Abstract
A simple and sensitive liquid chromatography method with diode array detector was established for simultaneous determination of 11 components (geniposidic acid, chlorogenic acid, caffeic acid, geniposide, luteoloside, isochlorogenic acid C, baicalin, luteolin, wogonoside, baicalein and wogonin) in various commercial Yinzhihuang preparations and their herbs by optimizing the extraction, separation and analytical conditions. Eleven components were identified on the basis of their retention times and mass spectra. Chromatographic separation was performed on a C18 analytical column with a gradient elution of acetonitrile and 0.1% formic acid water solution at a flow rate of 1.0 mL/min. The linearity, precision and accuracy of the data obtained were acceptable. The method was used to analyze four Yinzhihuang preparations (powder, capsule, oral liquid and injection) and related herbs (Radix Scutellariae, Flos Lonicerae, Herba Artemisiae Scopariae and Fructus gardeniae). Results suggested that the optimized method could be considered as a good approach to control the quality of Yinzhihuang preparations and their herbs.
Introduction
Yinzhihuang (YZH) preparations (granule, capsule, injection and oral liquid) are wildly used in Asia to prevent and treat neonatal jaundice and hepatitis (1, 2). All of YZH preparations are composed of four medicinal herbs: Radix Scutellariae, Flos Lonicerae, Herba Artemisiae Scopariae and Fructus gardeniae. In China, there are 15 pharmaceutical manufactures that produce YZH preparations and 276 manufactures that produce Chinese patent drugs related to Herba Artemisiae Scopariae, Fructus gardeniae, Flos Lonicerae or Radix Scutellariae. Effective quality control is the premise of safety, efficacy and therapeutic reproducibility of those drugs. As the origin herbs from those preparations grow in different regions of China, the contents of the bioactive components vary greatly depending on the geographical locations and climate (3, 4). For catering to the existing quality control standards, some foreign compounds may be sneaked in those Chinese drugs (5). Therefore, how to effectively control the quality of those Chinese drugs has become one of the most important problems to be resolved.
Two methods including biothermal active fingerprint (6) and combination of biological and chemical fingerprint (7) to detect the quality fluctuation of YZH injection have been reported. However, fingerprint only shows the calculated results of similarity based on the relative value with the selected marker compound as reference standard. It does not reveal the content variation of important ingredients. Lack of quantitative study would lead to the imperfection of quality control. For multi-ingredients analysis of Chinese drugs, high-performance liquid chromatography (HPLC) is the most important technique. Although studies had found that there are many bioactive ingredients in YZH preparations (8, 9), only a few chromatographic methods have been documented for the determination of one or two components presented in YZH preparations by liquid chromatography (10, 11). In China pharmacopoeia (2010) (Ch. P. (2010)), only baicalin and geniposide are chosen as the index for the quality control of YZH oral liquid (12). It is well known that the curative effects of medicinal herbs and their preparations are principally based on the synergic effect (13) of their multi-ingredients and multi-target, in contrast to synthetic drugs that often focus on a single chemical entity (14). So, utilizing one or two components as markers to control the quality of herbs or Chinese patent drugs is fundamentally flawed (15–18). For complex systems, selecting a variety of compounds as the index of quality control and using an effective method to simultaneously detect them may be a wise choice. To our knowledge, no method is available for the parallel quantification of 11 components in YZH preparations and their herbs by HPLC.
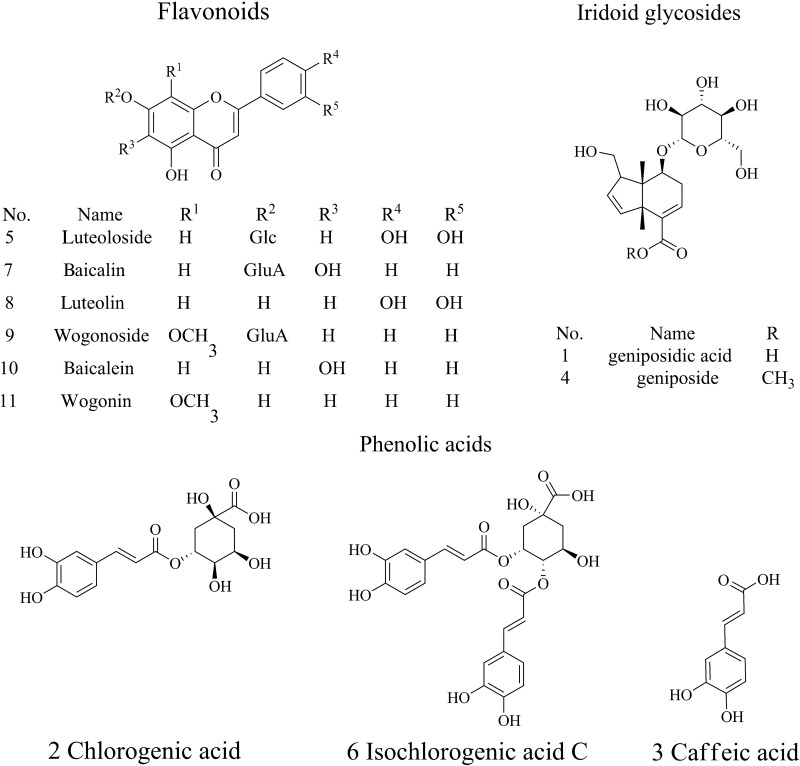
In this study, a rapid and reliable HPLC coupled with diode array detector (DAD) method was developed to analyze 11 compounds, namely geniposidic acid, chlorogenic acid, caffeic acid, geniposide, luteoloside, isochlorogenic acid C, baicalin, luteolin, wogonoside, baicalein and wogonin (Figure 1). Chromatographic peaks of selected markers were confirmed by electrospray ionization tandem mass spectrometry (ESI-MS-MS). Subsequently, validated method was performed for the quantitative assessment of YZH granule, capsule, injection and oral liquid. In addition, the origin and amounts of 11 markers in four constituent herbs (Herba Artemisiae Scopariae, Fructus gardeniae, Flos Lonicerae and Radix Scutellariae) of YZH preparations were studied. The proposed method enables both qualitative and quantitative analyses and could be developed as a new, efficient tool for the quality evaluation of YZH preparations and their constituent herbs.
