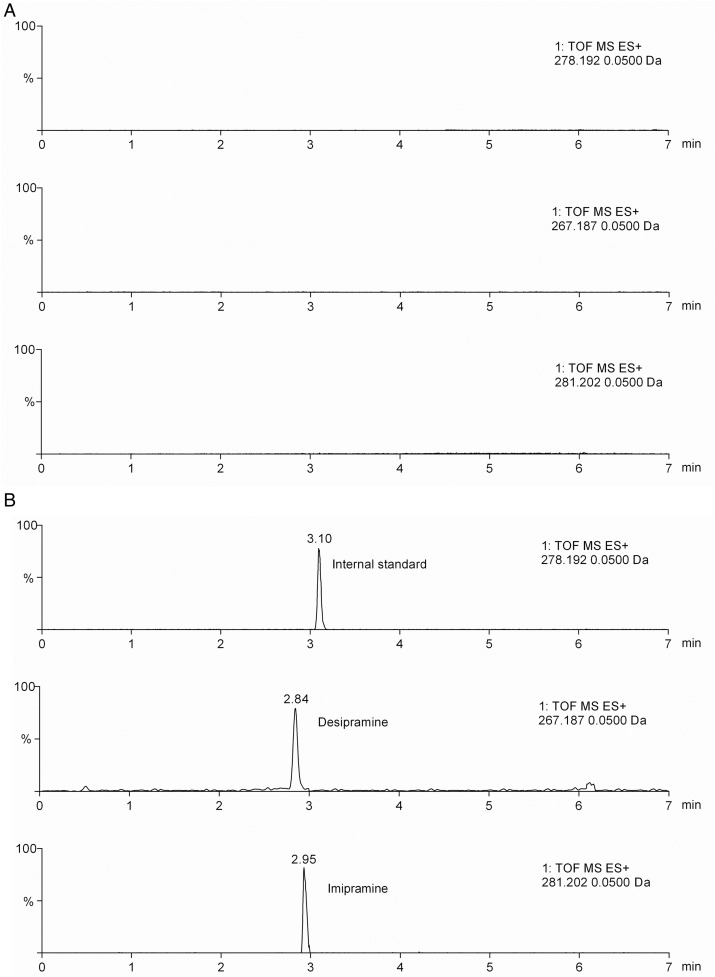
Based on literature and for simplicity, ACN and methanol were tested for protein precipitation (37–42), whereas hexane, EtOAc and TBME were compared for LLE (43–47). Effectiveness was evaluated based on the peak areas from triplicate injections of samples prepared from spiked mouse sera containing the analytes at a concentration of 250 ng mL−1. Our results showed that protein precipitation using ACN was the most effective among the different methods tested (Supplementary Figure S1). In addition to a higher response, ACN-based protein precipitation was much simpler and more rapid than LLE. Accordingly, protein precipitation with ACN was selected as the sample preparation method. After deproteination, the extract was dried and reconstituted prior to injection to the UHPLC system. It was found that a minimum of 100 µL of 50% ACN was needed to completely reconstitute the dried residues, which produced a volume of reconstituted extract enough for filtration. Conditions for UHPLC and Q-TOF-MS analysis were carefully optimized as described in the Experimental section. MS data for imipramine and desipramine are displayed in Table I. The total chromatography time was 10 min; imipramine and desipramine were eluted within 3 min, after which the column was washed and re-equilibrated to the initial condition. Representative chromatograms of spiked serum samples are shown in Figure 1. The resulting method required <20 min for the entire analysis including the sample preparation and the chromatographic analysis. This method was validated as described below.
Table I.
MS Data for Imipramine and Desipramine
| Analyte | Molecular formula | Exact mass ([M+H]+) | Measured mass | Mass error (ppm/mDa) | Mass fragment |
|---|---|---|---|---|---|
| Imipramine | C19H24N2 | 281.2018 | 281.2017 | −0.4/−0.1 | 236.1442, 208.1126, 193.0891 |
| Desipramine | C18H22N2 | 267.1861 | 267.1871 | 3.7/1.0 | 236.1404, 208.1133, 193.0903 |
Figure 1.
Chromatograms obtained from a control mouse. EICs were obtained by the analysis of blank serum (A) and serum spiked with imipramine and desipramine at the LOQ for each compound (B).
