Abstract
Palm oil is the richest source of natural carotenes, comprising 500–700 ppm in crude palm oil (CPO). Its concentration is found to be much higher in oil extracted from palm-pressed fiber, a by-product from the milling of oil palm fruits. There are 11 types of carotenes in palm oil, excluding the cis/trans isomers of some of the carotenes. Qualitative separation of these individual carotenes is particularly useful for the identification and confirmation of different types of oil as the carotenes profile is unique to each type of vegetable oil. Previous studies on HPLC separation of the individual palm carotenes reported a total analyses time of up to 100 min using C30 stationary phase. In this study, the separation was completed in <5 min. The qualitative separation was successfully carried out using a commonly used stationary phase, C18.
Introduction
Oil palm is the largest source of natural carotenes (1). There are 500–700 ppm of carotenes in crude palm oil (CPO) and 4,000–6,000 ppm in the oil obtained from the palm-pressed fiber, a by-product from the oil palm fruits milling (1–7). The individual carotenes in both CPO and PFO comprise 11 types (2, 3, 8, 9) (Table I). These carotenes, however, comprise different composition in the CPO and PFO (3). Palm oil can be distinguished from other types of oil by reviewing its carotenes content.
Table I.
Composition of Carotenes in CPO and PFO
| Carotenes | CPO (%) | PFO (%) |
|---|---|---|
| Phytoene | 1.27 | 11.87 |
| Phytofluene | 0.06 | 0.40 |
| β-Carotene | 56.02 | 30.95 |
| α-Carotene | 35.06 | 19.45 |
| cis-α-Carotene | 2.49 | 1.17 |
| ξ-Carotene | 0.69 | 7.56 |
| γ-Carotene | 0.33 | 2.70 |
| δ-Carotene | 0.83 | 6.94 |
| Neurosporene | 0.29 | 3.38 |
| β-Zeacarotene | 0.74 | 0.37 |
| α-Zeacarotene | 0.23 | trace |
| Lycopene | 1.30 | 14.13 |
Separation of the individual carotenes in palm oil is based on structural differences, such as the conjugation of double bonds and end groups resulting in differences in polarity. Analysis of the individual carotenes in palm oil is a challenge as each of these individual carotenes absorbs UV at different wavelength and thus has different λmax (4, 10–13) (Table II). In addition, not every type of these individual carotene is available in the form of standard reference material. The qualitative analysis of the individual carotenes in palm oil, however, has been reported in the past (4, 6, 11). These analyses were carried out using high performance liquid chromatography (HPLC) (5, 6, 11, 13–16). Different stationary and mobile phases were in used in these reports. The similarity in all these methods, however, is that a photodiode array detector (PDA) is used. The PDA is the most suitable detector for the detection of carotenes as different wavelengths can be monitored simultaneously in a single injection (5, 9, 11–18).
Table II.
Maximum Absorption Wavelengths (λmax) of Palm Carotenes in Hexane
| Carotene | Yap, 1991 (λmax) |
Tay, 1999 (λmax) |
||||||
|---|---|---|---|---|---|---|---|---|
| Phytoene | 276 | 286 | 297 | 276 | 287 | 299 | ||
| Phytofluene | 331 | 347 | 366 | 330 | 343 | 360 | ||
| β-Carotene | 426 | 429 | 477 | 430 | 444 | 480 | ||
| α-Carotene | 420 | 440 | 471 | 425 | 444 | 475 | ||
| cis-α-Carotene | 330 | 415 | 438 | 470 | 330 | 415 | 438 | 470 |
| ζ-Carotene | 380 | 401 | 426 | 383 | 406 | 420 | ||
| γ-Carotene | 435 | 462 | 490 | 436 | 461 | 488 | ||
| Neurosporene | 416 | 438 | 468 | 416 | 448 | 467 | ||
| β-Zeacarotene | 404 | 426 | 452 | 404 | 426 | 452 | ||
| α-Zeacarotene | 398 | 420 | 448 | 401 | 424 | 449 | ||
| Lycopene trans- | 444 | 470 | 500 | 446 | 470 | 503 | ||
| cis- | 362 | 438 | 464 | 495 | 349 | 438 | 467 | 488 |
This paper reports on a new method for the qualitative analyses of carotenes in palm oil using ultra performance liquid chromatography (UPLC). This method offers a more efficient and time-saving analyses as opposed to the HPLC methods reported in the past.
