Abstract
Organising pneumonia (OP) is a distinct but uncommon entity with characteristic clinicoradiological features and histological findings. When the aetiology of OP remains unknown, it is termed as cryptogenic OP (COP). COP is seen in the majority of patients with OP and usually observed in non/former smokers. A 54-year-old man, a smoker, presented with breathlessness, cough and mucoid sputum. Imaging demonstrated unilateral ‘Crazy-paving’ pattern in the left upper lobe and left-sided effusion. In addition, paraseptal emphysema and left lower lobe bullae along with very severe obstructive ventilatory defect and impaired diffusion suggested chronic obstructive pulmonary disease (COPD). Transbronchial biopsy was suggestive of OP. In the absence of a definite aetiology, a diagnosis of COP associated with COPD was established. COP presenting as a unilateral ‘Crazy-paving’ pattern is yet to be documented. To the best of our knowledge, this is the first detailed description of COP presenting as unilateral ‘Crazy-paving’ pattern associated with COPD.
Background
Organising pneumonia (OP), earlier known as bronchiolitis obliterans with OP (BOOP), is a specific clinicopathological entity characterised by presence of buds of granulation tissue within the alveolar ducts and distal airspaces. This is associated with varying degrees of bronchiolar involvement.1–3 OP has been classified as (A) idiopathic/cryptogenic and (B) secondary. Secondary OP may be caused by infections, drugs, radiation therapy, connective tissue disorders, collagen vascular disease, malignancies and organ transplantation.1–3 In the majority of patients, the cause of OP is unknown4 5 and this group is known as having ‘cryptogenic OP (COP)’, a term coined by Davison et al,6 in 1983. COP has been included as one of the idiopathic interstitial pneumonias (IIP) in the classification of major IIPs by the American Thoracic Society and European Respiratory Society.7 The term COP is preferred over BOOP, as it avoids any confusion with obliterative airway diseases such as constrictive bronchiolitis obliterans.3 8
Bilateral patchy consolidation is the most common radiological picture in COP.2 ‘Crazy-paving’ patterns been described in four patients with OP,9–11 but COP presenting as a unilateral ‘Crazy-paving’ pattern is yet to be documented. We report a case of a 54-year-old man, a smoker, with a confirmed diagnosis of COPD who had concomitant COP and presented on HRCT with a unilateral ‘Crazy-paving’ pattern. To the best of our knowledge, this is the first detailed description of the concomitant occurrence of both these clinical conditions with this distinct radiological appearance.
Case presentation
A 54-year-old HIV negative man, a farmer, was referred to our Institute, for evaluation of breathlessness, cough and mucoid sputum of 6 years’ duration. His clinical course was characterised by progressive exertional dyspnoea along with wheezing. A fortnight prior to presentation, he had experienced low-grade intermittent fever along with chills and rigors, which lasted a week and prompted the referral. He was a current smoker and had smoked 10 bidis per day for 40 years (pack-years: 40). There was neither haemoptysis, chest pain, palpitations nor pain in the small joints. There was no history of medications, intravenous drug abuse or exposure to toxic fumes.
General physical examination revealed a middle-aged man in respiratory distress with use of accessory muscles for respiration. He was tachypnoeic with a respiratory count of 26/min and afebrile on presentation. There was neither cyanosis, clubbing nor pallor. Diaphragmatic excursion was comparable on both sides. Vesicular breath sounds of equal intensity with prolonged expiration and polyphonic ronchi were audible bilaterally.
