Abstract
A rapid and sensitive method based on HPLC–DAD-MS was developed for quantitative analysis of two flavonoids and chemical fingerprint analysis to evaluate the quality of Receptaculum Nelumbinis. The analysis was conducted on a Poroshell 120 C18 column (100 × 4.6 mm, 2.7 μm) with 0.2% formic acid buffer solution and methanol as mobile phases with gradient elution. This method displayed good linearity with R2 at >0.9999 and limits of quantity <0.37 μg mL−1. Relative standard deviation values for intra- and interday precision were <0.82 and 1.03%, respectively. The mean recovery of hyperoside was 95.54% and of isoquercitrin was 92.10%. Hyperoside and isoquercitrin were determined simultaneously, and 12 peaks in the chemical fingerprint were identified. The chemometric methods, including similarity analysis, hierarchical clustering analysis and principal component analysis, were applied to distinguish 11 batches of Receptaculum Nelumbinis samples. The above results could validate each other and successfully divide these samples into two groups. Moreover, hyperoside and isoquercitrin could be selected as chemical markers to evaluate the quality of Receptaculum Nelumbinis from different localities. This study demonstrated that the developed method was a powerful and beneficial tool to carry out the quality control of Receptaculum Nelumbinis.
Introduction
Traditional Chinese Medicine (TCM) has been attracting more and more attention because of its complementary therapeutic effects to western medicines with few side effects, but the quality control of TCM is far from sufficient to meet the criteria needed to support its use worldwide (1). The chemical quality control of some TCMs is still relatively limited. Nowadays, simultaneous determination of multiple components and chemical fingerprint analysis is the development trend of controlling TCM quality (2). The fingerprint chromatograms are complex multivariate datasets due to sophisticated chemical constituents, and minor differences between very similar chromatograms might be missed (3). Thus, the chemometric methods, such as similarity analysis (SA), hierarchical clustering analysis (HCA) and principal component analysis (PCA), etc. should be taken into consideration for reasonable definition of the class of TCM.
Receptaculum Nelumbinis (Lianfang in Chinese), the dried receptacle of Nelumbo nucifera, is one kind of commonly used TCMs. It shows good efficacy for excessive menstrual bleeding and irregular genital bleeding, dehydration caused by diarrhea in summer and miscarriage (4). Existing studies showed that flavonoids were the characteristic chemical constituents of Receptaculum Nelumbinis, which exhibited promising bioactivities, including antioxidation, antitumor, radioprotective activities, protective effects against experimental myocardial injury and ischemia, and improving learning and memory abilities (5–9). High-performance liquid chromatography–mass spectrometry (HPLC–MS) has been widely applied in the analysis of flavonoids during recent years, which can provide accurate mass measurements and formulae of nontarget compounds and tentatively identify nontarget compounds. Recently, 12 flavonoids were identified in lotus petals, and 5 of these flavonoids were also found in lotus fruit coats by HPLC–MS (10–13). HPLC–MS has played an important role and demonstrated its great advantages for the structural analysis of chemical constituents in medicinal herbs with high sensitivity, short time and low consumption of sample (14).
Receptaculum Nelumbinis is officially recorded in the Chinese Pharmacopoeia (2010 Version) with the simple character identification (15). And the chemical quality evaluation of Receptaculum Nelumbinis is still in blank, so it is necessary to develop a method for evaluating its chemical quality. Some assays with HPLC–UV methods have been described for quality control of Receptaculum Nelumbinis, but these investigations suffer from no other detailed information about the flavonoids in Receptaculum Nelumbinis. Because the therapeutic effect of TCM is usually attributed to the synergy of multiple bioactive compounds, single active compound could not be responsible for the overall pharmacological activities of the medicines (16, 17). So, there is a solid need to use more advanced equipment to investigate the complicated chemical constituents in Receptaculum Nelumbinis.
In the present study, a HPLC–DAD-MS method was developed for simultaneous quantitative and chemical fingerprint analysis of Receptaculum Nelumbinis for the first time. Two flavonoids were determined simultaneously, and 12 peaks in the chemical fingerprint were identified. Moreover, the chemical fingerprints of Receptaculum Nelumbinis from various sources were investigated by chemometric analysis.