Figure 1.
Structures of the 11 compounds.
Experimental
Chemicals, reagents and materials
Reference standards including geniposidic acid, chlorogenic acid, caffeic acid, geniposide, luteoloside, isochlorogenic acid C, baicalin, luteolin, wogonoside, baicalein and wogonin were all obtained from National Institute for the Control of Pharmaceutical and Biological Products (NICPP, Beijing, China). HPLC-grade methanol and acetonitrile, and MS-grade formic acid were purchased from Fisher Scientific (Waltham, MA, USA). Acetic acid and other reagents were of analytical grade. Deionized (18 MΩ/cm) water was generated in-house using a Milli-Q water purification system from Millipore (Bedford, MA, USA). Commercial products of YZH were separately purchased from four pharmaceutical companies in China. The detailed information of samples was summarized in Table I. YZH granule (Lot No. 1009746) was selected as the sample for the development and validation of chromatographic conditions. Four herbs including Radix Scutellariae, Flos Lonicerae, Herba Artemisiae Scopariae and Fructus gardeniae were all collected from Xuzhou Pharmaceutical Co. Ltd and authenticated by Professor Wang from the Department of Pharmacognosy, Xuzhou Medical College (Xuzhou, China).
Table I.
Summary of the Tested YZH Commercial Products
| Preparations | Manufacturers | Lot No. |
|---|---|---|
| Granule | Lunan Pharmaceutical Group Co., Linyi, China | 1009746 |
| Capsule | Biokin Pharmaceutical Co., Ltd, Chengdu, China | 110502 |
| Oral liquid | Beijing Shuanghe Gaoke Natural Drug Co., Ltd, Beijing, China | 272112 |
| Injection | Shineway Pharmaceutical Co., Ltd, Shijiazhuang, China | 10101231 |
Chromatographic system and MS
Quantitative analysis was carried out on a Shimadzu LC-20A Series system (Shimadzu, Kyoto, Japan), consisted of LC-20AD pump, DGU-20A5 degasser, SIL-20A autosampler, CTO-20AC column oven and SPD-M20A diode array detector (DAD). Identification of standard solution was achieved on Agilent LC–MS-MS system (Agilent Technologies, USA), consisted of G1367E autosampler, G1312B pumps and a 6460 triple quadrupole mass spectrometer equipped with an ESI source. Chromatography was performed on an Agilent Zorbax SB-C18 (4.6 mm × 250 mm, 5 μm). The mobile phase was composed of acetonitrile (A) and water with 0.1% formic acid (B) in a gradient elution mode. The elution program was optimized and conducted as follows: 0–10 min, 7–10% A; 10–14 min, 10% A; 14–23 min, 10–19% A; 23–34 min, 19–24% A; 34–52 min, 24–35% A; 52–70 min, 35–48% A; 70–75 min, 48–90% A; 75–95 min, 90% A. The flow rate was 1.0 mL/min. The DAD spectra were recorded between 190 and 400 nm. In the quantitative analysis, wavelengths were set at 238, 280 or 350 nm according to the absorption properties of the analyzed compounds. The column temperature was kept at 30°C and the sample injection volume was 20 μL.
For MS analysis, the conditions of ESI source were as follows: source voltage, 3,500 V; drying gas (N2) flow rate, 11.0 L/min; drying gas temperature, 350°C; nebulizer, 15 psi. The MS data were acquired from m/z 100–1,000 in negative- or positive-ion modes.
Preparation of standard solutions
Stock solutions were prepared from the above-mentioned standard chemicals by dissolving their appropriate amounts in methanol and stored at 4°C before use. Working standards at the concentration of the calibration range were prepared by stepwise dilution of their stock solutions with acetonitrile–water (50 : 50, v/v). Before analysis, they were filtered through a 0.45-μm membrane filter.
Pretreatment of YZH preparations and their herbs
Before pretreatment, YZH granule was grounded into powder and YZH capsule content was taken out. 0.5 g powder, 0.024 g capsule's content, 3.6 mL oral liquid and 1.8 mL injection were soaked in methanol–water (50 : 50, v/v) in a volumetric flask of 50 mL and then pretreated in an ultrasonic water bath for 30 min, respectively. Every supernatant was filtered through a 0.45-μm membrane filter and 20 μL of each solution was injected into HPLC system.
Four herbs of Radix Scutellariae, Flos Lonicerae, Herba Artemisiae Scopariae and Fructus gardeniae were treated according to the prescription and preparation protocol of YZH formula recorded by Ch. P. (2010) to get their extract (12). The extract was dissolved in methanol–water (50 : 50, v/v) with the help of ultrasonic wave for 30 min to get the solution at concentration of 0.16, 0.49, 7.5 and 0.17 mg/mL for Radix Scutellariae, Flos Lonicerae, Herba Artemisiae Scopariae and Fructus gardeniae, respectively.