Validation of the established method
Linearity and sensitivity
Linearity of the calibration curves was verified using spiked mouse sera. The determined parameters for calibration curves are summarized in Table II. Correlation coefficients (r2) were above 0.999 for the range of 5.0–1,000.0 ng mL−1 for imipramine and 5.0–250.0 ng mL−1 for desipramine. The LOQ value as a measure of sensitivity was 5.0 ng mL−1 for both compounds. The signal-to-noise ratios at the LOQ were 12.0 and 10.3 for imipramine and desipramine, respectively.
Table II.
Calibration Parameters and LOQ of the Developed Method
| Analytes | Calibration curve | r2 | Linear range (ng mL−1) | LOQ (ng mL−1) |
|---|---|---|---|---|
| Imipramine | y = 0.01453x− 0.03326 | 0.9993 | 5.0–1000.0 | 5.0 |
| Desipramine | y = 0.01039x− 0.00408 | 0.9990 | 5.0–250.0 | 5.0 |
Accuracy and precision
The intraday accuracy and precision for imipramine were 96.0–97.9 and 2.8–4.6%, respectively (Table III). The interday accuracy varied between 97.0 and 106.6% and the precision ranged from 2.6 to 5.0%. In the case of desipramine, the intraday accuracy ranged between 87.9 and 102.0%, whereas the precision ranged between 3.1 and 5.8%. The interday accuracy and precision were 95.5–104.9 and 2.0–8.4%, respectively.
Table III.
Intra- and Interday Assay Accuracies and Precisions of the Developed Method
| Analyte | Concentration (ng mL−1) | Intraday assay (n = 5) |
Interday assay (n = 9) |
||
|---|---|---|---|---|---|
| Accuracy (%) | Precision (% RSD) | Accuracy | Precision (% RSD) | ||
| Imipramine | 5.0 | 104.4 | 3.6 | 106.6 | 3.9 |
| 50.0 | 94.4 | 2.2 | 98.8 | 2.6 | |
| 500.0 | 93.6 | 2.6 | 97.0 | 5.0 | |
| Desipramine | 5.0 | 106.4 | 4.2 | 95.5 | 8.4 |
| 50.0 | 94.1 | 1.7 | 100.4 | 2.0 | |
| 250.0 | 95.6 | 2.7 | 104.9 | 5.3 | |
Absolute recovery and stability
The absolute mean recoveries ranged from 96.0 to 97.6% for imipramine and from 87.0 to 99.5% for desipramine (Table IV). Results for the stability of spiked serum extract containing imipramine and desipramine are summarized in Table V. Imipramine and desipramine were relatively stable in the reconstituted extract at 4°C for at least 48 h. After 48 h, the concentration of imipramine decreased between 3.9 and 8.0% compared with the corresponding fresh sample concentrations. For desipramine, the decrease in concentration ranged from 3.6 to 8.3%.
Table IV.
Absolute Recovery of the Developed Method
| Analyte | Concentration (ng mL−1) | Recovery (%, n = 6) | % RSD |
|---|---|---|---|
| Imipramine | 5.0 | 97.2 | 2.1 |
| 50.0 | 96.0 | 4.0 | |
| 500.0 | 97.6 | 3.0 | |
| Desipramine | 5.0 | 87.1 | 12.1 |
| 50.0 | 92.3 | 7.7 | |
| 250.0 | 99.5 | 2.0 |
Table V.