Experimental
Chemical and materials
Hyperoside and isoquercitrin were obtained from Phytomarker Ltd. (Tianjin, China). HPLC-grade methanol, acetonitrile (Honeywell Burdick & Jackson, Muskegon, USA), formic acid and acetic acid (Tianjin Guang Fu Fine Chemical Research Institute, Tianjin, China) were used for the HPLC analysis. Analytical grade of methanol was purchased from Beijing Chemical Works (Beijing, China). Pure water (18.2 MΩ) for the HPLC analysis was obtained from a Milli-Q System (Millipore, Billerica, MA, USA).
Eleven batches of Receptaculum Nelumbinis samples were collected from different localities of Jianning County in Fujian province of China. All air-dried samples were ground and sieved (65-mesh), respectively.
Preparation of sample solutions
All Receptaculum Nelumbinis samples were oven-dried at 45°C until constant weight was reached. One gram of the sample was accurately weighed and extracted with 50 mL methanol by refluxing for 1.5 h (15). The weight loss in the refluxing procedure was compensated, and then filtered through 0.22 μm membrane filter prior to injection into the HPLC system.
Instrumentation and chromatographic conditions
HPLC analysis was performed on an Agilent 1260 HPLC system coupled with diode array detector (Agilent Technologies, Palo Alto, CA, USA). Chromatographic data were processed by Agilent ChemStation software. Chromatographic separation was conducted on a Poroshell 120 C18 column (100 × 4.6 mm, 2.7 μm, Agilent Technologies) with a column temperature of 38°C. The mobile phases consisted of 0.2% formic acid in water (A) and methanol (B), and the flow rate was at 0.8 mL min−1. The eluting conditions were optimized as follows: 0–10 min at 30% B, 10–13 min from 30 to 37.5% B, 13–15 min from 37.5 to 45% B and 15–20 min at 45% B. This was followed by a 6-min equilibration period prior to the injection of each sample. The detection wavelength was set at 360 nm with the sample injection volume of 2 μL.
Identification of flavonoids
Flavonoids in the methanol extract of Receptaculum Nelumbinis were identified by using an Applied Biosystem 3200 Q-Trap mass spectrometer (Applied Biosystems, Foster City, California, USA) connected to an Agilent 1200 HPLC system via electrospray ionization interface. The chromatographic conditions were described as above. Electrospray ionization was applied in negative-ion (NI) modes for MS and MS/MS to give fragmentation information on the molecular weights, aglycone groups and glycosylation patterns. The mass spectrometric parameters were optimized by using the two flavonoid standards listed in “Chemical and materials.” The parameters used in NI mode were as follows: ion spray voltage 4,000 V, curtain gas 10 psi, nebulizer gas 60 psi, auxiliary gas 40 psi, ion source tem 400°C and scan range m/z 100–700 units. Ultrapure nitrogen was used as nebulizer, heater, curtain and collision-activated dissociation gas. Data were processed by the Analyst 1.4 software (Applied Biosystems/MDSSciex). MS data, retention times and UV–vis spectra were used to identify the flavonoids contained in Receptaculum Nelumbinis. The assignments were also validated by coelution with the corresponding standards and by comparison with published data.
Method validation
The analytical method was validated in terms of linearity, precision and accuracy according to ICH guidelines (18). Each standard was accurately weighed, dissolved in methanol and the standard solutions were then diluted to generate an appropriate concentration range to establish calibration curves. All calibration curves were constructed by using five different concentrations of mixed standards in triplicate. Analytical method was validated by the calibration curves, limit of detection (LOD), limit of quantitation (LOQ), repeatability, stability, accuracy and recovery of the two flavonoids.
Chemometric analysis
The chemometric analysis was applied to demonstrate the variability of 12 batches of Receptaculum Nelumbinis samples. SA was performed by the Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine (Version 2004A), which was recommended by China Food and Drug Administration (CFDA). The HCA of 12 samples was performed using PASW Statistics (Version 19.0). The PCA was performed on the common chromatographic peaks in the HPLC fingerprints by using the Unscrambler X 10.0 software from Camo AS (Trondheim, Norway). The main chemical markers that had the major influence on distinguishing different samples were found with the results of PCA loading plots.
Results
Optimization of HPLC conditions
To give the comprehensive chemical information and best separation in the chromatograms, the column, mobile phase, flow rate, elution condition, column temperature and detection wavelength were investigated. Through a comparative study of three different chromatographic columns—Agilent Eclipse XDB C18 column (250 × 4.6 mm, 5 μm), Agilent Zorbax SB C18 column (250 × 4.6 mm, 5 μm) and Agilent Poroshell 120 C18 column (100 × 4.6 mm, 2.7 μm)—the platform of Agilent 1260 combined with Agilent Poroshell 120 C18 column (100 × 4.6 mm, 2.7 μm) exhibited best separation efficiency allowing target compound identification in a shorter time with little solvent consumption.
