Abstract
The aquaporin-1 (AQP1) water channel is a potentially important drug target, as AQP1 inhibition is predicted to have therapeutic action in edema, tumor growth, glaucoma, and other conditions. Here, we measured the AQP1 inhibition efficacy of 12 putative small-molecule AQP1 inhibitors reported in six recent studies, and one AQP1 activator. Osmotic water permeability was measured by stopped-flow light scattering in human and rat erythrocytes that natively express AQP1, in hemoglobin-free membrane vesicles from rat and human erythrocytes, and in plasma membrane vesicles isolated from AQP1-transfected Chinese hamster ovary cell cultures. As a positive control, 0.3 mM HgCl2 inhibited AQP1 water permeability by >95%. We found that none of the tested compounds at 50 µM significantly inhibited or increased AQP1 water permeability in these assays. Identification of AQP1 inhibitors remains an important priority.
Introduction
The aquaporins (AQPs) are a family of small, plasma membrane proteins that transport water and/or small polar solutes such as glycerol (Carbrey and Agre, 2009; Verkman, 2012). The AQPs are a potentially important class of targets for drug development (Jeyaseelan et al., 2006; Wang et al., 2006; Frigeri et al., 2007; Verkman et al., 2014; Beitz et al., 2015), though there has been limited progress to date on the discovery and validation of AQP inhibitors. Challenges in the identification of AQP inhibitors include difficulties in assaying water permeability and the structural features of the AQPs, which include a narrow pore that excludes small molecules.
Aquaporin-1 (AQP1), originally identified as responsible for high water permeability in erythrocytes (Preston and Agre, 1991), is a particularly compelling drug target. In addition to erythrocytes, AQP1 is expressed in various fluid secreting and absorbing epithelia in kidneys, gastrointestinal organs, the central nervous system, the eye, and others as well in most microvascular endothelia including tumor microvessels (Hasegawa et al., 1994a; Nielsen et al., 1995; Mobasheri and Marples, 2004). Data largely from AQP1 knockout mice has suggested the potential utility of AQP1 inhibitors in the treatment of edema (Schnermann et al., 1998), tumors (Saadoun et al., 2005; Esteva-Font et al., 2014), and glaucoma (Zhang et al., 2002), and potentially for brain swelling (Oshio et al., 2005) and other conditions.
High-resolution structural data show that AQP1 monomers consist of six transmembrane helical segments and two partially spanning segments that surround a central aqueous pore (de Groot et al., 2001; Sui et al., 2001). AQP1 monomers further assemble in membranes as homotetramers (Verbavatz et al., 1993). Molecular dynamics modeling supports the conclusion that the single-file passage of water through the central pore of an AQP1 monomer, as well as interactions with residues lining the pore, is responsible for AQP1 water selectivity (Hub and de Groot, 2008). The narrowest section of the pore, named the ar/R constriction, consists of aromatic and arginine residues and has a diameter of 2.8 Å (Sui et al., 2001). The AQP1 pore excludes passage of small molecules and even protons, with the latter related to lack of stabilization of hydronium and/or geometric constraints (Gonen and Walz, 2006; Kato et al., 2006).
Heavy-metal, sulfhydryl-reactive small molecules such as HgCl2 inhibit AQP1 water permeability by interaction with residue cysteine 187 near the extracellular surface of the AQP1 aqueous pore (Preston et al., 1993; Zhang et al., 1993). However, because of their lack of selectivity and toxicity, heavy metals are not drug development candidates.