Sample Stability for Imipramine and Desipramine
| Storage time | 0 h | 24 h |
48 h |
||
|---|---|---|---|---|---|
| Analyte | Concentration (ng mL−1) | Concentration (ng mL−1) | % Change | Concentration (ng mL−1) | % Change |
| Imipramine | 4.9 | 4.6 | −5.6 | 4.5 | −8.0 |
| 50.6 | 48.3 | −4.7 | 47.3 | −6.6 | |
| 498.9 | 489.6 | −1.9 | 479.5 | −3.9 | |
| Desipramine | 5.0 | 4.9 | −3.2 | 4.6 | −8.3 |
| 49.5 | 48.3 | −2.5 | 46.5 | −6.1 | |
| 248.1 | 241.0 | −2.9 | 239.1 | −3.6 | |
Application of method to analysis of real samples
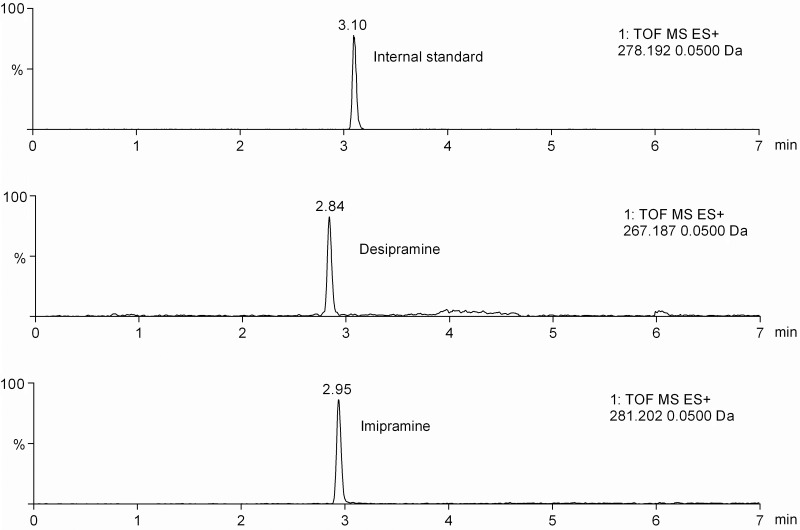
The developed method was applied to real serum samples from three different 8-week-old male ICR mice after imipramine administration at a dose of 10 mg kg−1 by intraperitoneal injection. Imipramine and its primary metabolite, desipramine, were clearly detected at 2.94 and 2.84 min, respectively, from the serum sample taken 6 h after drug administration (Figure 2). The determined serum concentrations were 51.4–64.0 and 10.5–17.8 ng mL−1 for imipramine and desipramine, respectively (Table VI). The therapeutic range of imipramine is 45–150 ng mL−1, and 75–250 ng mL−1 for desipramine (15).
Figure 2.
Chromatograms obtained from a mouse treated with imipramine. EICs were obtained by the analysis of real serum samples taken 6 h after drug administration.
Table VI.
Determined Concentrations of Imipramine and Desipramine in Mice 6 h After Imipramine Administration
| Imipramine (ng mL−1) | Desipramine (ng mL−1) | |
|---|---|---|
| Mouse 1 | 64.0 (±2.7) | 17.8 (±1.2) |
| Mouse 2 | 51.4 (±3.3) | 10.5 (±1.2) |
| Mouse 3 | 61.2 (±2.3) | 11.5 (±1.5) |
The values given in parenthesis denote standard deviation (n = 3).
Discussion
Sample preparation is required for sample clean-up and/or preconcentration in drug analysis in biological matrices. Particularly in LC–MS-based techniques, protein precipitation and LLE are probably the two most commonly employed methods, because they are relatively simple and require no special equipment. In the present study, protein precipitation using ACN was selected as the sample preparation method based on which it yielded a high response and it was much simpler and more rapid than LLE. The sample preparation procedures could be completed within 10 min. The mass spectrometric detection using Q-TOF-MS enabled highly selective and sensitive determination of imipramine and desipramine using a low volume of serum. In fact, the sample volume (50 µL) in this study was generally low compared with previous LC–MS methods (Supplementary Table SI). The optimized analytical conditions for sample preparation and chromatography were very simple and efficient. Imipramine and desipramine were eluted within 3 min. The entire analysis time was <20 min, requiring ∼9 min for sample preparation and 10 min for LC–MS analysis. Exact mass qualitative and quantitative experiments could be performed in one injection cycle using MSE scan function. The narrow mass range (<0.05 Da) helped improve selectivity for the target compounds.
Method validation parameters were evaluated for linearity, LOQ, precision, accuracy, absolute recovery and stability. On the basis of the LOQ value (5.0 ng mL−1), the current method exhibited comparable sensitivity to previous reports (Supplementary Table SI). The good intra- and interassay precisions and accuracies indicated that our method was reliable for analysis of the two compounds. The absolute recovery of our method is generally higher than that of previous reports (14, 17–19). The high absolute recovery values with low % RSD values throughout a wide range of concentrations indicated that the established sample preparation procedure was suitable for negligible matrix effects, reasonable repeatability and good extraction efficiency. Stability tests showed that the prepared samples were stable for 2 days at 4°C before LC–MS analysis, which suggests that a large number of samples can be analyzed in a batch without concerning degradation of the analytes. Using the developed method, real serum samples from several mice administered with imipramine were successfully analyzed.