Investigations
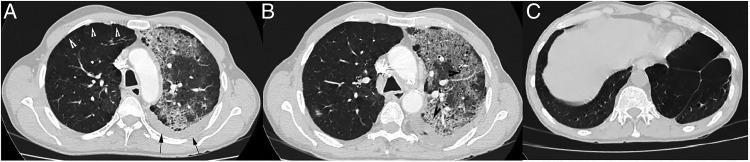
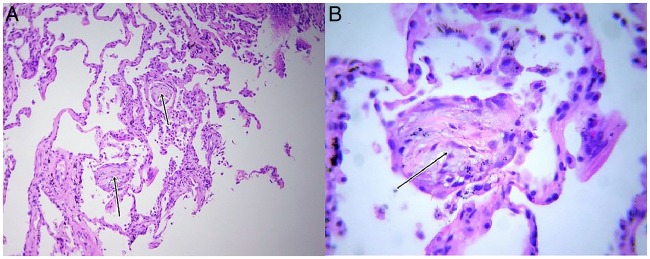
Oxygen saturation at room air was 90% with pH-7.37, pCO2—41.2 mm Hg, pO2—46 mm Hg and HCO3—25 mEq/L. Total leucocyte count was 14 600 cells/mm3 with neutrophils constituting 86%. ECG, urine analyses, blood sugar levels and renal and hepatic functions were within normal limits. Chest radiograph, performed on presentation, showed left upper and mid-zone consolidation with blunting of left costophrenic angle (figure 1). Sputum stains and cultures for Mycobacterium tuberculosis, fungi and other aerobic organisms were negative. Contrast-enhanced high-resolution CT (HRCT) of the thorax revealed a classic unilateral ‘Crazy-paving’ pattern in the left upper lobe, with minimal left-sided pleural effusion. On a background of ground-glass opacities (GGOs), thickened interlobular septa and intralobular lines with distinct geographic margins were seen. Bilateral upper lobe paraseptal emphysema and left lower lobe bullae were also noted (figures 2A–C). On spirometry, the forced vital capacity (FVC) was 2.06 L (51% of predicted), forced expired volume in the 1 s (FEV1) was 0.59 L (18% of predicted) and the FEV1/FVC ratio was 0.29. This was indicative of very severe obstructive ventilatory defect with no bronchodilator response. Complete pulmonary function test revealed a residual volume (RV) of 2.13 L (109% of predicted), total lung capacity (TLC) of 4.23 L (71% of predicted) and RV/TLC of 50% (161% of predicted). Diffusion capacity for carbon monoxide was 11.82 (43% of predicted). This was suggestive of mild restriction with severely impaired diffusion capacity. Antinuclear antibody, rheumatoid factor and anti-cyclic citrullinated peptide antibodies were not detected. Fibreoptic bronchoscopy visualised no gross abnormality. Transbronchial biopsies from the left lung demonstrated alveolar spaces with focal rounded bodies and spindle-shaped cells in myxoid stroma. Mononuclear infiltrate in the stroma along with focal areas of dense anthracotic pigmentation and fibrosis was the other histopathological finding. These findings were suggestive of OP (figure 3A, B). Stains and cultures of the bronchial aspirate were negative for M. tuberculosis as well as for any other aerobic organism or fungi. The aspirate was also negative for GeneXpert. A definite aetiology for OP could not be detected.