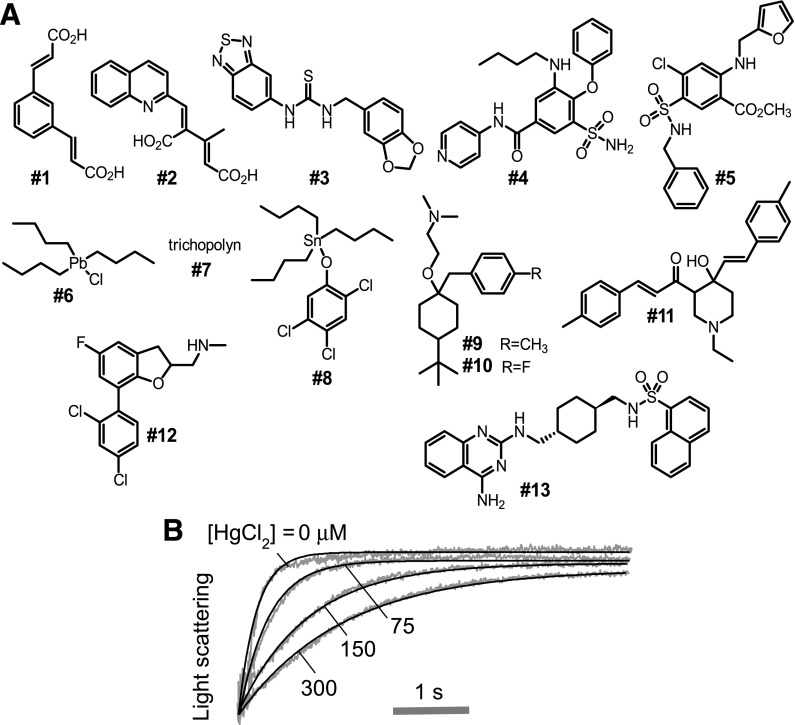
Early studies reported AQP1 inhibition by carbonic anhydrase inhibitors such as acetazolamide and K+ channel blockers such as tetraethylammonium (Brooks et al., 2000; Detmers et al., 2006), though subsequent reevaluation using different assays did not confirm AQP1 water transport inhibition by these molecules (Yang et al., 2006, 2008; Søgaard and Zeuthen, 2008; Yamaguchi et al., 2012). More recently, a variety of approaches, including high-throughput screening and computational chemistry, have yielded compounds with reported AQP1 inhibition or activation activity (Migliati et al., 2009; Mola et al., 2009; Seeliger et al., 2013; Yool et al., 2013; To et al., 2015; Patil et al., 2016), as summarized in Fig. 1 and Table 1. Motivated by the high potential clinical utility of small-molecule AQP1 inhibitors, here we tested these various proposed AQP1 inhibitors, comparing their water transport inhibition activity in different cellular systems with that of HgCl2.
Fig. 1.
Chemical structures of putative AQP1 inhibitors. (A) Structures of inhibitors reported by Migliati et al. (2009), Mola et al. (2009), Yool et al. (2013), Seeliger et al. (2013), To et al. (2015), and Patil et al. (2016) (see Table 1). (B) Osmotic water permeability in human erythrocytes as measured from the time course of scattered light intensity in response to a 250 mM inwardly directed sucrose gradient at room temperature. Erythrocytes were incubated with 0, 75, 150, or 300 µM HgCl2 for 5 minutes before measurement.
TABLE 1.
Reported small-molecule AQP1 modulators
| Compound |
Reference | Identification Method | Reported IC50 (µM) | |
|---|---|---|---|---|
| Code | Name | |||
| Inhibitor | ||||
| Compound 1 | 1,3-Phenylenediacrylic acid | Seeliger et al., 2013 | Xenopus laevis oocytes | 8 |
| Compound 2 | (E,Z)-3-methyl-4-(2-quinolinylmethylene)-2-pentenedioic acid disodium salt | Seeliger et al., 2013 | Xenopus laevis oocytes | 17 |
| Compound 3 | N-(1,3-benzodioxol-5-ylmethyl)-Nʹ-2,1,3-benzothiadiazol-5-yl-thiourea | Seeliger et al., 2013 | Xenopus laevis oocytes | 18 |
| AqB013 (Compound 4) | 3-Butylamino-4-phenoxy-N-pyridin-4-yl-5-sulfamoyl-benzamide | Migliati et al., 2009 | Virtual screen, Xenopus laevis oocytes | 20 |
| NSC168597 (Compound 6) | Tributyl lead chloride | Mola et al., 2009 | Calcein cell-based assay | 49 |
| NSC301460 (Compound 7) | Trichopolyn I | Mola et al., 2009 | Calcein cell-based assay | 28 |
| NSC164914 (Compound 8) | Tributyl-(2,4,5-trichlorophenoxy) stannane | Mola et al., 2009 | Calcein cell-based assay | 40 |
| NSC670229 (Compound 9) | 2-[4-Tert-butyl-1-[(4-methylphenyl) methyl] cyclohexyl] oxy-N,N-dimethylethanamine | Mola et al., 2009 | Calcein cell-based assay | 27 |
| NSC670226 (Compound 10) | 2-[4-Tert-butyl-1-[(4-fluorophenyl) methyl] cyclohexyl] oxy-N,N-dimethylethanamine | To et al., 2015 | Yeast freeze-thaw assay | 20 |
| NSC657298 (Compound 11) | (E)-1-[1-ethyl-4-hydroxy-4-[(E)-2-(4-methylphenyl) ethenyl] piperidin-3-yl]-3-(4-methylphenyl) prop-2-en-1-one | To et al., 2015 | Yeast freeze-thaw assay | 48 |
| Compound 12 | 1-(7-(2,4-Dichlorophenyl)-5-fluoro-2,3-dihydrobenzofuran-2-yl)-N-methylmethanamine | Patil et al., 2016 | Calcein cell-based assay | 10 |
| Compound 13 | N-[[trans-4-[[(4-amino-2-quinazolinyl) amino] methyl] cyclohexyl] methyl]-1-naphthalenesulfonamide | Patil et al., 2016 | Calcein cell-based assay | 3 |
| Activator | Reported EC50 (µM) | |||
| AqF026 (Compound 5) | 4-Chloro-2-[(2-furanylmethyl) amino]-5-[[(phenylmethyl) amino] sulfonyl]-benzoic acid methyl ester | Yool et al., 2013 | Virtual screen, Xenopus laevis oocytes | 3.3 |
Materials and Methods
Compounds.