The chromatographic conditions were optimized. Two kinds of acid modifiers (formic acid and acetic acid) were examined. Different concentrations of acid modifiers as buffers in the mobile phases were compared to improve the separation efficiency, and it was found that 0.2% formic acids achieved the best separation and suppressed the tailing of the peaks.
From the results of comparative study of flow rates of 0.6, 0.8 and 1.0 mL min−1 and column temperatures of 32, 35 and 38°C, the best separation was achieved in flow rate at 0.8 mL min−1 and the column temperature of 38°C. Three different wavelengths at 254, 282 and 360 nm were monitored and compared, and 360 nm was selected as the detection wavelength because more characteristic peaks could be obtained, and the baseline was well improved on the chromatographic profiles.
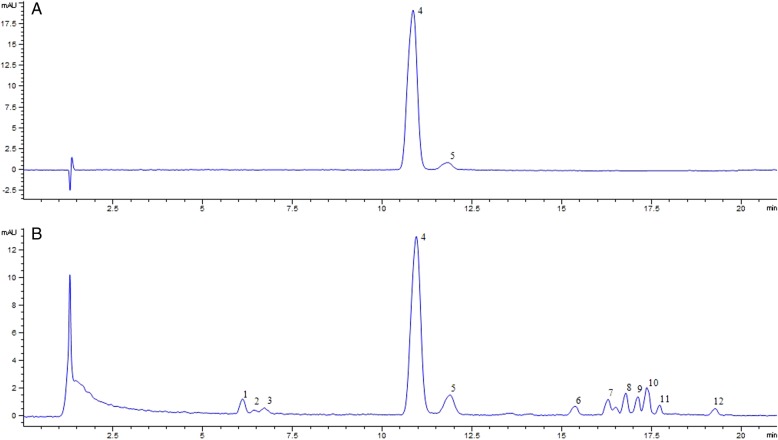
The optimized chromatographic conditions of Receptaculum Nelumbinis were as described in “Instrumentation and chromatographic conditions,” and both the two flavonoids and other compounds in the methanol extract of Receptaculum Nelumbinis were well separated as shown in Figure 1.
Figure 1.
Typical chromatograms for chemical analysis of mixed standards (A) and Receptaculum Nelumbinis (B). This figure is available in black and white in print and in color at JCS online.
Validation of quantitative analysis method
The results of method validation for two external standards (hyperoside and isoquercitrin) are given in Table I. The linearity of the calibration curves was verified by correlation study and the correlation coefficients were all better than 0.9999 within test ranges. The LODs and LOQs were <0.11 and 0.38 μg mL−1, which were determined at a signal-to-noise (S/N) ratio of 3 and 10, respectively.
Table I.
Linearity, LODs and LOQs of Hyperoside and Isoquercitrin
| Compounds | Calibration curve | r2 | Linear range (μg mL−1) | LOD (μg mL−1) | LOQ (μg mL−1) |
|---|---|---|---|---|---|
| Hyperoside | y = 4.10x + 2.98 | 1.0000 | 0.38–240.00 | 0.11 | 0.38 |
| Isoquercitrin | y = 5.43x + 4.86 | 0.9999 | 0.29–180.00 | 0.07 | 0.29 |
y, peak area; x, compound concentration (μg mL−1); LOD = limit of detection, S/N = 3; LOQ = limit of quantitation, S/N = 10.
As given in Table II, the precision based on peak area measurement of the two flavonoids was found to be <1.03% with RSD (n = 5). The developed method had good repeatability and stability with an RSD of <1.42%. The recoveries of the two flavonoids were 92.10–95.54% and their RSD values were <1.18% (Table II). All these indicated that the developed method was precise, accurate and sensitive enough for simultaneous quantitative determination of two flavonoids in Receptaculum Nelumbinis.
Table II.
