Abstract
We describe a case of a 62-year-old woman with a history of chronic obstructive pulmonary disease and gastro-oesophageal reflux disease who presented to the emergency department with left lower quadrant abdominal pain, flank pain with nausea and no history of preceding trauma. The patient had finished a course of azithromycin and oral methylprednisolone 1 day prior to presentation. Abdominal and pelvic CT scan identified changes suggestive of bilateral adrenal haemorrhage. The patient did not show signs of acute adrenal insufficiency but was started on steroid replacement therapy because of concerns about possible disease progression. All recognised causes of adrenal haemorrhage were excluded suggesting this was a case of spontaneous idiopathic bilateral adrenal haemorrhage, a rarely reported phenomenon in the literature. The patient was discharged after clinical improvement following 6 days in hospital, taking oral steroid replacement.
Background
Bilateral adrenal haemorrhage is difficult to diagnose clinically due to its relatively non-specific presentation. The clinical concern is that this diagnosis may be overlooked, leading to acute adrenal insufficiency that could be fatal.1 This case highlights the importance of considering bilateral adrenal haemorrhage in the differential diagnosis of patients with abdominal or flank pain of unclear aetiology. We also raise the question of a possible association between glucocorticoid use and adrenal haemorrhage, because of the prothrombotic effects of steroids, as has been reported previously in the literature.2
Case presentation
A 62-year-old woman with a history of well-controlled gastro-oesophageal reflux disease on pantoprazole and mild chronic obstructive pulmonary disease (COPD) on inhaled albuterol (as needed), presented to the emergency department of our hospital, with left lower quadrant abdominal pain that had started 8 h prior to presentation. She described the pain as crampy in nature, 9/10 in severity, and radiating to the left flank and left lower chest wall. She gave a history of nausea, while the review of systems was otherwise unremarkable. The patient had no history of trauma, antiplatelet therapy or anticoagulant use. She was a current smoker but had no history of illicit drug use. Her initial vital signs: blood pressure 155/80 mm Hg (not orthostatic), heart rate 78 bpm, respiratory rate 16/min, temperature 36.2°C and oxygen saturation of 95% on room air. On examination, she had mild-to-moderate tenderness in the left lower quadrant and flank area but was otherwise normal.
One week prior to this presentation, the patient had been seen by her family physician and treated for a respiratory tract infection and mild COPD exacerbation. She was then started on azithromycin 500 mg for 5 days and oral methylprednisolone 12 mg on day 1 reducing down to zero over the next 6 days.
Investigation
The initial laboratory investigations identified a random serum cortisol level of 15.2 µg/dL (normal:10–18 µg/dL), white cell count 17 500/µL (normal: 4800–10 800/µL, differential: 73% polymorphonuclear leucocytess, 1.2% eosinophils), sodium 138 meq/L (normal: 136–145 meq/L), potassium 4.7 meq/L (normal: 3.5–5.1 meq/L), random glucose (128 mg/dL), blood urea nitrogen 17 mg/dL (normal: 7–25 mg/dL), serum creatinine 0.90 mg/dL (normal: 0.60–1.30 mg/dL), haemoglobin 14.5 g/dL (normal: 12–16 g/dL) and erythrocyte sedimentation rate of 25 mm/h (range: 0–20 mm/h). The rest of the investigation was unremarkable including coagulation profile, liver function, ECG and a chest X-ray.