Validation of the method
The linearity of the method was obtained through calculating the regression equation about the peak areas versus the corresponding concentrations of all standards. Each calibration curve chose at least seven appropriate concentrations for quantification in triplicate. The limit of detection (LOD) and limit of quantification (LOQ) were defined at the signal-to-noise ratio of 3 and 10, respectively. Intra- and interday variations were examined using low, medium and high concentrations of mixed standard solutions under the optimized conditions for five times on 1 day and repeated this experiment operating on three consecutive days. Relative standard deviation (RSD) of peak area for each compound was calculated and used to evaluate the precision of the method.
In order to evaluate the repeatability of the method, six independently prepared sample solutions of YZH granule were analyzed and the RSD values of 11 target compounds' contents were calculated to measure the repeatability of method. One of the solutions was tested at room temperature in triplicate at 0, 4, 8, 12 and 18 h, respectively. The RSD values of peak areas of 11 target compounds were taken as a measurement of stability.
Recovery test by the standard addition method was used to evaluate the accuracy of this method. It was determined by adding the standards with three different levels (high, middle and low) to YZH granule. Then the samples were extracted and analyzed with the proposed method, and triplicate experiments were performed at each level. The mean recoveries were estimated according to the following formula: recovery (%) = [(amount found − original amount)/amount spiked] × 100.
Results
Confirmation of target compounds in YZH preparations
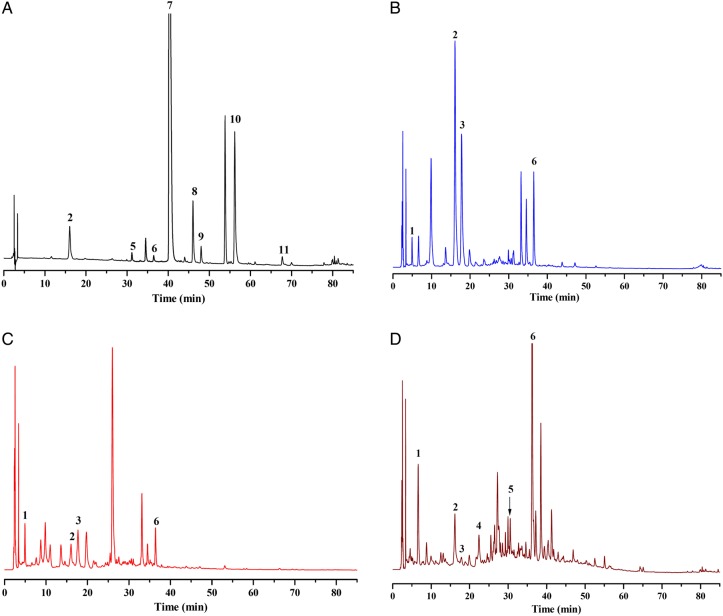
The quantification of constituents in YZH preparations was achieved at 238, 280 or 350 nm (geniposidic acid and geniposide at 238 nm; baicalin, wogonoside, baicalein and wogonin at 280 nm; chlorogenic acid, isochlorogenic acid C, caffeic acid, luteolin and luteoloside at 350 nm), where the UV spectra of the 11 analytes exhibited maximum absorbance, in which better response and less interference could be accomplished. The chromatograms of standards mixture and YZH granule were shown in Figure 2, in which peaks from 1 to 11 were corresponded to geniposidic acid, chlorogenic acid, caffeic acid, geniposide, luteoloside, isochlorogenic acid C, baicalin, luteolin, wogonoside, baicalein and wogonin, respectively. It was obvious that there were no interference for determination of the 11 compounds and the specificity was also verified by MS spectra.
Figure 2.
Typical HPLC chromatograms of standard compound (A) and YZH granule (B) and the amplified chromatogram of YZH granule from 0 to 39 min and from 42 to 80 min (B′). (1) Geniposidic acid, (2) chlorogenic acid, (3) caffeic acid, (4) geniposide, (5) luteoloside, (6) isochlorogenic acid C, (7) baicalin, (8) luteolin, (9) wogonoside, (10) baicalein and (11) wogonin. This figure is available in black and white in print and in color at JCS online.
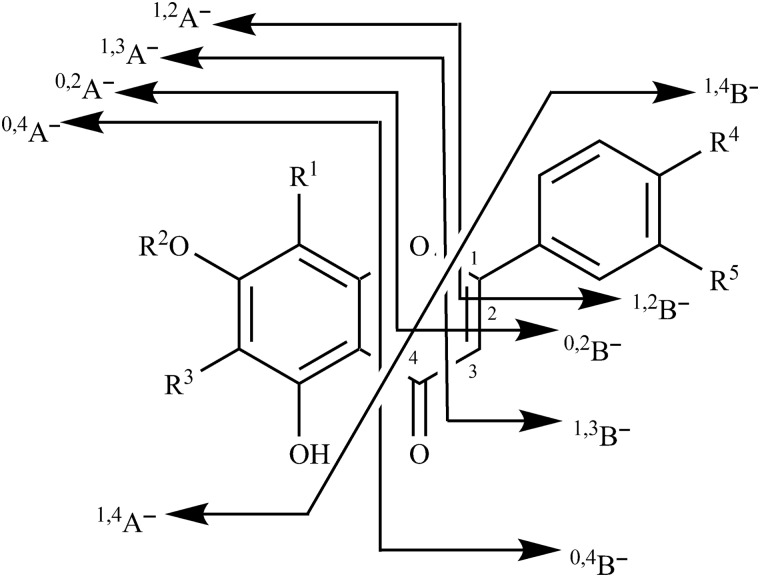
LC–MS-MS with ESI source was used to identify the 11 chemical constituents. Different scan mode was selected for MS analysis according to the number of peaks in positive or negative mode. MS parameters such as source voltage, drying gas flow rate and drying gas temperature were optimized, and the total ion current chromatogram was acquired. Figure 3 shows the various retrocyclization fragments observed in this study: the superscripts on the left of the A or B ring indicate the bonds that have been broken. These fragments can undergo further fragmentation. Table II lists the retention times (tR) and MS data of target compounds in YZH preparations. The mass spectra of those compounds were matched with those obtained from the pure standards for each of the compounds. According to the m/z values and retention features, the 11 compounds were identified and confirmed.