Figure 1.
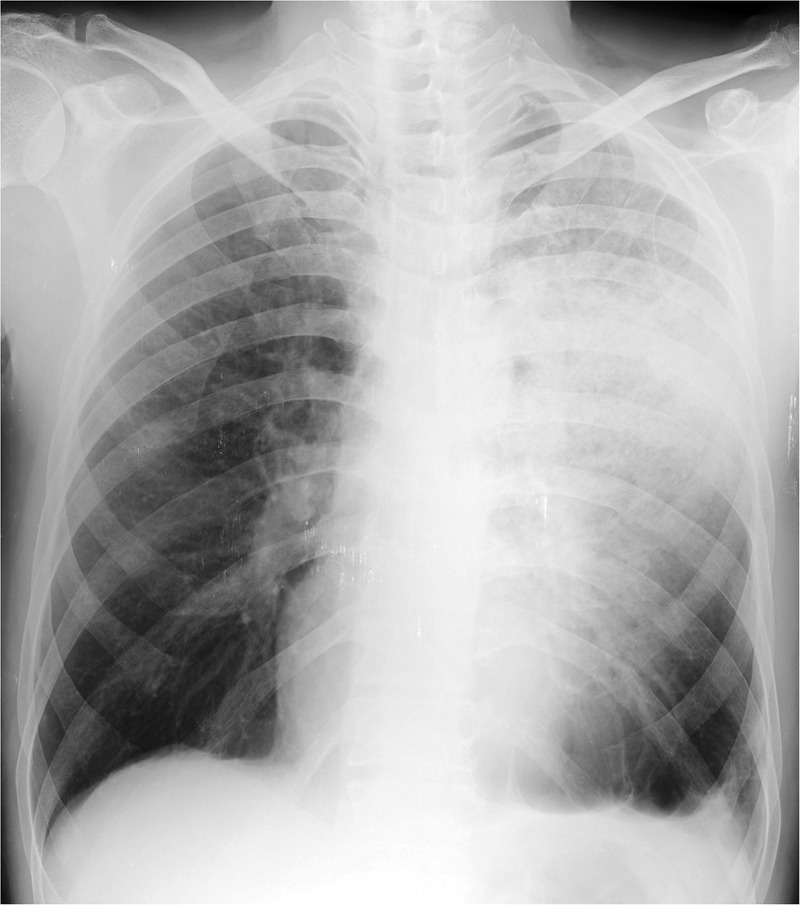
Chest X-ray on presentation showing patchy consolidation in left upper and middle zone with blunting of the left costophrenic angle.
Figure 2.
(A) High-resolution CT (HRCT) of the thorax (lung window) on presentation showing areas of paraseptal emphysema in right upper lobe (white arrowheads) with left-sided pleural effusion (black arrows). (B) HRCT of the thorax (lung window) on presentation showing characteristic ‘Crazy-paving’ pattern in left upper lobe (black arrowheads). (C) HRCT of the thorax (lung window) on presentation showing left lower lobe bullae.
Figure 3.
(A) Medium-power view (×10) of the patient's biopsy specimen on H&E stain showing dilated airspaces with focal fibrosis of septae. Two Masson's bodies are identified (black arrows). (B) High-power view (×40) of the patient's biopsy specimen on H&E stain showing Masson bodies and fibromyxoid cells (black arrow).
Diagnosis
A diagnosis of COPD with COP was made. The diagnosis of COPD was based on (A) a 40-pack-year history of smoking, (B) obstructive ventilatory defect on spirometry, (C) paraseptal emphysema and left lower lobe bullae visible on HRCT. The diagnosis of COP was supported by (A) ‘crazy-paving’ pattern on HRCT, (B) left-sided pleural effusion, (C) mild restriction along with a severe diffusion defect, (D) histopathology suggestive of OP and (E) absence of a definite aetiology for OP.
Treatment
The patient was initiated on tablet prednisolone 0.5 mg/kg body weight daily for 15 days followed by an alternate day regimen for 2 months. Simultaneously, treatment for COPD in the form of an inhaled long-acting β-2 agonist and muscarinic antagonist was started.
Outcome and follow-up
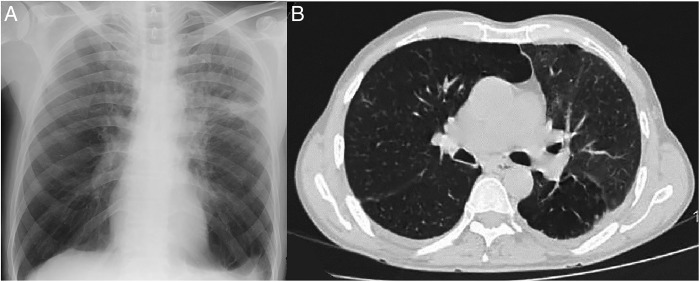
Within a fortnight, the patient experienced marked clinical improvement with symptoms being largely resolved. Chest imaging performed 2 months later (figures 4A, B) showed remarkable radiological clearance. The patient subsequently went back to his native place and was lost to follow-up.
Figure 4.
(A) Chest X-ray performed 2 months later showing resolution of the left upper and middle zone consolidation. (B) High-resolution CT of the thorax (lung window) performed 2 months later showing marked clearing of the lesion.