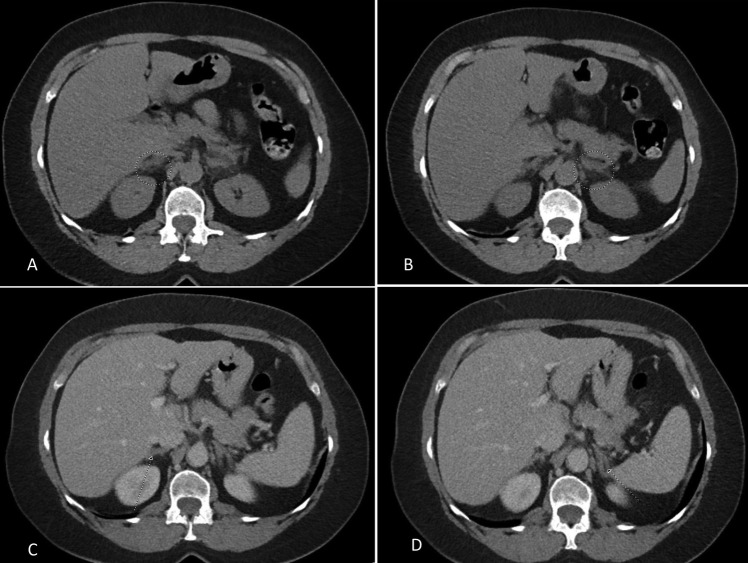
The initial contrast CT scan of the abdomen and pelvis identified mild soft tissue stranding and bilateral enlargement of the adrenal glands, greater on the left, suggestive of bilateral haemorrhage, as shown in figure 1. Causes of adrenal haemorrhage were entertained. Further work up included negative blood cultures, urine pneumococcal antigen, fourth generation HIV test, rapid plasma reagin, TB quantiFERON, serum cryptococcal antigen, urine histoplasma antigen, antinuclear antibody, anticardiolipin and anti-β2-glycoprotein antibodies, 21-hydroxylase antibodies and a negative search for malignancy including a normal upper gastro-oesophageal endoscopy. As a result of persistent pain, a repeat CT scan was performed on day 3 of her hospitalisation, which showed further enlargement and increased stranding in the soft tissue surrounding both adrenal glands suggesting progression of an underlying haemorrhagic process, as shown in figure 2.
Figure 1.
(A and B) (circle) Bilateral adrenal enlargement, greater on the left, with mild soft tissue stranding suggestive of haemorrhage. Comparison was made to a previous CT scan ((C and D)-arrows pointing to normal adrenals).
Figure 2.
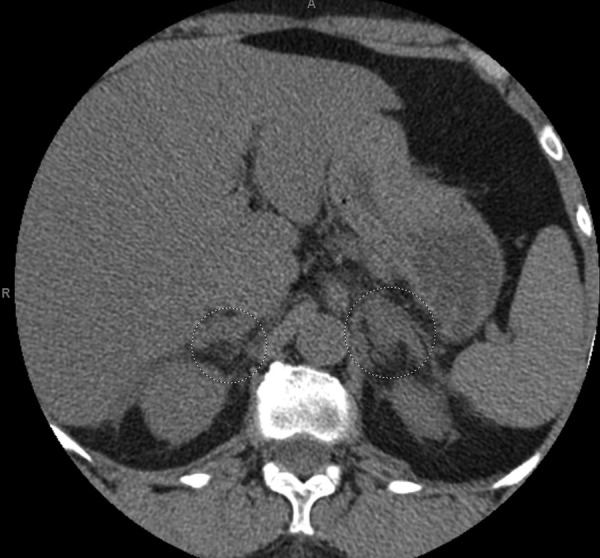
Repeated CT scan on day 3 showing further enlargement of both adrenals (circle), with increased stranding in the surrounding soft tissues suggesting worsening of the underlying haemorrhagic process.
Differential diagnosis
Potential causes of severe left lower abdominal pain radiating to the left flank and left lower chest wall in a 62-year-old woman include diverticulitis, nephrolithiasis, pyelonephritis, colitis (infectious or inflammatory) and, less commonly, myocardial infarction, among others. All were ruled out appropriately by history, physical examination, blood tests and imaging, making mild bilateral adrenal haemorrhage seen on the CT scan the most likely explanation of this patient's abdominal pain. The presence of neutrophilic leucocytosis may be explained by the recent use of oral steroids.
Treatment
Although the patient did not show signs of acute adrenal insufficiency and had a random cortisol level of 15.2 µg/dL (normal: 10–18 µg/dL), she was started by endocrinology on hydrocortisone 50 intravenous every 8 h due to the risk of disease progression and the development of adrenal insufficiency. The dose of hydrocortisone was decreased to 25 mg intravenous twice daily on day 2 of hospitalisation. The patient was subsequently switched to oral hydrocortisone 25 mg twice daily on day 4 of her stay.
Outcome and follow-up
The patient remained stable and showed no signs of adrenal insufficiency. She continued to have abdominal pain controlled with analgaesia; her pain had decreased in intensity by day 6 of hospitalisation. She was discharged on hydrocortisone 25 mg oral twice daily and a follow-up with endocrinology was scheduled in 1 week. On follow-up, the patient was asymptomatic and it was decided to pursue the adrenocorticotropic hormone (ACTH) stimulation test in 2 months, allowing for a slow steroid taper.