Precision, Stability, Repeatability and Recovery of Hyperoside and Isoquercitrin
| Compounds | Precisions (n = 6) |
Repeatability (n = 5) | Stability (n = 6) | Recovery | RSD (%) | |
|---|---|---|---|---|---|---|
| Intraday RSD (%) | Interday RSD (%) | RSD (%) | RSD (%) | |||
| Hyperoside | 0.47 | 0.53 | 0.82 | 0.20 | 95.54 | 1.18 |
| Isoquercitrin | 0.82 | 1.03 | 1.42 | 0.42 | 92.10 | 1.08 |
HPLC–DAD-MS identity confirmation
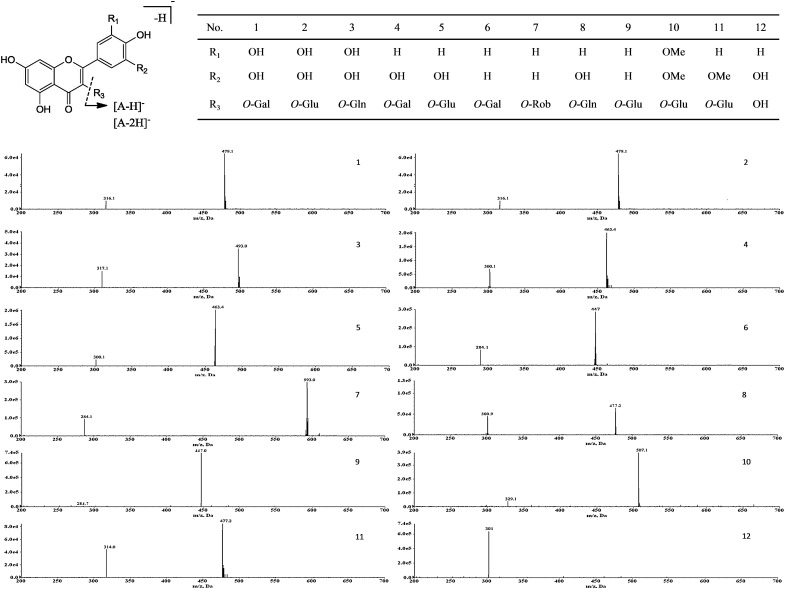
The chromatographic and MS data of flavonoids in Receptaculum Nelumbinis are listed in Table III. MS data in NI mode gave valuable information including the molecular weight, the information of 12 flavonoids aglycone and glycoside and the position of the link between them. The UV–vis spectrum and MS data of Peaks 1 and 2 (Figure 2 and Table III) indicated that their structures were determined to be a myricetin monosaccharide. Due to the neutral loss of a fragment ion of 162 mass units produced by protonated precursor ion 479 [M−H]− and produced aglycone ion at m/z 316 [A−2H]− in NI-MS/MS mode, Peaks 1 and 2 might contain a glucose or galactose conjugated at the 3-position (19). In addition, Peak 1 had a shorter retention time because glycoside linked with galactose was found to be eluted before glucose linkage (20). Thus, Peak 1 was identified as myricetin 3-O-galactoside and Peak 2 was identified as myricetin 3-O-glucoside, which was reported in lotus leaves (21). According to the data for aglycone ion, m/z 317 [A−H]− and loss of a 176 unit fragment produced from the ion 493 [M−H]− in NI-MS/MS mode process, Peak 3 was identified as myricetin 3-O-glucuronide (22).
Table III.
Identification of 12 Flavonoids in Receptaculum Nelumbinis
| Peak no. | Identification | RT (min) | λmax (nm) | NI | MS/MS |
|---|---|---|---|---|---|
| 1 | Myricetin 3-O-galactoside | 6.10 | 254.7, 356.3 | 479 [M−H]− | 316 [A−2H]− |
| 2 | Myricetin 3-O-glucoside | 6.43 | 254.3, 355.3 | 479 [M−H]− | 316 [A−2H]− |
| 3 | Myricetin 3-O-glucuronide | 6.69 | 259.5, 358.2 | 493 [M−H]− | 317 [A−H]− |
| 4 | Hyperoside | 10.95 | 252.4, 352.9 | 463 [M−H]− | 300 [A−2H]− |
| 5 | Isoquercitrin | 11.90 | 254.7, 352.9 | 463 [M−H]− | 300 [A−2H]− |
| 6 | Kaempferol 3-O-galactoside | 15.38 | 252.4, 354.1 | 447 [M−H]− | 284 [A−2H]− |
| 7 | Kaempferol 3-O-robinobioside | 16.30 | 267.8, 345.6 | 593 [M−H]− | 284 [A−H]− |
| 8 | Quercetin 3-O-glucuronide | 16.78 | 253.6, 352.9 | 477 [M−H]− | 301 [A−H]− |
| 9 | Kaempferol 3-O-glucoside | 17.12 | 253.6, 355.3 | 447 [M−H]− | 284 [A−2H]− |
| 10 | Syringetin 3-O-glucoside | 17.40 | 267.8, 340.8 | 507 [M−H]− | 329 [A−H-CH3]− |
| 11 | Isorhamnetin 3-O-glucoside | 17.73 | 264.3, 345.6 | 477 [M−H]− | 314 [A−2H]− |
| 12 | Quercetin | 19.27 | 264.3, 345.6 | 301 [A−H]− |
Figure 2.