Figure 3.
Nomenclature adopted for the different retrocyclization cleavages observed in this study.
Table II.
Identification of the 11 Target Compounds by LC–ESI-MS
| Compounds | Retention time (min) | Molecular weight | Parent ion (m/z) | Scan mode | Fragmentor (V) | Collision energy (V) | Product ion (m/z) |
|---|---|---|---|---|---|---|---|
| Geniposidic acid | 6.4 | 374 | 373 | − | 115 | 15 | 210.9 [M−H-GlcR]−; 166.7 [M−H-GlcR-CO2]−; 149.0 [M−H-Glc-COOH]− 122.9 [M−H-GlcR-CO2-CH3CHO]− |
| Chlorogenic acid | 15.9 | 354 | 353 | − | 105 | 8 | 190.9 [quinic acid-H]− |
| Caffeic acid | 17.6 | 180 | 179 | − | 100 | 15 | 135 [M−H-CO2]− |
| Geniposide | 22.2 | 404 | 411 | + | 162 | 31 | 249.3 [M+Na-GlcR]+; 231.0 [M+Na-Glc]+; 216.9 [M+Na-GlcR-CH3OH]+ 202.8 [Glc+Na]+ |
| Luteoloside | 33.1 | 448 | 447 | − | 228 | 26 | 284.9 [M−H-GlcR]−; 174.9 [M−H-GlcR-catechol]−; 151.1 [1,3A−]; 132.9 [1,3B−] |
| Isochlorogenic acid C | 36.4 | 516 | 515 | − | 130 | 18 | 352.9 [M−H-Caffeoyl]−; 190.7 [quinic acid-H]−; 178.8 [caffeoyl-H]− 172.8 [quinic acid-H-H2O]−; 134.7 [caffeoyl-H-CO2]− |
| Baicalin | 40.1 | 446 | 445 | − | 118 | 8 | 269.0 [M−H-GluAR]−; 174.7 [GluAR-H]−; 112.7 [GluAR-H-CO2-H2O]− |
| Luteolin | 45.8 | 286 | 285 | − | 180 | 34 | 174.9 [M−H-catechol]−; 132.9 [1,3B−]; 131.6 [1,3B−-H] |
| Wogonoside | 47.8 | 460 | 459 | − | 115 | 10 | 282.8 [M−H-GluAR]−; 267.9 [M−H-GluAR-CH3]−; 238.8 [M−H-GluA-CO]− 113.0 [GluA-H2O-H-CO2-H2O]− |
| Baicalein | 56.1 | 270 | 269 | − | 165 | 31 | 222.7 [M−H-H2O-CO]−; 166.8 [1,3A−]; 138.7 [1,4A−] |
| Wogonin | 67.6 | 284 | 283 | − | 115 | 15 | 268.0 [M−H-CH3]−; 162.8 [0,2A−-H2O] |
GlcR, glucose residue; GluAR, glucuronic acid residues.
Method validation of quantitative analysis
Good linearity (r > 0.9991) for all standards was observed within the investigated concentration ranges. The range of LOD and LOQ for all compounds was, respectively, from 0.00030 to 0.045 μg and 0.0010 to 0.05 μg. Detailed information were listed in Table III. Total intra- and interday variations (RSD) for evaluating precisions were from 0.05 to 1.3% (Table IV). The RSD values of 11 target compounds' contents for the repeatability evaluation were fluctuated in the range from 0.45 to 1.26%. The RSD values of peak area for the stability assessment were lower than 2.5% for all compounds. The mean recovery of the 11 compounds was in the range of 98.1–102.2% and their RSD values were <1.6% (Table V).
Table III.
Regression Equations, Correlation Coefficients, Linear Ranges and LOD and LOQ of 11 Target Compounds
| Compounds | λ (nm) | Linear range (μg) | Regression equations | Correlation coefficient (r) | LOD (μg) | LOQ (μg) |
|---|---|---|---|---|---|---|
| Geniposidic acid | 238 | 0.088–8.8 | y = 1.54 × 106x − 7.20 × 104 | 0.9992 | 0.018 | 0.040 |
| Chlorogenic acid | 350 | 0.1–10 | y = 1.76 × 106x − 1.35 × 105 | 0.9996 | 0.015 | 0.05 |
| Caffeic acid | 350 | 0.004–0.4 | y = 7.64 × 106x − 9.77 × 103 | 0.9998 | 0.0054 | 0.0018 |
| Geniposide | 238 | 0.013–1.3 | y = 1.39 × 106x + 1.69 × 105 | 0.9992 | 0.0017 | 0.0057 |
| Luteoloside | 350 | 0.0055–0.55 | y = 2.47 × 106x − 3.19 × 102 | 0.9999 | 0.00084 | 0.0028 |
| Isochlorogenic acid C | 350 | 0.019–1.9 | y = 1.67 × 106x − 2.96 × 103 | 0.9997 | 0.0027 | 0.0088 |
| Baicalin | 280 | 0.15–21 | y = 2.66 × 106x − 1.79 × 106 | 0.9994 | 0.045 | 0.015 |
| Luteolin | 350 | 0.010–1.0 | y = 3.54 × 106x − 2.53 × 103 | 0.9997 | 0.0015 | 0.0051 |
| Wogonoside | 280 | 0.0069–0.69 | y = 1.56 × 106x − 6.91 × 103 | 0.9991 | 0.0010 | 0.0034 |
| Baicalein | 280 | 0.0084–0.84 | y = 4.70 × 106x − 6.80 × 104 | 0.9993 | 0.00090 | 0.0030 |
| Wogonin | 280 | 0.0022–0.22 | y = 6.06 × 106x − 1.15 × 104 | 0.9995 | 0.00030 | 0.0010 |
Table IV.