Compounds 1 [1,3-phenylenediacrylic acid], 2 [(E,Z)-3-methyl-4-(2-quinolinylmethylene)-2-pentenedioic acid disodium salt], and 3 [N-(1,3-benzodioxol-5-ylmethyl)-Nʹ-2,1,3-benzothiadiazol-5-yl-thiourea] were purchased from ChemBridge (San Diego, CA). Compounds 6 (NSC168597 [tributyl lead chloride]), 7 (NSC301460 [trichopolyn I]), 8 (NSC164914 [tributyl-(2,4,5-trichlorophenoxy) stannane]), 9 (NSC670229 [2-[4-tert-butyl-1-[(4-methylphenyl) methyl] cyclohexyl] oxy-N,N-dimethylethanamine]), 10 (NSC670226 [2-[4-tert-butyl-1-[(4-fluorophenyl) methyl] cyclohexyl] oxy-N,N-dimethylethanamine]), and 11 (NSC657298 [(E)-1-[1-ethyl-4-hydroxy-4-[(E)-2-(4-methylphenyl) ethenyl] piperidin-3-yl]-3-(4-methylphenyl) prop-2-en-1-one]) were purchased from the National Cancer Institute. Compound 13 [N-[[trans-4-[[(4-amino-2-quinazolinyl) amino] methyl] cyclohexyl] methyl]-1-naphthalenesulfonamide] was purchased from Tocris Biosciences (Denver, CO). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Compounds 4 (AqB013 [3-butylamino-4-phenoxy-N-pyridin-4-yl-5-sulfamoyl-benzamide]), 5 (AqF026 [4-chloro-2-[(2-furanylmethyl) amino]-5-[[(phenylmethyl) amino] sulfonyl]-benzoic acid methyl ester]), and 12 [1-(7-(2,4-dichlorophenyl)-5-fluoro-2,3-dihydrobenzofuran-2-yl)-N-methylmethanamine] were synthesized. Compound 5 (AqF026) was synthesized from furosemide as reported (Yool et al., 2013), and the analytic data matched with the reported data.
Compound 4 (AqB013) was synthesized from bumetanide through an imidazolide intermediate (Migliati et al., 2009). To a suspension of bumetanide (100 mg, 0.27 mmol) in ethyl acetate (1 mL) was added 1,1ʹ-carbonyldiimidazole (50 mg, 0.3 mmol) under N2. The mixture was heated for 1 hour and cooled to room temperature. The resulting precipitate was filtered and washed with ethyl acetate and dried in vacuo to give intermediate 3-(butylamino)-5-(1H-imidazole-1-carbonyl)-2-phenoxybenzenesulfonamide as a white solid (105 mg, 90%). To this compound (10 mg, 0.024 mmol) in tetrahydrofuran (1 mL) was added 4-aminopyridine (4 mg, 0.042 mmol) and trifluoroacetic acid (catalytic amount) and stirred overnight. The reaction mixture was evaporated under reduced pressure. Compound 4 was obtained as a yellowish white solid (6 mg, 56%) after silica gel column chromatography. 1H-NMR (300 MHz, CD3OD): δ 8.19 (brs, 1H), 7.62 (m, 2H), 7.35–7.29 (m, 2H), 7.26 (d, 1H, J = 2.0 Hz), 7.15–7.13 (m, 1H), 7.08 (t, 1H, J = 6.3 Hz), 6.95 (d, 2H, J = 7.7 Hz), 3.08 (t, 2H, J = 6.9 Hz), 1.46–1.36 (m, 2H), 1.17–1.09 (m, 2H), 0.79 (t, 2H, J = 7.3 Hz); liquid chromatography with mass spectrometry (electrospray ionization): 441 (M+H)+.