Chemical structures and MS data of 12 flavonoids identified in Receptaculum Nelumbinis.
Peaks 4 and 5 had the same molecular ion m/z 463 [M−H]− and the aglycone ion m/z 300 [A−2H]− in NI mode (Table III). It was revealed that a hexose was conjugated to quercetin at the 3-position in these two compounds. By comparing the retention time and coelution with standards, Peaks 4 and 5 were identified as hyperoside and isoquercitrin, respectively, which had been reported previously in lotus leaves (12, 23).
The molecular weight of the compound corresponding to Peak 8 was found to be 478, and an aglycone ion was observed at m/z 301 [A−H]− in NI-MS/MS. The fragment loss of 176 mass unit from the ion 477 [M−H]− in NI-MS/MS mode validated that it was a glucuronide substituent at the 3-position linkage. Thus, it was deduced as quercetin 3-O-glucuronide, which had been reported previously in lotus leaves (22).
The compounds corresponding to Peaks 6 and 9 were identified that a hexose was conjugated to kaempferol at the 3-position in the two compounds by their ions at m/z 447 [M−H]− and m/z 284 [A−2H]− in NI mode. Peak 6 was identified as kaempferol 3-O-galactoside because of a shorter retention time, and Peak 9 was identified as kaempferol 3-O-glucoside, which was in agreement with previous reports on flavonoids in lotus petals (23).
As illustrated in Table III and Figure 2, integrating the precursor and product ion in NI-MS/MS, Peak 7 was identified as kaempferol 3-O-robinobioside (23). Based on the UV–vis spectrum and MS data, Peak 10 was proved to be syringetin 3-O-glucoside, which had been reported from lotus flower petal by HSCCC-NMR (24). Peak 11 was determined to be monosaccharide glycoside with its MS data, and was identified as isorhamnetin derivative on the basis of aglycone fragment ions at m/z 314 [A−2H]− in NI mode, and was deduced as isorhamnetin 3-O-glucoside, which had been reported in lotus petals (23).
Sample analysis
Quantitative analysis
Two flavonoids in 11 batches of Receptaculum Nelumbinis samples were analyzed with the newly developed method and the data are presented in Table IV. The content range was 1.88–3.31 mg g−1 for hyperoside and 0.11–0.41 mg g−1 for isoquercitrin. The content of the two flavonoids in different sources was significantly different, and the content of hyperoside in all samples was the most abundant. The sample collected from Qi village (S9) had the highest contents of total flavonoids, whereas the sample from Xiyuan village (S6) had the opposite result. The results illustrated that the internal quality of 11 batches of Receptaculum Nelumbinis samples from different geographical sources was variant.
Table IV.
Content (mg·100 g−1) of Hyperoside and Isoquercitrin in Receptaculum Nelumbinis Collected from Different Localities (n = 3)
| No. | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Origin (village) | Xianhe | Yuanli | Shangli | Dayuan | Xianhe | Xiyuan | Lanxi | Dayuan | Qi | Xiuzhu | Hedong |
| Hyperoside | 308.62 | 202.22 | 238.69 | 275.85 | 309.47 | 188.15 | 198.19 | 230.64 | 330.54 | 285.28 | 298.86 |
| Isoquercitrin | 34.72 | 15.27 | 20.99 | 18.19 | 25.19 | 11.10 | 11.77 | 22.06 | 40.68 | 27.47 | 38.00 |
| Total | 343.34 | 217.49 | 259.67 | 294.04 | 334.67 | 199.25 | 209.96 | 252.70 | 371.21 | 312.75 | 336.87 |
Similarity analysis
Twelve peaks existing in the chromatographic profiles of 11 samples were assigned as “Characteristic peaks.” Similarity evaluation system for chromatographic fingerprint of TCM (Version 2004A) was used to analyze the chromatographic fingerprint of Receptaculum Nelumbinis samples from various sources. The similarity values were 0.994, 0.973, 0.982, 0.991, 0.995, 0.972, 0.974, 0.986, 0.999, 0.992 and 0.996, respectively. The results indicated that the samples shared different values of similarity, showing that the internal quality of these samples was different. Samples with similar similarity values, such as S1, S4, S5, S9, S10 and S11, illustrated that the chemical constituents of these samples were generally consistent and stable.