In summary, accurate quantification was possible using a small volume of serum (50 µL), allowing for efficient use of limited blood samples. The developed method is highly likely to be applicable to human serum samples for imipramine/desipramine and even other antidepressants. These results indicated that the developed method can serve as a simple, rapid and reliable method, and therefore, it can be readily applicable for preclinical or clinical studies such as TDM or bioequivalence studies of imipramine and desipramine. In addition, the current method could be applied to the quantitative analysis of other popular antidepressants by using EICs at relevant m/z values because EICs of narrow mass ranges can possibly ensure selectivity for the new target compounds.
Conclusion
A UHPLC–Q-TOF-MS method for simultaneous quantification of imipramine and desipramine in serum was developed with high sensitivity and good recovery. The current study is the first report of a reliable, simple, rapid and efficient method using UHPLC–Q-TOF-MS for quantification of imipramine and desipramine. More broadly, these results suggest that UHPLC–Q-TOF-MS methods can be a valuable approach for the quantitation of pharmaceuticals and their metabolites for applications requiring routine bioanalysis such as TDM.
Supplementary Material
Funding
This study was supported by two grants (Nos. 2011-0024225 and 2011-0029199) from the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (MEST), Republic of Korea.
Supplementary Material
References
- 1.Baldessarini R.J., Kando J.C., Centorrino F.; Hospital use of antipsychotic agents in 1989 and 1993: stable dosing with decreased length of stay; American Journal of Psychiatry, (1995); 152(7): 1038–1044. [DOI] [PubMed] [Google Scholar]
- 2.Breaud A.R., Harlan R., Di Bussolo J.M., McMillin G.A., Clarke W.; A rapid and fully-automated method for the quantitation of tricyclic antidepressants in serum using turbulent-flow liquid chromatography-tandem mass spectrometry; Clinica Chimica Acta, (2010); 411(11–12): 825–832. [DOI] [PubMed] [Google Scholar]
- 3.Rudorfer M.V., Potter W.Z.; Metabolism of tricyclic antidepressants; Cellular and Molecular Neurobiology, (1999); 19(3): 373–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dargan P.I., Colbridge M.G., Jones A.L.; The management of tricyclic antidepressant poisoning: the role of gut decontamination, extracorporeal procedures and fab antibody fragments; Toxicological Reviews, (2005); 24(3): 187–194. [DOI] [PubMed] [Google Scholar]
- 5.Touw D.J., Neef C., Thomson A.H., Vinks A.A.; Cost-effectiveness of therapeutic drug monitoring: a systematic review; Therapeutic Drug Monitoring, (2005); 27(1): 10–17. [DOI] [PubMed] [Google Scholar]
- 6.Kirchherr H., Kuhn-Velten W.N.; Quantitative determination of forty-eight antidepressants and antipsychotics in human serum by HPLC tandem mass spectrometry: a multi-level, single-sample approach; Journal of Chromatography B, (2006); 843(1): 100–113. [DOI] [PubMed] [Google Scholar]
- 7.Uddin M.N., Samanidou V.F., Papadoyannis I.N.; Development and validation of an HPLC method for the determination of benzodiazepines and tricyclic antidepressants in biological fluids after sequential SPE; Journal of Separation Science, (2008); 31(13): 2358–2370. [DOI] [PubMed] [Google Scholar]
- 8.Bakkali A., Corta E., Ciria J.I., Berrueta L.A., Gallo B., Vicente F.; Solid-phase extraction with liquid chromatography and ultraviolet detection for the assay of antidepressant drugs in human plasma; Talanta, (1999); 49(4): 773–783. [DOI] [PubMed] [Google Scholar]