Intraday and Interday Precision of 11 Compounds
| Compound | Nominal concentration (μg) | Intraday (n = 6) |
Interday (n = 3) |
||||
|---|---|---|---|---|---|---|---|
| Found (μg) | RSD (%) | Accuracy (%) | Found (μg) | RSD (%) | Accuracy (%) | ||
| Geniposidic acid | 0.100 | 0.098 | 1.0 | 98.0 | 0.098 | 1.1 | 98.0 |
| 4.00 | 4.1 | 0.79 | 102.5 | 4.12 | 0.60 | 103.0 | |
| 8.00 | 7.94 | 0.31 | 99.2 | 7.98 | 0.40 | 99.8 | |
| Chlorogenic acid | 0.200 | 0.203 | 0.80 | 101.5 | 0.203 | 1.1 | 101.5 |
| 2.00 | 2.052 | 0.32 | 102.6 | 2.058 | 0.86 | 102.9 | |
| 3.50 | 3.476 | 0.07 | 99.3 | 3.481 | 0.11 | 99.4 | |
| Caffeic acid | 0.005 | 0.00495 | 1.3 | 99.0 | 0.00506 | 1.3 | 101.2 |
| 0.200 | 0.195 | 0.66 | 97.5 | 0.199 | 0.34 | 99.5 | |
| 0.360 | 0.362 | 0.58 | 100.6 | 0.362 | 0.19 | 100.6 | |
| Geniposide | 0.010 | 0.0102 | 0.94 | 102 | 0.0104 | 0.95 | 104.0 |
| 0.800 | 0.785 | 0.53 | 98.1 | 0.788 | 0.55 | 98.5 | |
| 1.20 | 1.208 | 0.14 | 100.7 | 1.21 | 0.16 | 100.8 | |
| Luteoloside | 0.008 | 0.00794 | 1.1 | 99.2 | 0.00805 | 1.2 | 100.6 |
| 0.300 | 0.306 | 0.86 | 102 | 0.31 | 0.63 | 103.3 | |
| 0.500 | 0.498 | 0.75 | 99.6 | 0.505 | 0.21 | 101.0 | |
| Isochlorogenic acid C | 0.250 | 0.254 | 0.87 | 101.6 | 0.258 | 1.2 | 103.2 |
| 1.00 | 1.012 | 0.76 | 101.2 | 1.02 | 0.60 | 102.0 | |
| 1.80 | 1.781 | 0.32 | 98.9 | 1.79 | 0.29 | 99.4 | |
| Baicalin | 0.200 | 0.195 | 1.40 | 97.5 | 0.199 | 1.1 | 99.5 |
| 10.0 | 9.87 | 0.90 | 98.7 | 10.05 | 0.36 | 100.5 | |
| 20.0 | 20.13 | 0.18 | 100.7 | 20.18 | 0.32 | 100.9 | |
| Luteolin | 0.020 | 0.0205 | 1.04 | 102.5 | 0.0204 | 1.2 | 102.0 |
| 0.500 | 0.497 | 0.84 | 99.4 | 0.498 | 1.0 | 99.6 | |
| 0.900 | 0.876 | 0.48 | 97.3 | 0.906 | 0.42 | 100.7 | |
| Wogonoside | 0.100 | 0.102 | 0.85 | 102.0 | 0.102 | 1.2 | 102.0 |
| 0.400 | 0.397 | 0.29 | 99.2 | 0.396 | 0.49 | 99.0 | |
| 0.600 | 0.593 | 0.05 | 98.8 | 0.598 | 0.06 | 99.7 | |
| Baicalein | 0.010 | 0.0102 | 1.2 | 102.0 | 0.0102 | 0.61 | 102.0 |
| 0.400 | 0.387 | 0.74 | 96.8 | 0.387 | 0.54 | 96.8 | |
| 0.800 | 0.792 | 0.26 | 99.0 | 0.792 | 0.24 | 99.0 | |
| Wogonin | 0.004 | 0.00397 | 1.1 | 99.2 | 0.00397 | 0.80 | 99.2 |
| 0.100 | 0.103 | 0.44 | 103.0 | 0.103 | 0.45 | 103.0 | |
| 0.200 | 0.207 | 0.26 | 103.5 | 0.207 | 0.30 | 103.5 | |
Table V.