Hierarchical clustering analysis
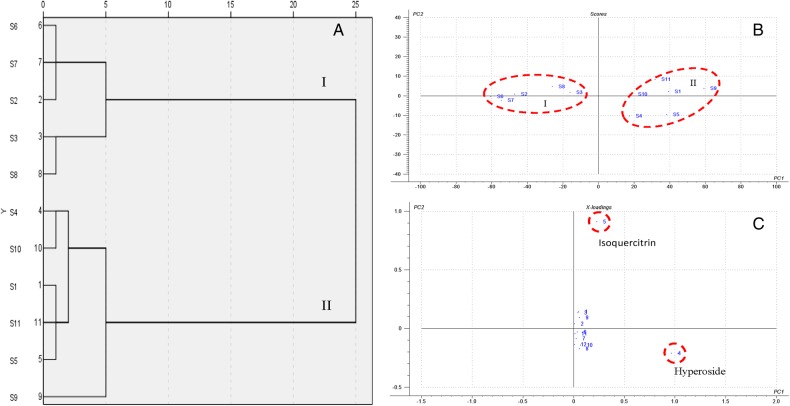
In order to validate the results of SA and further elucidate the resemblance relationship among these samples, HCA was performed. The relative peak areas of 12 characteristic peaks from diode array detector in 11 samples formed a matrix of 12 × 11, which was calculated by using the PASW Statistics (Version 19.0). The results of HCA showed that the similarity was revealed clearly, and 11 samples were divided into 2 clusters obviously (Figure 3). Group II was formed by the samples S1, S4, S5, S9, S10 and S11. Group I consisted of the remaining samples. Comparing with the samples of chromatographic profiles, it was easy to find that the samples in Group II showed better similarity, which indicated that the samples in Group II had the very similar chemical constituents. The results were very similar to the results of SA, so HCA was helpful to judge the consistency of Receptaculum Nelumbinis.
Figure 3.
Chemometric analysis of 11 batches of Receptaculum Nelumbinis samples. (A) Dendrogram for hierarchical clustering by PASW Statistics. (B) Scores plot of PCA by the Unscrambler. (C) Loadings plot of PCA for 12 peaks in their HPLC profiles by the Unscrambler. This figure is available in black and white in print and in color at JCS online.
Principal component analysis
In order to evaluate the discrimination ability of 12 characteristic peaks from diode array detector, PCA was employed by using the Unscrambler software in which the relative peak areas of the 12 peaks were chosen as input data instead of the full spectrum of fingerprints without any preprocessing. On the basis of eigenvalues of >1, the first two principal components PC1 and PC2 are often used to provide a convenient visual aid for identifying homogeneity in the datasets. The score plot of the first two principal components (Figure 3B) showed clear differentiation of 11 samples with different sources as grouped by HCA. The scatter points showed that the samples could be classified into two groups: Group I and Group II. The samples classified into the same group were associated with similar chemical properties.
Moreover, 12 characteristic peaks were obtained to find the possible chemical markers for the discrimination of different samples in PCA. For the log-centered dataset with 86.21% of explained variance by the first two principal components, the loading plot of the scores (Figure 3C) indicated that Peaks 4 and 5 might have more influence on the discrimination of the sample from different localities than other components. In other words, hyperoside and isoquercitrin could be selected as chemical markers to evaluate the quality of Receptaculum Nelumbinis from different localities.