Discussion
COP is an uncommon clinical entity that is generally seen in the sixth decade; it presents without any specific constellation of symptoms.2 The condition is more commonly observed in never-smokers and ex-smokers, and occurs twice as frequently in this group as compared with that of smokers.2 3 Although there is no gender predisposition, it occurs more frequently in females who are non-smokers.3 Most series on COP have shown an inverse relationship with smoking.4 12–14 However, an Italian study detected COP in 54% patients who were current smokers as compared with 32% never-smokers and 14% ex-smokers.15 Our patient too was a current smoker.
Chest X-ray is inevitably the first diagnostic clue for raising the suspicion of OP/COP. Five major radiological series5 16–19 have highlighted three characteristic radiological patterns with multiple areas of patchy consolidation—often migratory—being the most common presentations. These areas of consolidation correspond to the granulation tissue plugs within the alveoli. On HRCT scan, these appear as areas of GGOs or consolidation with air bronchograms in a bilateral and subpleural distribution. The next common radiological pattern is that of a solitary nodule or mass-like area of consolidation (focal OP/COP)—often localised in the upper lobes—which can cavitate.2 3 20 Non-resolving consolidation is not uncommon and not infrequently mimics a malignant lesion, as positron emission tomography scan can falsely be positive. Patients with focal OP/COP are often subjected to invasive diagnostic procedures to exclude lung cancer, which may result in a diagnosis of OP/COP.2 The third pattern described in OP/COP is infiltrative or progressive fibrotic OP/COP, characterised by a combination of interstitial and alveolar opacities, mostly seen in lower lung fields.2 3 20 This pattern is usually a pulmonary manifestation of idiopathic inflammatory myopathy, which may precede the muscular symptoms. In these patients, autoantibodies, especially antisynthetase antibodies, should be assayed.2 Apart from these three particular radiological descriptions, other imaging findings include bronchial wall thickening, centrilobular nodules, pleural effusion and ‘atoll’ sign.2 3 20 The ‘atoll sign’, also known as ‘reverse halo’ sign, has been recorded in up to 20% of patients and was once considered as a specific radiological sign in OP/COP.21 This sign has now been reported in tuberculosis, infectious pneumonia, paracoccidioidomycosis, invasive aspergillosis, granulomatosis with polyangiitis and pulmonary sarcoidosis.22 Pleural effusion has been reported in up to 22% of patients with OP23 and is more common in secondary OP as compared with COP.24 Our patient too had a left-sided pleural effusion.
The ‘Crazy-paving’ pattern is an unusual HRCT finding and a search of PubMed and other databases revealed four patients with OP9–11 who presented with this characteristic radiological image (table 1). In addition, there is mention of one patient in a series describing a ‘Crazy-paving’ pattern but details were not available.25 This distinctive pattern was first described in a patient with pulmonary alveolar proteinosis and is still considered as a diagnostic hallmark of the disease.26 However, this has now been documented in several other diseases.27 On HRCT, the ‘Crazy-paving’ pattern is seen as areas of GGOs superimposed with interlobular septal thickening and intralobular lines in a geographic distribution resembling irregularly laid cobblestones. These areas of air-space opacification are usually bilateral and sharply demarcated from the surrounding normal lung parenchyma.9 This unusual pattern is thought to occur due to alveolar filling processes, interstitial fibrotic processes or a combination of both.28 One of the differential diagnosis of the ‘Crazy-paving’ pattern can be a ‘Swiss-Cheese’ appearance. This pattern occurs when pneumonia develops in pulmonary emphysema. The parenchymal consolidation appears to be non-uniformly ‘perforated’, resembling multiple cavities. This is due to low attenuation areas forming a ‘Swiss-Cheese’ appearance, which could be mistaken for honeycombing.29
Table 1.