Recovery of 11 Compounds (n = 3)
| Compound | Original (μg) | Spiked (μg) | Found (μg) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Geniposidic acid | 0.7861 | 0.6300 | 1.4189 | 100.2 | 0.6 |
| 0.7875 | 1.5689 | 99.7 | |||
| 0.9450 | 1.7467 | 100.9 | |||
| Chlorogenic acid | 0.4171 | 0.3400 | 0.7677 | 101.4 | 1.1 |
| 0.4200 | 0.8321 | 99.4 | |||
| 0.5000 | 0.9153 | 99.8 | |||
| Caffeic acid | 0.0227 | 0.0180 | 0.0402 | 98.8 | 0.3 |
| 0.0216 | 0.0435 | 98.2 | |||
| 0.0270 | 0.0491 | 98.8 | |||
| Geniposide | 0.0774 | 0.0612 | 0.1396 | 100.7 | 1.4 |
| 0.0765 | 0.1573 | 102.2 | |||
| 0.0918 | 0.1680 | 99.3 | |||
| Luteoloside | 0.0394 | 0.0330 | 0.0710 | 98.1 | 0.8 |
| 0.0385 | 0.0776 | 99.6 | |||
| 0.0473 | 0.0851 | 98.2 | |||
| Isochlorogenic acid C | 0.1038 | 0.0841 | 0.1889 | 100.5 | 1.4 |
| 0.1032 | 0.2085 | 100.7 | |||
| 0.1262 | 0.2258 | 98.2 | |||
| Baicalin | 4.2310 | 3.3696 | 7.5094 | 98.8 | 1.4 |
| 4.2432 | 8.4657 | 99.9 | |||
| 5.0752 | 9.4551 | 101.6 | |||
| Luteolin | 0.0466 | 0.0367 | 0.0834 | 100.1 | 1.1 |
| 0.0449 | 0.0898 | 98.1 | |||
| 0.0571 | 0.1035 | 99.8 | |||
| Wogonoside | 0.0397 | 0.0317 | 0.0719 | 100.7 | 1.2 |
| 0.0400 | 0.0787 | 98.7 | |||
| 0.0469 | 0.0852 | 98.4 | |||
| Baicalein | 0.0384 | 0.0302 | 0.0691 | 100.7 | 0.3 |
| 0.0386 | 0.0780 | 101.3 | |||
| 0.0454 | 0.0848 | 101.2 | |||
| Wogonin | 0.0176 | 0.0144 | 0.0315 | 98.4 | 1.6 |
| 0.0180 | 0.0351 | 98.6 | |||
| 0.0216 | 0.0397 | 101.3 |
Simultaneous quantification of 11 compounds in YZH preparation
Samples of four kinds of preparations including YZH granule, capsule, oral liquid and injection obtained from different pharmaceutical companies in China were prepared using the proposed method. An aliquot of 20 μL filtrate was injected into the HPLC system. Each sample was analyzed in triplicate to determine the mean content. The content of each analyte was calculated from the corresponding calibration curve. The typical chromatograms were shown in Figure 4 and the contents of 11 compounds were listed in Table VI.
Figure 4.
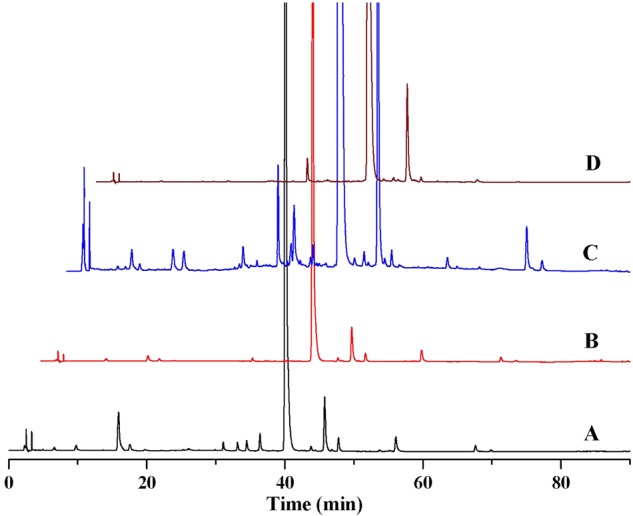
The chromatograms of YZH granule (A), capsule (B), oral liquid (C) and injection (D). This figure is available in black and white in print and in color at JCS online.
Table VI.
Content of 11 Target Compounds in Different YZH Preparations (n = 3)
| Compounds | Granule (mg/g) | Capsule (mg/g) | Injection (mg/mL) | Oral liquid (mg/mL) |
|---|---|---|---|---|
| Geniposidic acid | 15.72 | 6.433 | 0.08408 | 0.05219 |
| Chlorogenic acid | 8.342 | 14.22 | 0.1124 | 0.2268 |
| Caffeic acid | 0.4510 | 0.7639 | 0.00305 | 0.03808 |
| Geniposide | 1.541 | 3.972 | 0.03505 | 1.290 |
| Luteoloside | 0.7890 | 1.898 | 0.1769 | 0.4125 |
| Isochlorogenic acid C | 2.075 | 1.245 | 0.0086 | 0.1507 |
| Baicalin | 84.63 | 485.36 | 31.00 | 30.21 |
| Luteolin | 0.9280 | 5.101 | 0.1936 | 0.3911 |
| Wogonoside | 0.7850 | 9.833 | 0.07559 | 0.1242 |
| Baicalein | 0.7620 | 7.428 | 0.03987 | 0.04442 |
| Wogonin | 0.3520 | 1.925 | 0.01513 | 0.1120 |
Results showed that contents of all the 11 compounds in YZH capsule and granule were higher than those in oral and injection. Among them, baicalin had the highest content in all four preparations and even reached to 500 mg/g in YZH capsule. But the second was geniposidic acid, chlorogenic acid, luteolin and geniposide in YZH granule, capsule, oral liquid and injection, respectively. It indicated that different preparation should use different marker compounds for its quality control, even though their herbs were extracted with the same method.