Compound 12 was synthesized by Suzuki coupling of (7-bromo-5-fluoro-2,3-dihydrobenzofuran-2-yl)methyl-4-methylbenzenesulfonate and 2,4-dichlorophenylboronic acid under microwave irradiation, followed by alkylation with methyl amine at 60°C in dimethylsulfoxide (DMSO) overnight. 1H-NMR (300 MHz, CD3OD): δ 7.56 (dd, 1H, J = 1.7, 0.6 Hz), 7.39–7.37 (m, 2H), 7.05–7.02 (m, 1H), 6.80 (dd, 1H, J = 9.5, 2.7 Hz), 4.99–4.96 (m, 1H), 3.42 (m, 1H), 3.06–2.83 (m, 3H), 2.45 (s, 3H); 13C-NMR (75 MHz, CD3OD): δ 153.0, 152.4, 134.3, 133.8, 132.4, 128.9, 128.8, 126.8, 120.4, 120.3, 116.1, 114.7, 112.1, 81.9, 54.8, 34.4, 33.2; liquid chromatography with mass spectrometry (electrospray ionization): 326 (M+H)+.
Collection of Human and Rat Blood.
Human venous blood obtained from a single donor was collected into K3EDTA Vacutainers (Greiner, Kremsmunster, Austria). Whole rat blood was collected from adult Wistar rats (250–300 g) purchased from Charles River Laboratories (Wilmington, MA) by cardiac puncture under isoflurane anesthesia. Animal protocols were approved by the University of California, San Francisco Committee on Animal Research.
Preparation of Hemoglobin-Free Erythrocyte Ghosts.
Ghost membranes were prepared by the procedure of Zeidel et al. (1992), with modifications. Collected blood was washed 3 times with phosphate-buffered saline (PBS) by centrifugation at 800g for 5 minutes at 4°C. The erythrocyte pellet was resuspended in 0.1x PBS (hypotonic buffer), and the membranes were washed twice in the same buffer by centrifugation at 30,000g for 10 minutes at 4°C. Hypertonic (10x) PBS was added to restore isotonicity, and membranes were incubated for 1 hour at 37°C to allow resealing. The resulting ghost membrane vesicles were resuspended at ∼0.4 mg protein/ml for stopped-flow measurements.
Erythrocyte Labeling.
Erythrocytes were washed 3 times with PBS (3000g, 15 minutes) and then fluorescently labeled by incubation with 15 μM calcein-AM (Invitrogen, Carlsbad, CA) at 37°C for 1.5 hours. Erythrocytes were then washed twice with PBS (3000g, 10 minutes) to remove extracellular dye and diluted 15-fold in PBS.
Cell Culture.
Chinese hamster ovary (CHO) cells stably expressing human AQP1 or AQP4-M23, generated as reported (Ma et al., 1993, Phuan et al., 2012), and nontransfected CHO cells were grown in Ham’s F-12 nutrient medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. Geneticin (500 μg/ml) was used as a selection marker for the cells expressing AQP1 and AQP4-M23. Cells were grown at 37°C in 5% CO2 and 95% air.
Preparation of Plasma Membrane Vesicles from CHO Cells.
Enriched plasma membrane fractions were prepared as described elsewhere (Rossi et al., 2012). CHO cells from 10 confluent 175 cm2 flasks (Thermo Scientific, Rochester, NY) were homogenized by 20 strokes of a glass Dounce homogenizer in 250 mM sucrose, 10 mM Tris-HCl, and 1 mM EDTA containing protease inhibitors (Complete Mini; Roche Diagnostics, Mannheim, Germany). The homogenate was then centrifuged at 4000g for 15 minutes at 4°C, and the enriched plasma membrane fraction was obtained by centrifugation at 17,000g for 45 minutes. The resultant pellet was suspended in PBS for stopped-flow measurements.
Stopped-Flow Measurements.
Osmotic water permeability was measured by stopped-flow light scattering (or fluorescence) using a Hi-Tech Sf-51 instrument (Wiltshire, United Kingdom) as described by Jin et al. (2015). Intact erythrocytes (hematocrit ∼0.5%), hemoglobin-free erythrocyte ghost membranes (∼0.4 mg protein/ml), plasma membrane vesicles from CHO cells (∼0.8 mg protein/ml), or calcein-labeled erythrocytes were suspended in PBS and subjected to a 250 mOsm inwardly directed gradient of sucrose. Some experiments were performed with a 150 mOsm outwardly directed NaCl gradient produced by mixing equal volumes of the membrane suspension in PBS with distilled water. The resultant kinetics of cell volume were measured from the time course of 90° scattered light intensity at 530 nm (or calcein fluorescence) in which increasing scattered light intensity corresponds to decreasing cell volume.