Discussion
Adrenal haemorrhage is an uncommon cause of adrenal insufficiency. The exact incidence of adrenal haemorrhage in the general population is unknown. Older postmortem studies reported prevalence around 0.14–1.1%. The clinical presentation of adrenal haemorrhage is often non-specific. Patients most commonly present with flank pain and abdominal pain, while symptoms of acute adrenal insufficiency such as nausea, vomiting, fatigue, dizziness and change in mental status can be present as well. Rarely, patients can be entirely asymptomatic.3 4 Laboratory findings can include hyponatraemia, hyperkalaemia, hypoglycaemia, azotaemia and eosinophilia. A drop in haemoglobin can be seen in cases of haemorrhagic adrenal insufficiency.
Spontaneous bilateral adrenal haemorrhage can be divided into traumatic or non-traumatic aetiologies. Non-traumatic causes include anticoagulant use (mostly heparin-induced thrombocytopaenia), thromboembolic disease, antiphospholipid syndrome (APS), severe sepsis/septic shock due to meningococcaemia, Streptococcus pneumoniae and Haemophilus influenza, among others, and malignancy, haematological disorders, pregnancy and adrenal masses, inter alia.3–9 All were essentially ruled out, making this a rare case of spontaneous idiopathic bilateral adrenal haemorrhage.10 11 Although our patient had mild respiratory tract infection symptoms 1 week prior to presentation, they were not significant enough to cause severe stress precipitating adrenal haemorrhage.
To understand the underlying mechanism in cases of adrenal haemorrhage it is important to review the adrenal gland anatomy. The glands are well supplied by three suprarenal arteries forming a rich plexus of arterioles and poorly drained by a single adrenal vein effectively creating a ‘vascular dam’. It is proposed that, in cases of prothrombotic states such as heparin-induced thrombocytopenia, APS and malignancy, the adrenal vein may thrombose, creating intravascular pressure within the gland, leading to haemorrhage.3 5 8 9 Interestingly, adrenal haemorrhage is often bilateral, although the pathophysiology of this is not explained in the current literature. Our patient received azithromycin and a short course of low-dose oral glucocorticoids before presenting with adrenal haemorrhage. Our literature search identified no reported association of adrenal haemorrhage with a short course of oral steroids in apparently healthy participants, nor with azithromycin. However, we propose that, if adrenal haemorrhage in heparin-induced thrombocytopaenia, malignancy and APS occurs because of a prothrombotic state, then a similar association could be made with a short course of oral glucocorticoids, based on the available literature. In a recent study by Isidori et al,2 it was proposed that exogenous glucocorticoids increase von Willebrand factor levels, factor VII, VIII, XI, fibrinogen levels and possible increase in plasminogen activator inhibitor-1, leading to a decrease in fibrinolysis and an overall state of hypercoagulability. Some of the studies yielded contradictory results and hence the authors concluded that there are variable changes in thrombotic factors after short-term administration of glucocorticoids. Similarly, a rise in factor VII, VIII, XI and fibrinogen levels was found in a trial that randomised 24 healthy participants to dexamethasone 5 mg twice daily versus placebo, for 5 days.12 In a Danish population-based case–control study by Johannesdottir et al,13 the authors found that systemic glucocorticoids increase risk of venous thromboembolism among new users of glucocorticoids.
CT scan of the adrenals is the most important test in establishing the diagnosis of bilateral adrenal haemorrhage.14 Random cortisol levels are not helpful in diagnosis as the levels may vary with the type of disease and its associated severity. However, a random cortisol level below 15 µg/dL has been proposed as being that where patients would benefit from steroid replacement therapy.15 The ACTH stimulation test has several limitations in acute adrenal processes and should ideally be performed before the initiation of steroids.15 Unfortunately, our patient was already started on treatment and hence the test could not be performed.
In summary, after reviewing the available literature on possible causes of spontaneous bilateral adrenal haemorrhage, we propose that the aetiology in our case was most likely idiopathic. We have suggested a possible association with steroid use. Glucocorticoids are used for a wide variety of indications including adrenal insufficiency; hence further studies would be needed to determine if such an association exists.
Learning points.
Adrenal haemorrhage should be considered in the differential diagnosis of patients with abdominal or flank pain of unclear aetiology because of the risks associated with delayed diagnosis.