Experimental
Instrumentation and reagents
CPO and palm-pressed fiber were obtained from POMTEC in Labu, Negri Sembilan.
Palm-pressed fiber oil (PFO) is obtained by soaking the fresh palm-pressed fiber overnight in hexane, followed by filtration. Excess solvent is removed by way of rotary evaporation.
Mobile phase: all solvents were of chromatographic grade and obtained from Merck (Darmstadt, Germany).
Waters UPLC with Acquity H class Quaternary Solvent Manager, Acquity Sample Manager-FTN and Acquity PDA ɛλ Detector were used for the qualitative analyses of palm carotenes. Column used was Acquity UPLC BEH C18 1.7 µm 2.1 × 50 mm.
Method
CPO was dissolved in acetonitrile to make into concentration of 2 mg/mL and injected into UPLC. Injection volume was 10 µL. Mobile phase was acetonitrile and dichloromethane at the ration (98.5:1.5). Flowrate was 0.6 mL/min.
Above procedure was repeated with PFO.
Results
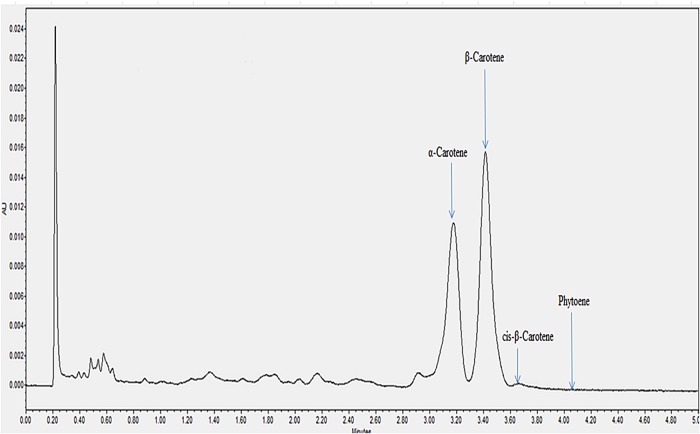
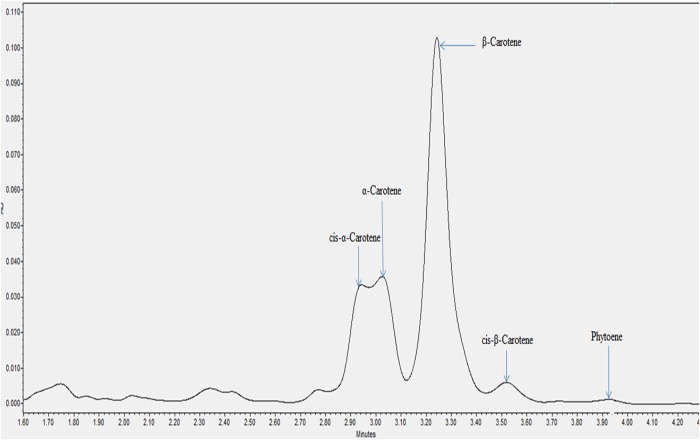