For the testing of putative AQP1 modulators, compounds in DMSO (0.5% final DMSO concentration) were incubated with cell or membrane suspensions for >10 minutes at 50 µM before stopped-flow measurement. Relative osmotic water permeability was determined by exponential regression using Prism software (GraphPad Software, San Diego, CA).
Erythrocyte Hemoglobin Release.
Compounds (50 µM, 0.5% final DMSO concentration) were incubated with a freshly prepared erythrocyte suspension for 15 minutes and centrifuged at 3000 rpm for 10 minutes. Hemoglobin released in the supernatant was measured by absorbance at 540 nm. In some studies, erythrocyte morphology was assessed by phase-contrast microscopy at 1000× magnification.
Statistical Analysis.
Data are presented as the mean standard error of the mean (S.E.M.) of at least four independent experiments, and were analyzed with paired Student’s t test or one-way analysis of variance (ANOVA).
Results
Figure 1A shows chemical structures of the 12 putative AQP1 inhibitors and one AQP1 activator studied here. HgCl2 was used as a positive control for inhibition. Figure 1B shows HgCl2 concentration-dependent inhibition of water permeability in human erythrocytes, which natively express AQP1. Osmotic water permeability was measured by the established stopped-flow light-scattering method in which a dilute erythrocyte suspension was mixed rapidly with an anisosmolar solution to impose a 250 mM inwardly directed sucrose gradient. The sucrose gradient causes osmotic water efflux and cell shrinkage, seen as increasing scattered light intensity at 530 nm wavelength. The IC50 for HgCl2 inhibition of erythrocyte AQP1 water permeability was ∼85 µM.
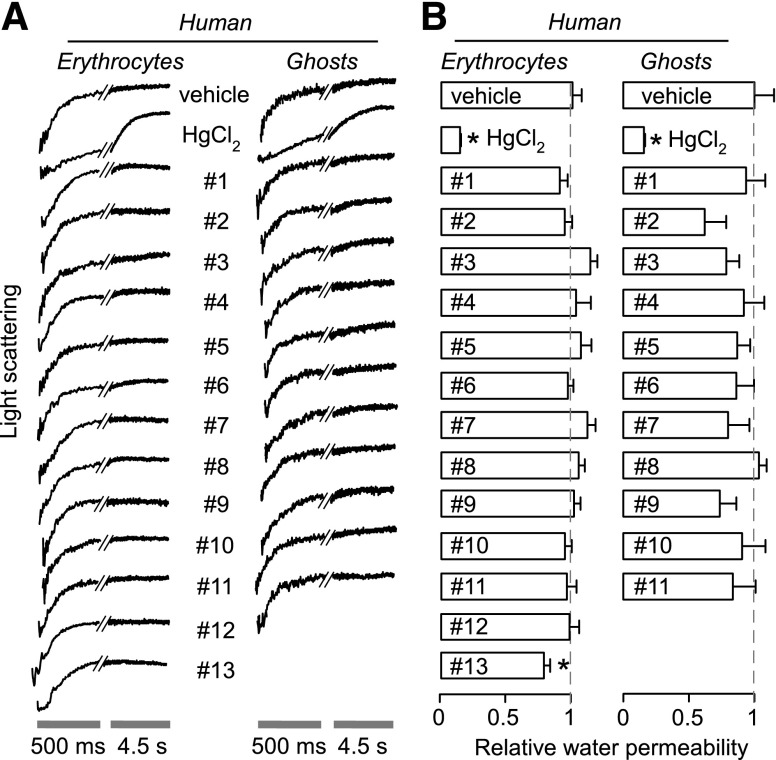
Similar studies were performed for the putative AQP1 modulators at 50 µM, a concentration predicted from published data to strongly inhibit (or weakly activate) AQP1 water permeability (published IC50 values listed in Table 1). Compounds were incubated with the erythrocyte suspension for at least 15 minutes before stopped-flow measurements. Representative light-scattering curves are shown for human erythrocytes in Fig. 2A (left) with averaged data (± S.E.M.) summarized in Fig. 2B (left). Whereas HgCl2 strongly inhibited osmotic water permeability in human erythrocytes, no significant effect was seen for any of the 13 test compounds.
Fig. 2.
Osmotic water permeability in human erythrocytes and hemoglobin-free ghost membranes derived therefrom. Osmotic water permeability was measured from the time course of scattered-light intensity in response to a 250 mM inwardly directed sucrose gradient. (A) Representative time course data for negative control (0.5% DMSO vehicle alone), 0.3 mM HgCl2 (positive control), and indicated compounds (each 50 μM). Cells and ghosts were incubated with test compounds for 15 minutes before measurements. (B) Relative osmotic water permeability (S.E., n = 4). *P < 0.05 compared with control.