Discussion
A HPLC–DAD-MS method was developed to evaluate the quality of Receptaculum Nelumbinis for the first time. In this study, chemometric analysis such as SA, HCA and PCA was used to distinguish samples objectively and the results showed a good consistency. Eleven samples were divided into two clusters by chemometric analysis, with considerable differences between them. The clustering result by HCA was approximate to the results of SA. The samples in Group II, which was formed with S1, S4, S5, S9, S10 and S11, have better quality than those in Group I. The chemical constituents of the samples in the same group were generally consistent and stable. As for the contents of the two flavonoids, it could be seen that the samples in Group II had higher contents than those in Group I, and this was probably because of the different ecological conditions of the producing areas, cultivated techniques, processed methods, etc. The score plot of the first two principal components by PCA showed a clear differentiation among the 11 samples, which was in accord with that grouped by HCA. The results of HCA and PCA could validate each other and provide more references for the quality evaluation of Receptaculum Nelumbinis. PCA's loading plot further revealed that Compounds 4 and 5 might have more influence on the discrimination of the samples from different producing areas than other compounds. Hyperoside and isoquercitrin could be deemed to be the main chemical markers in Receptaculum Nelumbinis, which could be helpful to further evaluate the quality of Receptaculum Nelumbinis. So, it is demonstrated that the quantitative analysis of multiple characteristic chemical makers coupled with qualitative analysis of chromatographic fingerprinting is a powerful, practical tool for the comprehensive quality analysis of TCM.
In this study, Agilent 1260 combined with Poroshell 120 C18 column (100 × 4.6 mm, 2.7 μm) enjoyed the practical advantages of shorter analysis time and less solvent consumption, and exhibited the effect of UPLC. These factors made the platform an attractive alternative to conventional HPLC technique in routine TCM fingerprinting analysis. Hence, we applied this platform to evaluate the chemical quality of Receptaculum Nelumbinis, and the developed method would provide an important reference to other related TCMs.
Conclusion
Two flavonoids were determined simultaneously, and 12 peaks in the chemical fingerprint were identified. Eleven batches of Receptaculum Nelumbinis could be successfully divided into two groups, and six batches showed good similarity on chemical constituents according to the results of chemometric analysis. Hyperoside and isoquercitrin could be selected as chemical markers to evaluate the quality of Receptaculum Nelumbinis with different sources.
References
- 1.Liang Y.Z., Xie P.S., Chan K.; Quality control of herbal medicines; Journal of Chromatography B, (2004); 1: 53–70. [DOI] [PubMed] [Google Scholar]
- 2.Liang X.M., Jin Y., Wang Y.P., Jin G.W., Fu Q., Xiao Y.S.; Qualitative and quantitative analysis in quality control of traditional Chinese medicines; Journal of Chromatography A, (2009); 11: 2033–2044. [DOI] [PubMed] [Google Scholar]
- 3.Xu C.J., Liang Y.Z., Chau F.T., Heyden Y.V.; Pretreatments of chromatographic fingerprints for quality control of herbal medicines; Journal of Chromatography A, (2006); 1: 253–259. [DOI] [PubMed] [Google Scholar]
- 4.Ishida H., Umino T., Tsuji K., Kosuge T.; Studies on antihemorrhagic substances in herbs classified as hemostatics in Chinese medicine. VII. On the antihemorrhagic principle in Cirsium japonicum DC; Chemical & Pharmaceutical Bulletin, (1988); 2: 4585–4587. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y.B., Zheng L.J., Yi J., Wu J.G., Tan C.J., Chen T.Q. et al. A comparative study on antioxidant activity of ten different parts of Nelumbo nucifera Gaertn. Afr; Journal of Pharmacy and Pharmacology, (2011); 22: 2454–2461. [Google Scholar]
- 6.Gong Y.S., Liu L.G., Xie B.J., Liao Y.C., Yang E.L., Sun Z.D.; Ameliorative effects of lotus seedpod proanthocyanidins on cognitive deficits and oxidative damage in senescence-accelerated mice; Behavioural Brain Research, (2008); 1: 100–107. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X.H., Zhang B., Gong P.L., Zeng F.D.; Protective effect of procyanidins from the seedpod of the lotus on myocardial ischemia and its mechanisms; Acta Pharmacologica Sinica, (2004); 10: 747–750. [PubMed] [Google Scholar]