Spontaneous adrenal haemorrhage can occur in patients with no recognised risk factors.
CT scan is the most useful test in the diagnosis of bilateral adrenal haemorrhage and immediate treatment with steroids should be considered in cases of bilateral adrenal haemorrhage.
Acknowledgments
The authors would like to acknowledge the patient for her consent to submit this article for publication.
Footnotes
Contributors: SN wrote the main draft of the manuscript. SS was involved in direct patient care and editing of the manuscript. VF and EY were involved in direct patient care and final review of the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Rao RH, Vagnucci AH, Amico JA. Bilateral massive adrenal hemorrhage: early recognition and treatment. Ann Intern Med 1989;110:227–35. 10.7326/0003-4819-110-3-227 [DOI] [PubMed] [Google Scholar]
- 2.Isidori AM, Minnetti M, Sbardella E et al. Mechanisms in endocrinology: the spectrum of haemostatic abnormalities in glucocorticoid excess and defect. Eur J Endocrinol 2015;173:R101–13. 10.1530/EJE-15-0308 [DOI] [PubMed] [Google Scholar]
- 3.Vella A, Nippoldt TB, Morris JC. Adrenal hemorrhage: a 25-year experience at the Mayo Clinic. Mayo Clin Proc 2001;76:161–8. 10.1016/S0025-6196(11)63123-6 [DOI] [PubMed] [Google Scholar]
- 4.Marti JL, Millet J, Sosa JA et al. Spontaneous adrenal hemorrhage with associated masses: etiology and management in 6 cases and a review of 133 reported cases. World J Surg 2012;36:75–82. 10.1007/s00268-011-1338-6 [DOI] [PubMed] [Google Scholar]
- 5.Saleem N, Khan M, Parveen S et al. Bilateral adrenal haemorrhage: a cause of haemodynamic collapse in heparin-induced thrombocytopaenia. BMJ Case Rep 2016;2016;▪▪▪ 10.1136/bcr-2016-214679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahasrabudhe N, Byers R. Massive haemorrhagic adrenal metastases leading to sudden death: a case report. BMJ Case Rep 2009;2009; ▪▪▪ 10.1136/bcr.06.2008.0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keizer AL, Peters LW, de Vries C et al. Spontaneous adrenal haemorrhage in early pregnancy. BMJ Case Rep. 2013;2013; ▪▪▪ 10.1136/bcr-2012-008062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleanthous A, Koushiappi E, Herodotou Y et al. Acute adrenal insufficiency as a first presentation of myelodysplastic syndrome and sigmoid colon adenocarcinoma: a case report. Oxf Med Case Rep 2014;2014:89–92. 10.1093/omcr/omu034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Presotto F, Fornasini F, Betterle C et al. Acute adrenal failure as the heralding symptom of primary antiphospholipid syndrome: report of a case and review of the literature. Eur J Endocrinol 2005;153:507–14. 10.1530/eje.1.02002 [DOI] [PubMed] [Google Scholar]
- 10.Tan PL, Moore NR. Spontaneous idiopathic bilateral adrenal haemorrhage in adults. Clin Radiol 2003;58:890–2. 10.1016/S0009-9260(03)00338-6 [DOI] [PubMed] [Google Scholar]
- 11.Ogino J, Toda J, Onitsuka S et al. Idiopathic bilateral adrenal haemorrhage related to acute adrenal insufficiency. BMJ Case Rep 2013;2013; ▪▪▪ 10.1136/bcr-2013-009626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brotman DJ, Girod JP, Posch A et al. Effects of short-term glucocorticoids on hemostatic factors in healthy volunteers. Thromb Res 2006;118:247–52. 10.1016/j.thromres.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 13.Johannesdottir SA, Horváth-Puhó E, Dekkers OM et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med 2013;173:743–52. 10.1001/jamainternmed.2013.122 [DOI] [PubMed] [Google Scholar]
- 14.Sacerdote MG, Johnson PT, Fishman EK. CT of the adrenal gland: the many faces of adrenal hemorrhage. Emerg Radiol 2012;19:53–60. 10.1007/s10140-011-0989-9 [DOI] [PubMed] [Google Scholar]
- 15.Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med 2003;348:727–34. 10.1056/NEJMra020529 [DOI] [PubMed] [Google Scholar]