Reasoning that the lack of inhibition might be due to the presence of hemoglobin in the erythrocyte cytoplasm, which potentially could bind compounds, we performed similar studies in sealed, hemoglobin-free ghost membranes prepared from human erythrocytes. Similar to the results in Fig. 2A, no significant effect on osmotic water permeability by the test compounds was seen in ghost membranes, with HgCl2 showing strong inhibition as positive control.
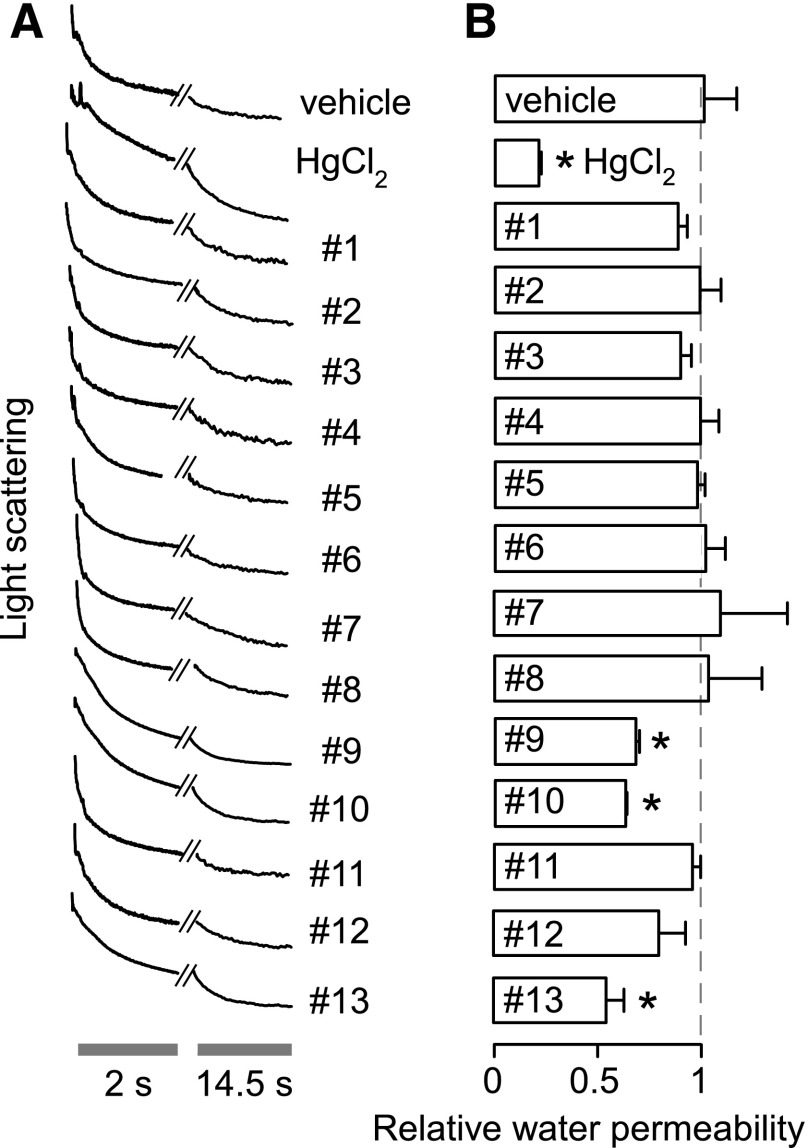
As it is possible, though unlikely, that inhibition efficacy could depend on the direction of water flow, compounds were also tested in human erythrocytes using a stopped-flow light-scattering assay of osmotic swelling in which cells were exposed to 50% hypotonic saline. Compounds 9, 10, and 13 showed apparent weak inhibition of water permeability (30%–40% at 50 µM) whereas the other compounds had no effect on water permeability (Fig. 3).
Fig. 3.
Osmotic swelling of human erythrocytes. Osmotic water permeability was measured from the time course of scattered-light intensity in response to a 150 mOsm outwardly directed osmotic gradient. (A) Representative time course data for negative control (0.5% DMSO vehicle alone), 0.3 mM HgCl2 (positive control), and indicated compounds (each 50 μM). (B) Relative osmotic water permeability (S.E., n = 4). *P < 0.05 compared with negative control (0.5% DMSO vehicle alone). Cells and ghosts were incubated with test compounds for ∼15 minutes before measurements.