- 9.Kerr G.W., McGuffie A.C., Wilkie S.; Tricyclic antidepressant overdose: a review; Emergency Medicine Journal, (2001); 18(4): 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilkaya F., Bilge S.S., Bozkurt A., Bas D.B., Erdal A., Ciftcioglu E. et al. ; The antinociceptive effect of intravenous imipramine in colorectal distension-induced visceral pain in rats: the role of serotonergic and noradrenergic receptors; Pharmacology, Biochemistry and Behavior, (2014); 122: 1–6. [DOI] [PubMed] [Google Scholar]
- 11.Cantu M.D., Toso D.R., Lacerda C.A., Lancas F.M., Carrilho E., Queiroz M.E.; Optimization of solid-phase microextraction procedures for the determination of tricyclic antidepressants and anticonvulsants in plasma samples by liquid chromatography; Analytical and Bioanalytical Chemistry, (2006); 386(2): 256–263. [DOI] [PubMed] [Google Scholar]
- 12.Kubera M., Grygier B., Arteta B., Urbanska K., Basta-Kaim A., Budziszewska B. et al. ; Age-dependent stimulatory effect of desipramine and fluoxetine pretreatment on metastasis formation by B16F10 melanoma in male C57BL/6 mice; Pharmacological Reports, (2009); 61(6): 1113–1126. [DOI] [PubMed] [Google Scholar]
- 13.Johnson-Davis K.L., Juenke J.M., Davis R., McMillin G.A.; Quantification of tricyclic antidepressants using UPLC-MS/MS; Methods in Molecular Biology, (2012); 902: 175–184. [DOI] [PubMed] [Google Scholar]
- 14.de Castro A., Concheiro M., Quintela O., Cruz A., Lopez-Rivadulla M.; LC-MS/MS method for the determination of nine antidepressants and some of their main metabolites in oral fluid and plasma. Study of correlation between venlafaxine concentrations in both matrices; Journal of Pharmaceutical and Biomedical Analysis, (2008); 48(1): 183–193. [DOI] [PubMed] [Google Scholar]
- 15.de Castro A., Ramirez Fernandez Mdel M., Laloup M., Samyn N., De Boeck G., Wood M. et al. ; High-throughput on-line solid-phase extraction-liquid chromatography-tandem mass spectrometry method for the simultaneous analysis of 14 antidepressants and their metabolites in plasma; Journal of Chromatography A, (2007); 1160(1–2): 3–12. [DOI] [PubMed] [Google Scholar]
- 16.Zhao C., Wu Z., Xue G., Wang J., Zhao Y., Xu Z. et al. ; Ultra-high capacity liquid chromatography chip/quadrupole time-of-flight mass spectrometry for pharmaceutical analysis; Journal of chromatography A, (2011); 1218(23): 3669–3674. [DOI] [PubMed] [Google Scholar]
- 17.Rani S., Kumar A., Malik A.K., Singh B.; Quantification of tricyclic and nontricyclic antidepressants in spiked plasma and urine samples using microextraction in packed syringe and analysis by LC and GC-MS; Chromatographia, (2011); 74: 235–242. [Google Scholar]
- 18.Zhang H., Heinig K., Henion J.; Atmospheric pressure ionization time-of-flight mass spectrometry coupled with fast liquid chromatography for quantitation and accurate mass measurement of five pharmaceutical drugs in human plasma; Journal of Mass Spectrometry, (2000); 35(3): 423–431. [DOI] [PubMed] [Google Scholar]
- 19.Chen A.G., Wing Y.K., Chiu H., Lee S., Chen C.N., Chan K.; Simultaneous determination of imipramine, desipramine and their 2- and 10-hydroxylated metabolites in human plasma and urine by high-performance liquid chromatography; Journal of Chromatography B, (1997); 693(1): 153–158. [DOI] [PubMed] [Google Scholar]
- 20.Chaves A.R., Silva S.M., Queiroz R.H., Lancas F.M., Queiroz M.E.; Stir bar sorptive extraction and liquid chromatography with UV detection for determination of antidepressants in plasma samples; Journal of Chromatography B, (2007); 850(1–2): 295–302. [DOI] [PubMed] [Google Scholar]
- 21.Jemal M.; High-throughput quantitative bioanalysis by LC/MS/MS; Biomedical Chromatography, (2000); 14(6): 422–429. [DOI] [PubMed] [Google Scholar]
- 22.Jemal M., Ouyang Z., Xia Y.Q.; Systematic LC-MS/MS bioanalytical method development that incorporates plasma phospholipids risk avoidance, usage of incurred sample and well thought-out chromatography; Biomedical Chromatography, (2010); 24(1): 2–19. [DOI] [PubMed] [Google Scholar]