Summary of the four documented patients with organising pneumonia/cryptogenic organising pneumonia presenting as a ‘Crazy-paving’ pattern
| S No | Author, year of publication, country | Number of patients/age/sex | Symptoms | Radiology | Histopathology | Aetiology | Management |
|---|---|---|---|---|---|---|---|
| 1 | Rossi et al, 2003, Argentina9 | 2 (a) 45/female (b) 44/female |
Dyspnoea diagnosed with small cell cancer Dry cough, dyspnoea diagnosed with Hodgkin's lymphoma |
HRCT chest: RUL: diffuse ground-glass attenuation and septal thickening in ‘Crazy-paving’ pattern HRCT chest: B/L ‘Crazy-paving’ pattern |
Wedge resection biopsy of RUL: scattered interstitial inflammation, occlusion of terminal bronchioles and alveolar ducts by plugs of loose connective tissue Transthoracic biopsy: organising pneumonia |
Secondary: topotecan-induced Secondary: bleomycin-induced |
NA Improvement postdiscontinuation of bleomycin and initiation of corticosteroids |
| 2 | De Wever et al, 2011, Belgium10 | 1/24/female | Case of B/L lung transplantation | HRCT chest: B/L ‘Crazy-paving’ pattern | Diagnosis on clinical basis | Secondary: postorgan transplantation | NA |
| 3 | Utrilla Contreras et al, 2013, Spain11 | 1/56/female | Dry cough, dyspnoea: 1 month | CXR: Poorly defined, bilateral alveolar opacities Chest CT: B/L lower lobes: ground-glass opacities with interlobular septal thickening (‘Crazy-paving’ pattern) |
Open lung biopsy: interstitial inflammation, opacification of terminal bronchioles and alveolar ducts by granulation tissue. Masson's trichrome stain: thickened interalveolar septa, infiltration by lymphocytes, plasma cells | NA | Mechanical ventilation |
| 4 | Present case | 1/54/male | Cough, dyspnoea | CXR: left upper and mid zone consolidation Chest HRCT: unilateral ‘Crazy-paving’ pattern in left lower lobe |
Transbronchial biopsy: alveolar spaces with focal rounded bodies and spindle-shaped cells in myxoid stroma | Idiopathic/cryptogenic | Oral corticosteroids for 2 months following which patient was lost to follow-up |
*Johkoh et al,25 Japan, 1999: no details available.
B/L, bilateral; CXR, chest X-ray; HRCT, high-resolution CT; NA, not available; RUL, right upper lobe.
All four patients diagnosed with OP and a ‘Crazy-paving’ pattern were females in the age group ranging from 24 to 56 years.9–11 In three of four patients, both lungs had the same appearance9–11 while only one had a unilateral ‘Crazy-paving’ pattern affecting the right upper lobe.9 Our patient was a male with a unilateral ‘crazy-paving’ pattern involving the left upper lobe. In three of four patients, the diagnosis was established with biopsies9 11 while one had a clinical diagnosis.10 None of the patients had a diagnosis of COP. In a review of five radiological series documenting 153 patients with OP/COP, a ‘Crazy-paving’ pattern was not reported in any patient.5 16–19
Although open lung biopsy is still considered the gold standard for diagnosis, in practice, transbronchial biopsy is often of help.30 Histopathologically, there is presence of buds of granulation tissue (Masson bodies) consisting of fibroblasts and myofibroblasts embedded in connective tissue.2
Lung function tests in COP show a mild to moderate restrictive ventilatory pattern with an impaired diffusion capacity.2 However, patients with a history of smoking and underlying COPD may have an obstructive ventilatory defect,2 as was seen in our patient.
Corticosteroids form the standard therapy for COP, with doses of prednisolone ranging from 0.5 to 1.5 mg/kg/day and tapered over 6 months. This often results in marked improvement, as was seen in our patient. Immunosuppressive agents may be used as an adjunct to steroids in case of persistent disease. Relapses are known to occur in COP and are seen in 13–58% of patients on decreasing the dose or stopping of corticosteroids. However, in patients with relapse, response to corticosteroids is dramatic.3
OP/COP is a rare but distinct clinical entity with well-defined imaging findings. However, a Crazy-paving’ pattern as a presentation of COP is rather exceptional.
Learning points.
Organising pneumonia is a distinct clinical entity with characteristic clinicoradiological and histological findings.