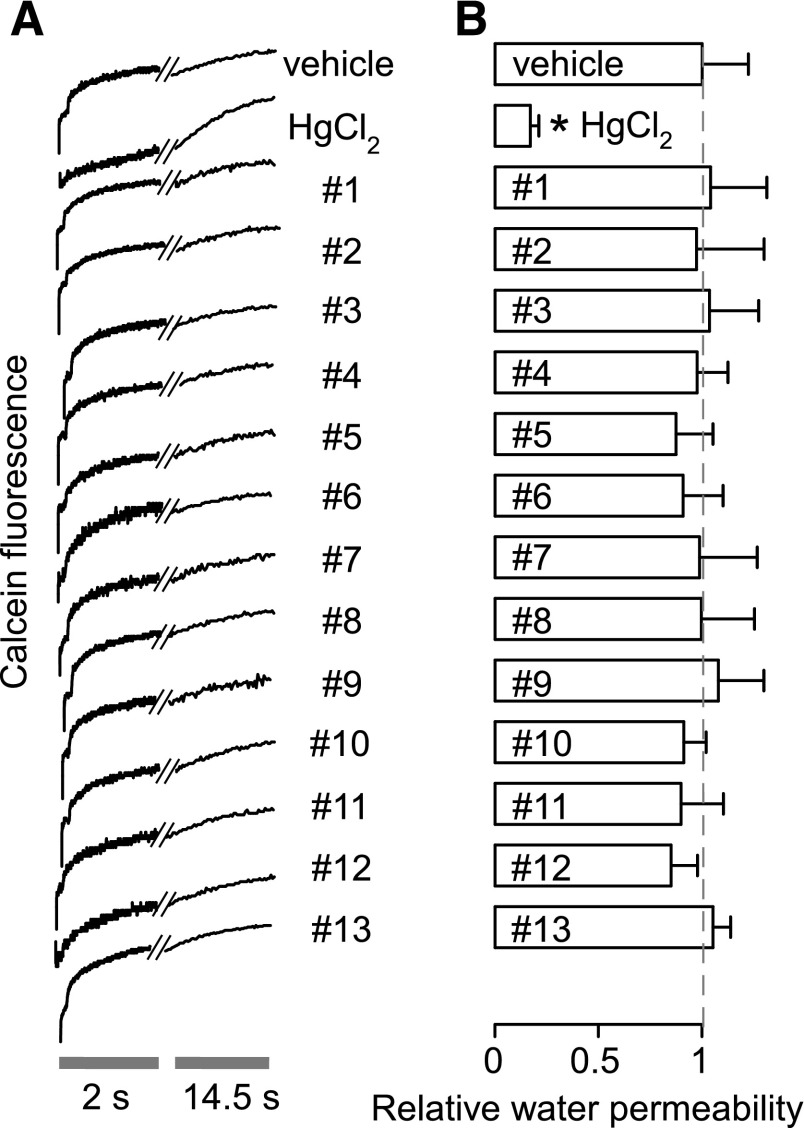
Because potential artifacts in light-scattering assays could be produced, for example, by erythrocyte crenation or aggregation, compounds were also tested using a calcein-quenching assay of osmotic swelling. This assay is based on volume-dependent calcein quenching, which is insensitive to factors such as cell shape that can affect light scattering. Figure 4 shows that none of the 13 compounds significantly affected erythrocyte water permeability. The reduced fluorescence signal intensity for compound 12 suggests partial erythrocyte lysis.
Fig. 4.
Osmotic swelling of calcein-labeled human erythrocytes. Osmotic water permeability was measured from the time course of intracellular calcein fluorescence in response to a 150 mOsm outwardly directed osmotic gradient. (A) Representative time course data for negative control (0.5% DMSO vehicle alone), 0.3 mM HgCl2 (positive control), and indicated compounds (each 50 μM). (B) Relative osmotic water permeability (S.E., n = 4). *P < 0.05 compared with negative control (0.5% DMSO vehicle alone).