- 23.Mortishire-Smith R.J., O'Connor D., Castro-Perez J.M., Kirby J.; Accelerated throughput metabolic route screening in early drug discovery using high-resolution liquid chromatography/quadrupole time-of-flight mass spectrometry and automated data analysis; Rapid Communications in Mass Spectrometry, (2005); 19(18): 2659–2670. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Marino I., Quintana J.B., Rodriguez I., Gonzalez-Diez M., Cela R.; Screening and selective quantification of illicit drugs in wastewater by mixed-mode solid-phase extraction and quadrupole-time-of-flight liquid chromatography-mass spectrometry; Analytical Chemistry, (2012); 84(3): 1708–1717. [DOI] [PubMed] [Google Scholar]
- 25.Ni S., Qian D., Duan J.A., Guo J., Shang E.X., Shu Y. et al. ; UPLC-QTOF/MS-based screening and identification of the constituents and their metabolites in rat plasma and urine after oral administration of Glechoma longituba extract; Journal of Chromatography B, (2010); 878(28): 2741–2750. [DOI] [PubMed] [Google Scholar]
- 26.Zhao M., Xu J., Qian D., Guo J., Jiang S., Shang E.X. et al. ; Ultra performance liquid chromatography/quadrupole-time-of-flight mass spectrometry for determination of avicularin metabolites produced by a human intestinal bacterium; Journal of Chromatography B, (2014); 949–950: 30–36. [DOI] [PubMed] [Google Scholar]
- 27.Tang D., Dong Y., Guo N., Li L., Ren H.; Metabolomic analysis of the polyphenols in germinating mung beans (Vigna radiata) seeds and sprouts; Journal of the Science of Food and Agriculture, (2014); 94(8): 1639–1647. [DOI] [PubMed] [Google Scholar]
- 28.Xie C., Zhong D., Yu K., Chen X.; Recent advances in metabolite identification and quantitative bioanalysis by LC-Q-TOF MS; Bioanalysis, (2012); 4(8): 937–959. [DOI] [PubMed] [Google Scholar]
- 29.Peixoto M.P., Kaiser S., Verza S.G., de Resende P.E., Treter J., Pavei C. et al. ; LC-UV assay method and UPLC/Q-TOF-MS characterisation of saponins from Ilex paraguariensis A. St. Hil. (mate) unripe fruits; Phytochemical Analysis, (2012); 23(4): 415–420. [DOI] [PubMed] [Google Scholar]
- 30.Lee J., Jung Y., Shin J.H., Kim H.K., Moon B.C., Ryu do H. et al. ; Secondary metabolite profiling of Curcuma species grown at different locations using GC/TOF and UPLC/Q-TOF MS; Molecules, (2014); 19(7): 9535–9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou M., Jiang M., Ying X., Cui Q., Han Y., Hou Y. et al. ; Identification and comparison of anti-inflammatory ingredients from different organs of Lotus nelumbo by UPLC/Q-TOF and PCA coupled with a NF-kappaB reporter gene assay; PLoS One, (2013); 8(11): e81971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao X., Guo M., Li Q., Peng L., Liu H., Zhang L. et al. ; Plasma metabolomic profiling to reveal antipyretic mechanism of Shuang-huang-lian injection on yeast-induced pyrexia rats; PLoS One, (2014); 9(6): e100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun H., Zhang A.H., Zou D.X., Sun W.J., Wu X.H., Wang X.J.; Metabolomics coupled with pattern recognition and pathway analysis on potential biomarkers in liver injury and hepatoprotective effects of yinchenhao; Applied Biochemistry and Biotechnology, (2014); 173(4): 857–869. [DOI] [PubMed] [Google Scholar]
- 34.Shen Y., Shi S., Tong H., Guo Y., Zou J.; Metabolomics analysis reveals that bile acids and phospholipids contribute to variable responses to low-temperature-induced ascites syndrome; Molecular BioSystems, (2014); 10(6): 1557–1567. [DOI] [PubMed] [Google Scholar]
- 35.Geng J.L., Dai Y., Yao Z.H., Qin Z.F., Wang X.L., Qin L. et al. ; Metabolites profile of Xian-Ling-Gu-Bao capsule, a traditional Chinese medicine prescription, in rats by ultra performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry analysis; Journal of Pharmaceutical and Biomedical Analysis, (2014); 96: 90–103. [DOI] [PubMed] [Google Scholar]