The aetiology in the vast majority of patients with organising pneumonia is unknown when it is known as cryptogenic organising pneumonia and is included in the classification of idiopathic interstitial pneumonias.
Cryptogenic organising pneumonia is usually reported in never-smokers or ex-smokers and can occasionally be seen in current smokers.
Oral corticosteroids elicit significant response in patients with organising pneumonia/cryptogenic organising pneumonia. However, relapses are not infrequent.
Organising pneumonia presenting radiologically as a ‘Crazy-paving’ pattern has rarely been documented in the literature. Our case highlights this singularly uncommon presentation in a patient with cryptogenic organising pneumonia.
Footnotes
Contributors: SK, VP and AS collected the clinical data and reviewed the literature. SJ reviewed the pathological aspects. SK, VP, SJ and AS drafted the manuscript. AS is responsible for the genuineness of the data and is also the guarantor of the manuscript, and conceived of the idea. All the authors have read and approved the final manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cordier JF. Bronchiolitis obliterans organizing pneumonia. Semin Respir Crit Care Med 2000;21:135–46. 10.1055/s-2000-9840 [DOI] [PubMed] [Google Scholar]
- 2.Cordier JF. Cryptogenic organising pneumonia. Eur Respir J 2006;28:422–46. 10.1183/09031936.06.00013505 [DOI] [PubMed] [Google Scholar]
- 3.Cottin V, Cordier JF. Cryptogenic organizing pneumonia. Semin Respir Crit Care Med 2012;33:462–75. 10.1055/s-0032-1325157 [DOI] [PubMed] [Google Scholar]
- 4.Lazor R, Vandevenne A, Pelletier A et al. Cryptogenic organizing pneumonia. Characteristics of relapses in a series of 48 patients. The Groupe d'Etudes et de Recherche sur les Maladles 'Orphelines’ Pulmonaires (GERM‘O'P). Am J Respir Crit Care Med 2000;162:571–7. [DOI] [PubMed] [Google Scholar]
- 5.Faria IM, Zanetti G, Barreto MM et al. Organizing pneumonia: chest HRCT findings. J Bras Pneumol 2015;41:231–7. 10.1590/S1806-37132015000004544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davison AG, Heard BE, McAllister WA et al. Cryptogenic organizing pneumonitis. Q J Med 1983;52:382–94. [PubMed] [Google Scholar]
- 7.American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277–304. [DOI] [PubMed] [Google Scholar]
- 8.Geddes DM. BOOP and COP. Thorax 1991;46:545–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi SE, Erasmus JJ, Volpacchio M et al. ‘Crazy-paving’ pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics 2003;23:1509–19. 10.1148/rg.236035101 [DOI] [PubMed] [Google Scholar]
- 10.De Wever W, Meersschaert J, Coolen J et al. The crazy-paving pattern: a radiological-pathological correlation. Insights Imaging 2011;2:117–32. 10.1007/s13244-010-0060-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Utrilla Contreras C, Fernández-Velilla Peña M, García Río F et al. Radiographic patterns in the diagnostic approach to organizing pneumonia. Rev Clin Esp (Barc) 2014;214:258–65. 10.1016/j.rce.2013.10.015 [DOI] [PubMed] [Google Scholar]
- 12.Costabel U, Teschler H, Greschuchna D et al. BOOP in Europe. Chest 1992;102(1 Suppl):14S–20S. 10.1378/chest.102.1.14S [DOI] [PubMed] [Google Scholar]
- 13.Izumi T, Kitaichi M, Nishimura K et al. Bronchiolitis obliterans organising pneumonia. Clinical features and differential diagnosis. Chest 1992;102:715–9. [DOI] [PubMed] [Google Scholar]