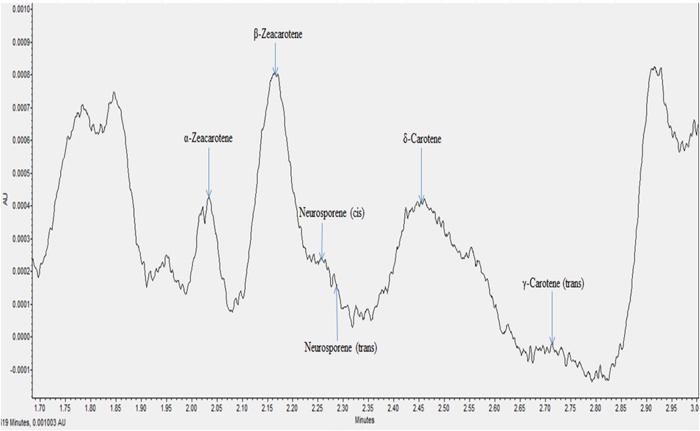
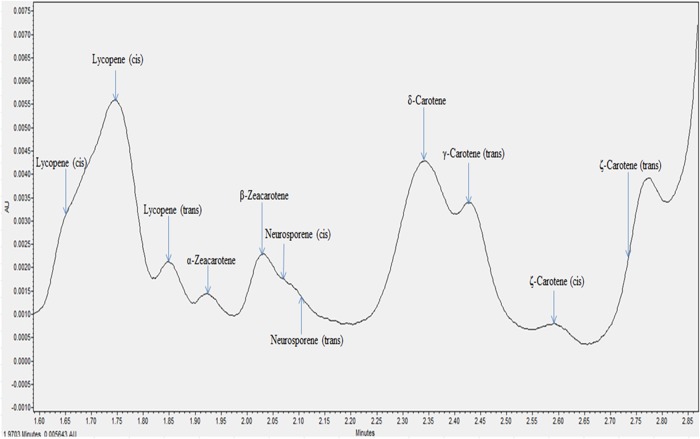
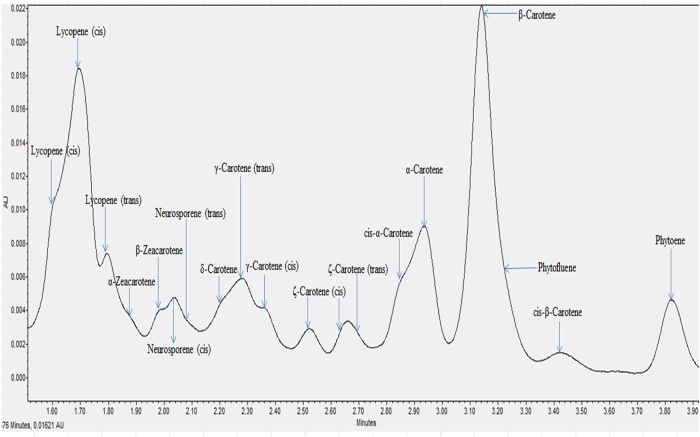
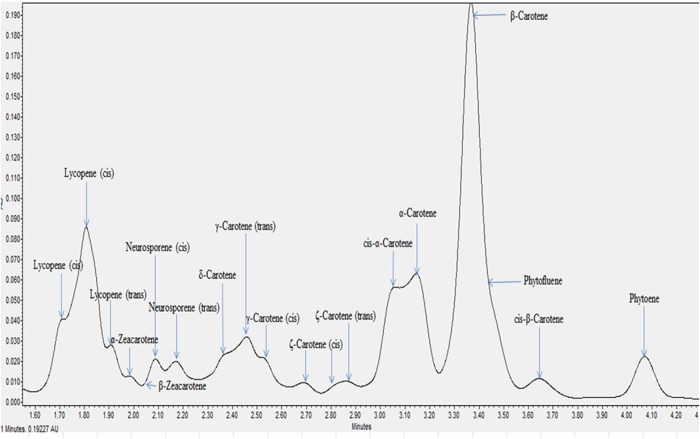
All the individual carotenes in palm oil were completely eluted in <5 min. Figures 1 and 2 depict the carotenes profile of CPO and unsaponifiable fraction of CPO. Figure 3 is an attenuation of Figure 1 between 0 and 2.85 min for the portrayal of the carotenes that are present in minute quantity compared with α- and β-carotene. Similarly, Figure 4 is an attenuation of Figure 2 of the same. Identification of the individual carotenes was carried out based on their λmax from previous study. The λmax value of each of the palm carotenes in this study when compared with previous literature is depicted in Tables III and IV. Specific assignment of the cis position was not carried out due to the unavailability of standards. Figures 5 and 6 depict the carotenes profile of PFO and unsaponifable fraction of PFO.