The source of 11 compounds and their content in herbs
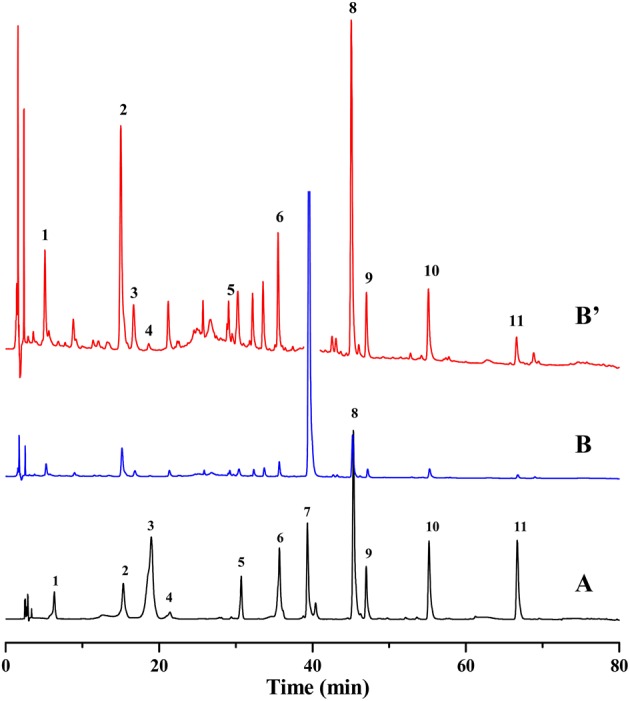
For controlling the quality of YZH preparations more efficiently, the herbs used in YZH preparations including Radix Scutellariae, Flos Lonicerae, Herba Artemisiae Scopariae and Fructus gardeniae were extracted according to the Ch. P. (2010) and analyzed by this proposed method. The chromatogram showed in Figure 5 and contents listed in Table VII exhibited that some components do not necessarily belong to one herb. For example, chlorogenic acid and isochlorogenic acid C could be detected in the four herbs, while geniposidic acid and caffeic acid were found in Flos Lonicerae, Herba Artemisiae Scopariae and Fructus gardeniae. Most of the tested compounds could be detected in Radix Scutellariae including chlorogenic acid, luteoloside, isochlorogenic acid C, baicalin, baicalein and wogonin. Comparing four herbs and YZH preparations, the total contents of 11 compounds were not consistent. It might result from some unknown chemical reaction existing in pharmaceutical process. It was just the fascination of Chinese drug that exhibited the synergistic effect not a simple mixing of herbs. So, strictly controlling the quality of preparations as well as the herbs was necessary. In the present work, the qualitative and quantitative analyses of different YZH preparations and their constituent herbs could be achieved in the same system, which undoubtedly decreased the analysis procedures and saved human and physical resources.
Figure 5.
Chromatograms of Radix Scutellariae (A), Flos Lonicerae (B), Herba Artemisiae Scopariae (C) and Fructus gardenia (D). (1) Geniposidic acid; (2) chlorogenic acid; (3) caffeic acid; (4) geniposide; (5) luteoloside; (6) isochlorogenic acid C; (7) baicalin; (8) luteolin; (9) wogonoside; (10) baicalein and (11) wogonin. This figure is available in black and white in print and in color at JCS online.
Table VII.
Contents of 11 Target Compounds in Four Herbs (mg/g)
| Compounds | Radix Scutellariae | Flos Lonicerae | Herba Artemisiae Scopariae | Fructus gardeniae |
|---|---|---|---|---|
| Geniposidic acid | – | 19.29 | 1.067 | 34.62 |
| Chlorogenic acid | 43.37 | 198.8 | 11.79 | 15.71 |
| Caffeic acid | – | 25.16 | 4.163 | 13.97 |
| Geniposide | – | – | – | 330.4 |
| Luteoloside | 3.132 | – | – | 15.34 |
| Isochlorogenic acid C | 3.572 | 66.74 | 5.920 | 15.69 |
| Baicalin | 844.1 | – | – | – |
| Luteolin | 4.296 | – | – | – |
| Wogonoside | 9.587 | – | – | – |
| Baicalein | 33.12 | – | – | – |
| Wogonin | 1.962 | – | – | – |
Discussion
In order to acquire a good separation, different columns packed with different materials, different mobile phases, elution modes and column temperature, different extraction solvents were all tested.
Four kinds of columns including Sepax GP-C18 (250 mm × 4.6 mm i.d., 5 μm), Agilent Zorbax SB-C18 (250 mm × 4.6 mm i.d., 5 μm), Aglient Zorbax Eclipse Plus C18 (250 mm × 4.6 mm i.d., 5 μm) and Kromasil C18 (250 mm × 4.6 mm i.d., 5 μm) were tested. All four types of column exhibited similar chromatography behavior and the backpressure were all in the range of 12.8–14.6 MPa. Agilent Zorbax SB-C18 was selected because it exhibited relatively shorter retention time than other columns.
Different mobile phase compositions including methanol–water or acetonitrile–water containing different concentrations of formic acid, acetic acid or phosphoric acid were studied. As a result, the optimal mobile phase system consisted of acetonitrile and water containing 0.1% formic acid was selected because it had greater baseline stability in chromatographic separation and ionization efficiency in MS analysis.
From results of comparative study of column temperature of 25, 30, 35 and 40°C, the column temperature was kept as 30°C considering the separation efficiency.
In order to achieve the optimum extraction efficiency, the main conditions such as solvent, solvent volume and extraction time were optimized. Pure and aqueous methanol or ethanol solutions were investigated. The best solvent was found to be 50% methanol that allowed efficient extraction of the compounds.
Conclusion
Simple, reliable and accurate HPLC-DAD and LC–MS-MS methods for the simultaneous quantitative and qualitative analyses of geniposidic acid, chlorogenic acid, caffeic acid, geniposide, luteoloside, isochlorogenic acid C, baicalin, luteolin, wogonoside, baicalein and wogonin were developed and validated for the quality control of YZH granule, capsule, injection and oral liquid, as well as their constituent herbs of Radix Scutellariae, Flos Lonicerae, Herba Artemisiae Scopariae and Fructus gardeniae. The method has been proved with good linearity, precision, repeatability, stability and recovery. Our results have demonstrated that the proposed method is a powerful and meaningful tool to comprehensively conduct the quality control of traditional Chinese medicines.