- 36.Ahmad N., Warsi M.H., Iqbal Z., Samim M., Ahmad F.J.; Quantification of curcumin, demethoxycurcumin, and bisdemethoxycurcumin in rodent brain by UHPLC/ESI-Q-TOF-MS/MS after intra-nasal administration of curcuminoids loaded PNIPAM nanoparticles; Drug Testing and Analysis, (2014); 6(3): 257–267. [DOI] [PubMed] [Google Scholar]
- 37.Shakleya D.M., Jansson L.M., Huestis M.A.; Validation of a LC-APCI-MS/MS method for quantification of methadone, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) and 2-ethyl-5-methyl-3,3-diphenylpyraline (EMDP) in infant plasma following protein precipitation; Journal of Chromatography B, (2007); 856(1–2): 267–272. [DOI] [PubMed] [Google Scholar]
- 38.Macek J., Klima J., Ptacek P.; Rapid determination of omeprazole in human plasma by protein precipitation and liquid chromatography-tandem mass spectrometry; Journal of Chromatography B, (2007); 852(1–2): 282–287. [DOI] [PubMed] [Google Scholar]
- 39.Macek J., Klima J., Ptacek P.; Rapid determination of valsartan in human plasma by protein precipitation and high-performance liquid chromatography; Journal of Chromatography B, (2006); 832(1): 169–172. [DOI] [PubMed] [Google Scholar]
- 40.Shin J., Pauly D.F., Johnson J.A., Frye R.F.; Simplified method for determination of clarithromycin in human plasma using protein precipitation in a 96-well format and liquid chromatography-tandem mass spectrometry; Journal of Chromatography B, (2008); 871(1): 130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wen J., Wu Y., Zhang L., Qi Y., Fan G., Li Z.; High-throughput determination of fudosteine in human plasma by liquid chromatography-tandem mass spectrometry, following protein precipitation in the 96-well plate format; Journal of Chromatography B, (2008); 867(1): 153–159. [DOI] [PubMed] [Google Scholar]
- 42.Rybak M.E., Pfeiffer C.M.; A simplified protein precipitation and filtration procedure for determining serum vitamin B6 by high-performance liquid chromatography; Analytical Biochemistry, (2009); 388(1): 175–177. [DOI] [PubMed] [Google Scholar]
- 43.Frahnert C., Rao M.L., Grasmader K.; Analysis of eighteen antidepressants, four atypical antipsychotics and active metabolites in serum by liquid chromatography: a simple tool for therapeutic drug monitoring; Journal of Chromatography B, (2003); 794(1): 35–47. [DOI] [PubMed] [Google Scholar]
- 44.Duverneuil C., de la Grandmaison G.L., de Mazancourt P., Alvarez J.C.; A high-performance liquid chromatography method with photodiode-array UV detection for therapeutic drug monitoring of the nontricyclic antidepressant drugs; Therapeutic Drug Monitoring, (2003); 25(5): 565–573. [DOI] [PubMed] [Google Scholar]
- 45.Aymard G., Livi P., Pham Y.T., Diquet B.; Sensitive and rapid method for the simultaneous quantification of five antidepressants with their respective metabolites in plasma using high-performance liquid chromatography with diode-array detection; Journal of Chromatography B, (1997); 700(1–2): 183–189. [DOI] [PubMed] [Google Scholar]
- 46.del Mar Ramirez Fernandez M., Wille S.M., Samyn N.; Quantitative method validation for the analysis of 27 antidepressants and metabolites in plasma with ultraperformance liquid chromatography-tandem mass spectrometry; Therapeutic Drug Monitoring, (2012); 34(1): 11–24. [DOI] [PubMed] [Google Scholar]
- 47.Remane D., Meyer M.R., Wissenbach D.K., Maurer H.H.; Full validation and application of an ultra-high performance liquid chromatographic-tandem mass spectrometric procedure for target screening and quantification of 34 antidepressants in human blood plasma as part of a comprehensive multi-analyte approach; Analytical and Bioanalytical Chemistry, (2011); 400(7): 2093–2107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.