Figure 1.
Carotenes profile of CPO. This figure is available in black and white in print and in color at JCS online.
Figure 2.
Carotenes profile of unsaponifiable fraction of CPO. This figure is available in black and white in print and in color at JCS online.
Figure 3.
Magnified carotenes profile of CPO. This figure is available in black and white in print and in color at JCS online.
Figure 4.
Magnified carotenes profile of unsaponifiable fraction from CPO. This figure is available in black and white in print and in color at JCS online.
Table III.
Main Absorption Maxima (nm) of Carotenes in CPO and Saponified CPO
| Carotenoids | CPO |
Saponified CPO |
||||||
|---|---|---|---|---|---|---|---|---|
| Lycopene (cis) | — | 361 | 442 | 467 | 496 | |||
| Lycopene (cis) | — | 362 | 443 | 470 | 499 | |||
| Lycopene (trans) | — | 444 | 471 | 501 | ||||
| α-Zeacarotene | 398 | 421 | 449 | 398 | 421 | 449 | ||
| β-Zeacarotene | 404 | 430 | 453 | 403 | 427 | 450 | ||
| Neurosporene (cis) | 331 | 412 | 433 | 464 | 331 | 412 | 433 | 464 |
| Neurosporene (trans) | 417 | 440 | 466 | 417 | 440 | 466 | ||
| δ-Carotene | 430 | 456 | 485 | 432 | 459 | 486 | ||
| γ-Carotene (trans) | — | 436 | 462 | 489 | ||||
| γ-Carotene (cis) | — | — | ||||||
| ζ-Carotene (cis) | — | 296 | 380 | 401 | 426 | |||
| ζ-Carotene (cis) | — | — | ||||||
| ζ-Carotene (trans) | 382 | 398 | 427 | 379 | 401 | 424 | ||
| cis-α-Carotene | — | 333 | 416 | 442 | 468 | |||
| α-Carotene | 420 | 444 | 472 | 420 | 445 | 472 | ||
| β-Carotene | 426 | 449 | 478 | 426 | 451 | 478 | ||
| Phytofluene | — | — | ||||||
| cis-β-Carotene | 334 | 419 | 444 | 473 | 334 | 419 | 444 | 473 |
| Phytoene | 276 | 287 | 299 | 276 | 287 | 299 | ||
Table IV.
Main absorption maxima (nm) of carotenes of unsaponifiable PFO and PFO
| Carotenoids | PFO |
Unsaponifiable PFO |
||||||
|---|---|---|---|---|---|---|---|---|
| Lycopene (cis) | 362 | 441 | 467 | 496 | 361 | 442 | 467 | 498 |
| Lycopene (cis) | 362 | 442 | 470 | 500 | 362 | 443 | 470 | 500 |
| Lycopene (trans) | 444 | 468 | 500 | 444 | 468 | 499 | ||
| α-Zeacarotene | 397 | 421 | 448 | 398 | 422 | 446 | ||
| β-Zeacarotene | 404 | 430 | 457 | 403 | 425 | 450 | ||
| Neurosporene (cis) | 330 | 415 | 439 | 468 | 328 | 414 | 433 | 462 |
| Neurosporene (trans) | 413 | 437 | 467 | 416 | 439 | 468 | ||
| δ-Carotene | 431 | 457 | 485 | 432 | 457 | 485 | ||
| γ-Carotene (trans) | 433 | 459 | 489 | 434 | 460 | 489 | ||
| γ-Carotene (cis) | 349 | 435 | 459 | 487 | 350 | 433 | 460 | 488 |
| ζ-Carotene (cis) | 296 | 380 | 401 | 426 | 296 | 380 | 400 | 426 |
| ζ-Carotene (cis) | 296 | 379 | 401 | 425 | 297 | 380 | 401 | 425 |
| ζ-Carotene (trans) | 380 | 402 | 426 | 381 | 402 | 426 | ||
| cis-α-Carotene | 332 | 417 | 442 | 469 | 332 | 415 | 441 | 468 |
| α-Carotene | 420 | 445 | 473 | 419 | 445 | 472 | ||
| β-Carotene | 424 | 450 | 477 | 424 | 450 | 477 | ||
| Phytofluene | 331 | 349 | 368 | 333 | 349 | 368 | ||
| cis-β-Carotene | 333 | 418 | 442 | 471 | 334 | 420 | 445 | 473 |
| Phytoene | 276 | 286 | 298 | 276 | 287 | 299 | ||
Figure 5.