Funding
The authors acknowledge support for this work by the National Science Foundation of China (81201696), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, Jiangsu Province Student Innovation Project (2012JSSPITP1831, 201310313004Z), the National Student Innovation Project (201210313022, 201310313004), Qing Lan Project of the Higher Education Institutions of Jiangsu Province and Zhenxing Project of Xuzhou Medical College.
Conflict of interest statement. None declared.
References
- 1.Chen S.P., Tian L.L., Liu F.L.; Clinical observation of Yinzhihuang oral liquid on prevention of the premature infantile jaundice; Chinese Journal of Integrative Medicine, (2009); 15: 299–302. [DOI] [PubMed] [Google Scholar]
- 2.Chen X., Krakauer T., Oppenheim J.J., Howard Z.; Yin Zhi Huang, an injectable multicomponent Chinese herbal medicine, is a potent inhibitor of T-cell activation; Journal of Alternative and Complementary Medicine, (2004); 10: 519–526. [DOI] [PubMed] [Google Scholar]
- 3.Chen H., Chen X., Han Q., Wu J., Tang D.Q., Du Q. et al. A new strategy for quality control and qualitative analysis of Yinhuang preparations by HPLC-DAD-MS/MS; Analytical and Bioanalytical Chemistry, (2012); 404: 1851–1865. [DOI] [PubMed] [Google Scholar]
- 4.Liao H.J., Lai Z.Q., Su J.Y., Yi Y.Y., Li Y.C., Lai X.P. et al. Fingerprinting and simultaneous determination of alkaloids in Picrasma quassioides from different locations by high performance liquid chromatography with photodiode array detection; Journal of Separation Science, (2012); 35: 2193–2202. [DOI] [PubMed] [Google Scholar]
- 5.Yang D.Z., An Y.Q., Jiang X.L., Tang D.Q., Gao Y.Y., Zhao H.T. et al. Development of a novel method combining HPLC fingerprint and multi-ingredients quantitative analysis for quality evaluation of traditional Chinese medicine preparation; Talanta, (2011); 85: 885–890. [DOI] [PubMed] [Google Scholar]
- 6.Ren Y.S., Zhang P., Yan D., Yan Y., Chen L.H., Qiu L.L. et al. Application of microcalorimetry of Escherichia coli growth and discriminant analysis to the quality assessment of a Chinese herbal injection (Yinzhihuang); Acta Pharmaceutica Sinica B, (2012); 2: 278–285. [Google Scholar]
- 7.Ren Y.S., Zhang P., Yan D., Wang J.B., Du X.X., Xiao X.H.; A strategy for the detection of quality fluctuation of a Chinese herbal injection based on chemical fingerprinting combined with biological fingerprinting; Journal of Pharmaceutical and Biomedical Analysis, (2011); 56: 436–442. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J.H., Shang H.C., Zheng W.K., Hu J., Xu H.J., Wang H. et al. Systematic review on the compatibility of Shuanghuanglian injection combined with western medical injections; Journal of Evidence-Based Medicine, (2010); 3: 27–36. [DOI] [PubMed] [Google Scholar]
- 9.Li B.Q., Dong X., Yang G.Q., Fang S.H., Gao J.Y., Zhang J.X. et al. Role of chlorogenic acid in the toxicity induced by Chinese herbal injections; Drug and Chemical Toxicology, (2010); 33: 415–420. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q.Y., Song Q.; HPLC determination of geniposide in Yinzhihuang soft capsules; Chinese Journal of Pharmaceutical Analysis, (2009); 3: 470–471. [Google Scholar]
- 11.Chen Q., Chen S.H., Xu X.F.; Determination of baicalin in Yinzhihuang injection by HPLC; Journal of Guangdong Pharmaceutical University, (2005); 21: 146–147. [Google Scholar]
- 12.National Pharmacopoeia Committee. Chinese pharmacopoeia. China Medico-Pharmaceutical Sciences and Technology Publishing House, Beijing, China, (2010), pp. 871–872. [Google Scholar]
- 13.Duan B.Z., Huang L.F., Chen S.L.; Chemical fingerprint analysis of Fritillaria delavayi Franch. by high-performance liquid chromatography; Journal of Separation Science, (2012); 35: 513–518. [DOI] [PubMed] [Google Scholar]
- 14.Xie P.S., Chen S.B., Liang Y.Z., Wang X.H., Tian R.T., Upton R.; Chromatographic fingerprint analysis-a rational approach for quality assessment of traditional Chinese herbal medicine; Journal of Chromatography A, (2006); 1112: 171–180. [DOI] [PubMed] [Google Scholar]
- 15.Liu E.H., Qi L.W., Li K., Chu C., Li P.; Recent advances in quality control of traditional Chinese medicines; Combinatorial Chemistry & High Throughput Screening, (2010); 13: 869–884. [DOI] [PubMed] [Google Scholar]
- 16.Wang S., Fu P., Liu L., Wang L., Peng C., Zhang W. et al. Simultaneous determination of fifteen constituents of jitai tablet using ultra high-performance liquid chromatography coupled with triple quadrupole electrospray tandem mass spectrometry; Molecules, (2014); 19: 1635–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong Y., Xiao X., Yang X., Yan D., Zhang C., Zou H. et al. Quality control of Lonicera japonica stored for different months by electronic nose; Journal of Pharmaceutical and Biomedical Analysis, (2014); 91: 68–72. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y., Cao C., Ji J.Y., Cong X.D., Wang S.B., Cai B.C.; Simultaneous chemical fingerprinting and quantitative analysis of crude and processed Radix Scrophulariae from different locations in China by HPLC; Journal of Separation Science, (2011); 34: 1429–1436. [DOI] [PubMed] [Google Scholar]