- 8.Duan Y., Zhang H., Xu F., Xie B., Yang X., Wang Y. et al. Inhibition effect of procyanidins from lotus seedpod on mouse B16 melanoma in vivo and in vitro; Food Chemistry, (2010); 1: 84–91. [Google Scholar]
- 9.Duan Y., Zhang H., Xu F., Xie B., Yang X., Wang Y. et al. Whole body radioprotective activity of an acetone-water extract from the seedpod of Nelumbo nucifera Gaertn; Food and Chemical Toxicology, (2010); 12: 3374–3384. [DOI] [PubMed] [Google Scholar]
- 10.Kredy H.M., Huang D., Xie B., He H., Yang E., Tian B. et al. Flavonols of lotus (Nelumbo nucifera, Gaertn.) seed epicarp and their antioxidant potential; European Food Research and Technology, (2010); 231: 387–394. [Google Scholar]
- 11.Yang R.Z., Wei X.L., Gao F.F., Wang L.S., Zhang H.J., Xu Y.J. et al. Simultaneous analysis of anthocyanins and flavonols in petals of lotus (Nelumbo) cultivars by high-performance liquid chromatography-photodiode array detection/electrospray ionization mass spectrometry; Journal of Chromatography A, (2009); 1216: 106–112. [DOI] [PubMed] [Google Scholar]
- 12.Goo H.R., Choi J.S., Na D.H.; Simultaneous determination of quercetin and its glycosides from the leaves of Nelumbo nucifera by reversed-phase high-performance liquid chromatography; Archives of Pharmacal Research, (2009); 32: 201–206. [DOI] [PubMed] [Google Scholar]
- 13.Liu J.S., Guo Y.J., Zhang J., Qi Y.D., Jia X.G., Gao G.F. et al. Systematic chemical analysis of flavonoids in the Nelumbinis stamen; Phytomedicine, (2014); 23: 1753–1758. [DOI] [PubMed] [Google Scholar]
- 14.Wang X., Wang H., Zhang A., Lu X., Sun H., Dong H. et al. Metabolomics study on the toxicity of aconite root and its processed products using ultraperformance liquid-chromatography/electrospray-ionization synapt high-definition mass spectrometry coupled with pattern recognition approach and ingenuity pathways analysis; Journal of Proteome Research, (2011); 11: 1284–1301. [DOI] [PubMed] [Google Scholar]
- 15.Chinese Pharmacopoeia Commission; Chinese pharmacopoeia. Chinese Medical Science Press, Beijing, (2010). [Google Scholar]
- 16.Wang C.L., Zhang X.L.; Determination of hyperin and quercetin in different processed products of Nelumbins Receptaculum by HPLC; Chinese Traditional Patent Medicine, (2010); 32: 1729–1732. [Google Scholar]
- 17.Wang J.S., Liu Y.P., Cheng B., Shi Y.H., Chen H.P.; The research on the isolation and identification of hyperin in Receptaculum Nelumbinis and quality standard of Receptaculum Nelumbinis; Journal of Chengdu Medical College, (2008); 3: 35–37. [Google Scholar]
- 18.ICH; Guidance for industry. Q2B validation of analytical procedures. Methodology. FDA, Rockville, (1996). [Google Scholar]
- 19.Ablajan K., Abliz Z., Shang X.Y., He J.M., Zhang R.P., Shi J.G.; Structural characterization of flavonol 3, 7-di-O-glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry; Journal of Mass Spectrometry, (2006); 3: 352–360. [DOI] [PubMed] [Google Scholar]
- 20.Wu X.L., Prior R.L.; Identification and characterization of anthocyanins by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry in common foods in the United States: vegetables, nuts, and grains; Journal of Agricultural and Food Chemistry, (2005); 8: 3101–3113. [DOI] [PubMed] [Google Scholar]
- 21.Huang B., Ban X.Q., He J.S., Tong J., Tian J., Wang Y.W.; Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaertn.) leaves; Food Chemistry, (2010); 3: 873–878. [Google Scholar]
- 22.Kredy H., Huang D., Xie B.J., He H., Yang E., Tian B.Q. et al. Flavonols of lotus (Nelumbo nucifera, Gaertn.) seed epicarp and their antioxidant potential; European Food Research and Technology, (2010); 3: 387–394. [Google Scholar]
- 23.Yang R.Z., Wei X.L., Gao F.F., Wang L.S., Zhang H.J., Xu Y.J. et al. Simultaneous analysis of anthocyanins and flavonols in petals of lotus (Nelumbo) cultivars by high-performance liquid chromatography-photodiode array detection/electrospray ionization mass spectrometry; Journal of Chromatography A, (2009); 1: 106–112. [DOI] [PubMed] [Google Scholar]
- 24.Guo X.F., Wang D.J., Duan W.J., Du J.H., Wang X.; Preparative isolation and purification of four flavonoids from the petals of nelumbo nucifera by high-speed counter-current chromatography; Phytochemical Analysis, (2010); 3: 268–272. [DOI] [PubMed] [Google Scholar]