Carotenes profile of PFO. This figure is available in black and white in print and in color at JCS online.
Figure 6.
Carotenes profile of unsaponifiable fraction from PFO. This figure is available in black and white in print and in color at JCS online.
The carotenes profile of palm oil follows the sequence of: cis lycopene, cis lycopene, lycopene, α-zeacarotene, β-zeacarotene, cis-neurosporene, neurosporene, δ-carotene, γ-carotene, cis γ-carotene, cis ξ-carotene, cis ξ-carotene, cis ξ-carotene, ξ-carotene, cis α-carotene, α-carotene, β-carotene, phytofluene, cis β-carotene and phytoene.
Discussion
There are 11 types of carotenes present in both CPO and PFO. The composition of these carotenes, however, differs in both types of oil. Although quantitative analysis of all the individual carotenes in palm oil has yet to be carried out successfully, the qualitative analysis of the carotenes is particularly useful as the wide range of individual carotenes present can be used as indication of the type of oil in the absence of proper labeling.
Due to the low concentration of carotenes in CPO, which is ∼500–700 ppm, the carotenes profile is not as prominent as that of PFO. Increasing the concentration of the sample resulted in saturation of the column. By removing the oil components through saponification, the concentration of carotenes in the unsaponifiable fraction of the CPO is greatly enhanced. This led to cleaner and sharper elution of the carotenes from the column. As the concentration of carotenes in PFO is already quite high, 4,000–6,000 ppm, its carotenes profile was more clearly defined.
Each carotene absorbs UV at wavelengths that are different from others. This is called the λmax and it is unique for each carotene. The λmax of each of the carotene depends on the structure of the molecule and the number of double bonds they contain. The λmax of each of the carotene in palm oil has been well documented in the past and it is used to identify the individual carotenes in palm oil in the absence of authentic standards (Table I).
In comparison with past reports using HPLC, UPLC is a fast and efficient method for the qualitative analyses of palm carotenes. The analysis was completed in <5 min, compared with HPLC which took more than 100 min. This is a definite improvement as it saves time and mobile phase consumption. The detection of the individual carotenes is sufficient to prove whether the oil is of palm origin as this carotenes profile is specific to palm oil.
Cis/trans isomer of the individual carotenes was resolved using C30 stationary phase in HPLC separation. In this study, using the UPLC, the C18 stationary phase is able to resolve the cis/trans isomers of the carotenes, which otherwise could not be done using HPLC. This shows that UPLC is a more powerful separation tool compared with HPLC.
Acknowledgments
The authors wish to thank the staff of the Clean and Emerging Technologies Group for their technical assistance.