- 14.Kim M, Cha SI, Seo H et al. Predictors of Relapse in Patients with Organizing Pneumonia. Tuberc Respir Dis (Seoul) 2015;78:190–5. 10.4046/trd.2015.78.3.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cazzato S, Zompatori M, Baruzzi G et al. Bronchiolitis obliterans-organizing pneumonia: an Italian experience. Respir Med 2000;94:702–8. 10.1053/rmed.2000.0805 [DOI] [PubMed] [Google Scholar]
- 16.Lee KS, Kullnig P, Hartman TE et al. Cryptogenic organizing pneumonia: CT findings in 43 patients. AJR Am J Roentgenol 1994;162:543–6. 10.2214/ajr.162.3.8109493 [DOI] [PubMed] [Google Scholar]
- 17.Ujita M, Renzoni EA, Veeraraghavan S et al. Organizing pneumonia: perilobular pattern at thin-section CT. Radiology 2004; 232: 757–61. 10.1148/radiol.2323031059 [DOI] [PubMed] [Google Scholar]
- 18.Lee JW, Lee KS, Lee HY et al. Cryptogenic organizing pneumonia: serial high-resolution CT findings in 22 patients. AJR Am J Roentgenol 2010;195:916–22. 10.2214/AJR.09.3940 [DOI] [PubMed] [Google Scholar]
- 19.Mehrian P, Shahnazi M, Dahaj AA et al. The spectrum of presentations of cryptogenic organizing pneumonia in high resolution computed tomography. Pol J Radiol 2014;79:456–60. 10.12659/PJR.891011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oikonomou A, Hansell DM. Organizing pneumonia: the many morphological faces. Eur Radiol 2002;12:1486–96. 10.1007/s00330-001-1211-3 [DOI] [PubMed] [Google Scholar]
- 21.Kim SJ, Lee KS, Ryu YH et al. Reversed halo sign on high-resolution CT of cryptogenic organizing pneumonia: diagnostic implications. AJR Am J Roentgenol 2003;180:1251–4. 10.2214/ajr.180.5.1801251 [DOI] [PubMed] [Google Scholar]
- 22.Baque-Juston M, Pellegrin A, Leroy S et al. Organising pneumonia: what is it? A conceptual approach and pictorial review. Diagn Interv Imaging 2014;95:771–7. 10.1016/j.diii.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 23.Lohr RH, Boland BJ, Douglas WW et al. Organizing pneumonia. Features and prognosis of cryptogenic, secondary, and focal variants. Arch Intern Med 1997;157:1323–9. [DOI] [PubMed] [Google Scholar]
- 24.Vasu TS, Cavallazzi R, Hirani A et al. Clinical and radiologic distinctions between secondary bronchiolitis obliterans organizing pneumonia and cryptogenic organizing pneumonia. Respir Care 2009;54:1028–32. [PubMed] [Google Scholar]
- 25.Johkoh T, Itoh H, Müller NL et al. Crazy-paving appearance at thin-section CT: spectrum of disease and pathologic findings. Radiology 1999;211:155–60. 10.1148/radiology.211.1.r99ap10155 [DOI] [PubMed] [Google Scholar]
- 26.Murch CR, Carr DH. Computed tomography appearances of pulmonary alveolar proteinosis. Clin Radiol 1989;40:240–3. 10.1016/S0009-9260(89)80180-1 [DOI] [PubMed] [Google Scholar]
- 27.Kunal S, Gera K, Pilaniya V et al. ‘Crazy-paving’ pattern: A characteristic presentation of pulmonary alveolar proteinosis and a review of the literature from India. Lung India 2016;33:335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maimon N, Heimer D. The crazy-paving pattern on computed tomography. CMAJ 2010;182:1545 10.1503/cmaj.091422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nambu A, Ozawa K, Kobayashi N et al. Imaging of community-acquired pneumonia: roles of imaging examinations, imaging diagnosis of specific pathogens and discrimination from noninfectious diseases. World J Radiol 2014;6:779–93. 10.4329/wjr.v6.i10.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jara-Palomares L, Gomez-Izquierdo L, Gonzalez-Vergara D et al. Utility of high-resolution computed tomography and BAL in cryptogenic organizing pneumonia. Respir Med 2010;104:1706–11. 10.1016/j.rmed.2010.06.008 [DOI] [PubMed] [Google Scholar]