References
- 1.Goh S.H., Choo Y.M., Ong A.S.H.; Minor constituents of palm oil; Journal of American Oil Chemists Society, (1985); 62(2): 237–240. [Google Scholar]
- 2.Ooi C.K., Choo Y.M., Yap S.C., Basiron Y., Ong A.S.H.; Recovery of carotenoids from palm oil; Journal of American Oil Chemists Society, (1994); 71(4): 423–426. [Google Scholar]
- 3.Choo Y.M., Yap S.C., Ooi C.K., Ma A.N., Goh S.H., Ong A.S.H.; Recovered oil from palm-pressed fiber: a good source of natural carotenoids, vitamin E and sterols; Journal of American Oil Chemists Society, (1996); 73(5): 599–602. [Google Scholar]
- 4.Yap S.C., Choo Y.M., Ooi C.K., Ong A.S.H., Goh S.H.; Carotenes in the oil from different palm species; Elaeis, (1991); 8(2): 369–378. [Google Scholar]
- 5.Tay B.Y.P., Choo Y.M.; Valuable minor constituents of commercial red palm olein: carotenoids, vitamin E, ubiquinones and sterols; Journal of Oil Palm Research, (2000); 12(1): 14–24. [Google Scholar]
- 6.Ng M.H., Choo Y.M., Ma A.N., Chuah C.H., Mohd A.H.; Isolation and identification of individual palm carotenes using supercritical fluid chromatography; Malaysian Journal of Science, (2006); 25(2): 139–145. [Google Scholar]
- 7.Ng M.H., Choo Y.M.; Isolation and recovery of phytonutrients in palm by isocratic and isobaric flash chromatography; Journal of Oil Palm Research, (2012); 25(2): 165–169. [Google Scholar]
- 8.Tay B.Y.P., Choo Y.M., EE G.C.L., Goh S.H.; Geometrical isomers of major provitamin A palm carotenes, α- and β-carotenes in the MEsocarp oil of fresh and sterilised palm fruits, crude palm oil and palm carotene-based products: red palm olein and carotene concentrates; Journal of Oil Palm Research, (2002); 13(2): 23–32. [Google Scholar]
- 9.Tan B., Grady C.M., Gawrenowski A.M.; Hydrocarbon carotenoid profiles of palm oil processed fractions; Journal of American Oil Chemists Society, (1986); 63: 1175–1179. [Google Scholar]
- 10.Britton G.; UV/Visible spectroscopy. In Britton, G., Liaaen-Jensen, S., and Pfander, Birkhauser-Verlag, H. (eds.) Carotenoids, Vol. 1B Birkhauser-Verlag, Basel, Switzerland, (1995), pp. 13–64. [Google Scholar]
- 11.Tay B.Y.P.; Palm carotenoids profile as a quality control tool for palm carotene producers: introducing an improvised method by HPLC-photodiode array and a C30 column; Journal of Oil Palm Research, (2006); 18: 253–259. [Google Scholar]
- 12.Tay B.Y.P., Choo Y.M.; Practical guide to establishing palm carotenoids profiles by HPLC with three dimensional diode array detector; Palm Oil Developments, (2000); 22: 13–17. [Google Scholar]
- 13.Maoka T., Fujiwara Y., Hashimoto K., Akimoto N.; Rapid identification of carotenoids in a combination of liquid chromatography/UV–visible absorption spectrometry by photodiode-array detector and atomospheric pressure chemical ionization mass spectrometry (LC/PAD/APCI-MS); Journal of Oleo Science, (2002); 51(1): 1–9. [Google Scholar]
- 14.Tay B.Y.P., Gwendoline E.C.L.; Identification of lutein in crude palm oil and evaluation of carotenoids at various ripening stages of the oil palm fruit; Journal of Oil Palm Research, (2003); 18: 189–197. [Google Scholar]
- 15.Pettersson A., Jonsson L.; Separation of cis–trans isomers of alpha and beta carotene by adsorption HPLC and identification with diode array detection; Journal of Micronutrient Analysis, (1990); 8: 23–41. [Google Scholar]
- 16.Emenhiser C., Sander L.C., Schwartz S.J.; Capability of a polymeric C30 stationary phase to resolve cis–trans carotenoid isomers in reversed-phase liquid chromatography; Journal of Chromatography A, (1995); 707: 205–216. [Google Scholar]
- 17.Ng J.H., Tan B.; Analysis of palm oil carotenoids by HPLC with diode array detection; Journal of Chromatographic Science, (1988); 26: 463–469. [DOI] [PubMed] [Google Scholar]
- 18.Darnoko D., Cheryan M., Moros E., Jerrel J., Perkins E.G.; Simultaneous HPLS analyses of palm carotenoids and tocopherols using a C30 Column and Photodiode Array Detector; Journal of Liquid Chromatography and Related Technologies, (2000); 23(12): 1873–1885. [Google Scholar]