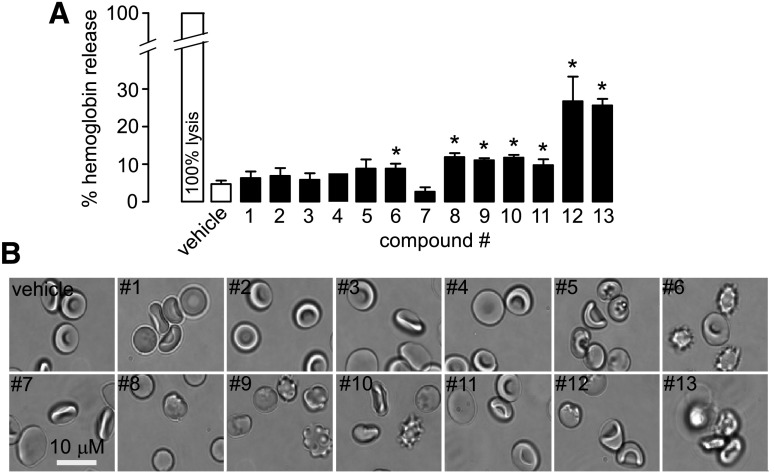
To investigate the possible reasons for the effects of compounds 9, 10, and 13 in the light-scattering assay of osmotic swelling, we evaluated erythrocyte toxicity by use of a hemoglobin-release assay and by cell morphology. Compounds 6 and 8 to 13 caused significantly greater hemoglobin release than the vehicle control. Direct examination of erythrocyte morphology at high magnification showed marked abnormalities for compounds 6, 9, 10, 12, and 13, with cell crenation and variable aggregation (Fig. 5B). These abnormalities may account for the apparent artifacts in the light-scattering assay of cell swelling.
Fig. 5.
Compound effects on hemoglobin release and erythrocyte morphology. (A) Hemoglobin release from human erythrocytes after 15 minutes of incubation with test compounds at 50 µM (S.E., n = 4). *P < 0.05 compared with control. (B) Representative phase-contrast photomicrographs of human erythrocytes after 15-minute incubations with test compounds.
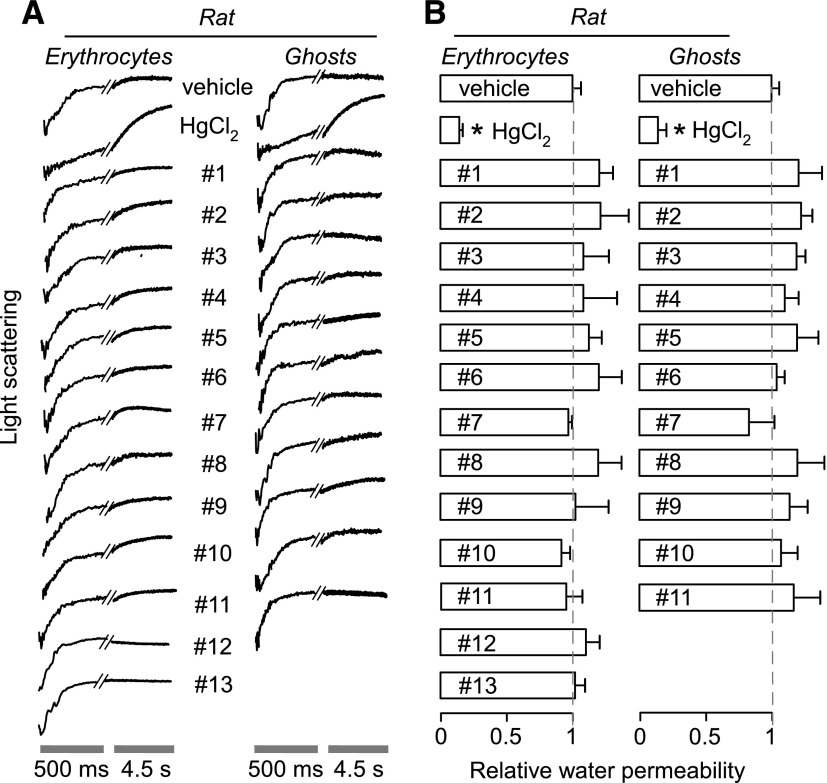
Stopped-flow light-scattering measurements were also performed using rat erythrocytes, which express rat AQP1, and sealed, hemoglobin-free ghost membranes prepared from rat erythrocytes (Fig. 6). As found with human erythrocytes and ghost membranes, no significant effect on osmotic water permeability was seen for any of the test compounds, with HgCl2 showing strong inhibition as positive control.
Fig. 6.
Osmotic water permeability in rat erythrocytes and hemoglobin-free ghost membranes derived therefrom. Osmotic water permeability was measured from the time course of scattered light intensity in response to a 250 mM inwardly directed sucrose gradient. (A) Representative time course data for negative control (0.5% DMSO vehicle alone), 0.3 mM HgCl2 (positive control), and indicated compounds (each 50 μM). Cells and ghosts were incubated with test compounds for ∼15 minutes before measurements. (B) Relative osmotic water permeability (S.E., n = 4). *P < 0.05 compared with control.
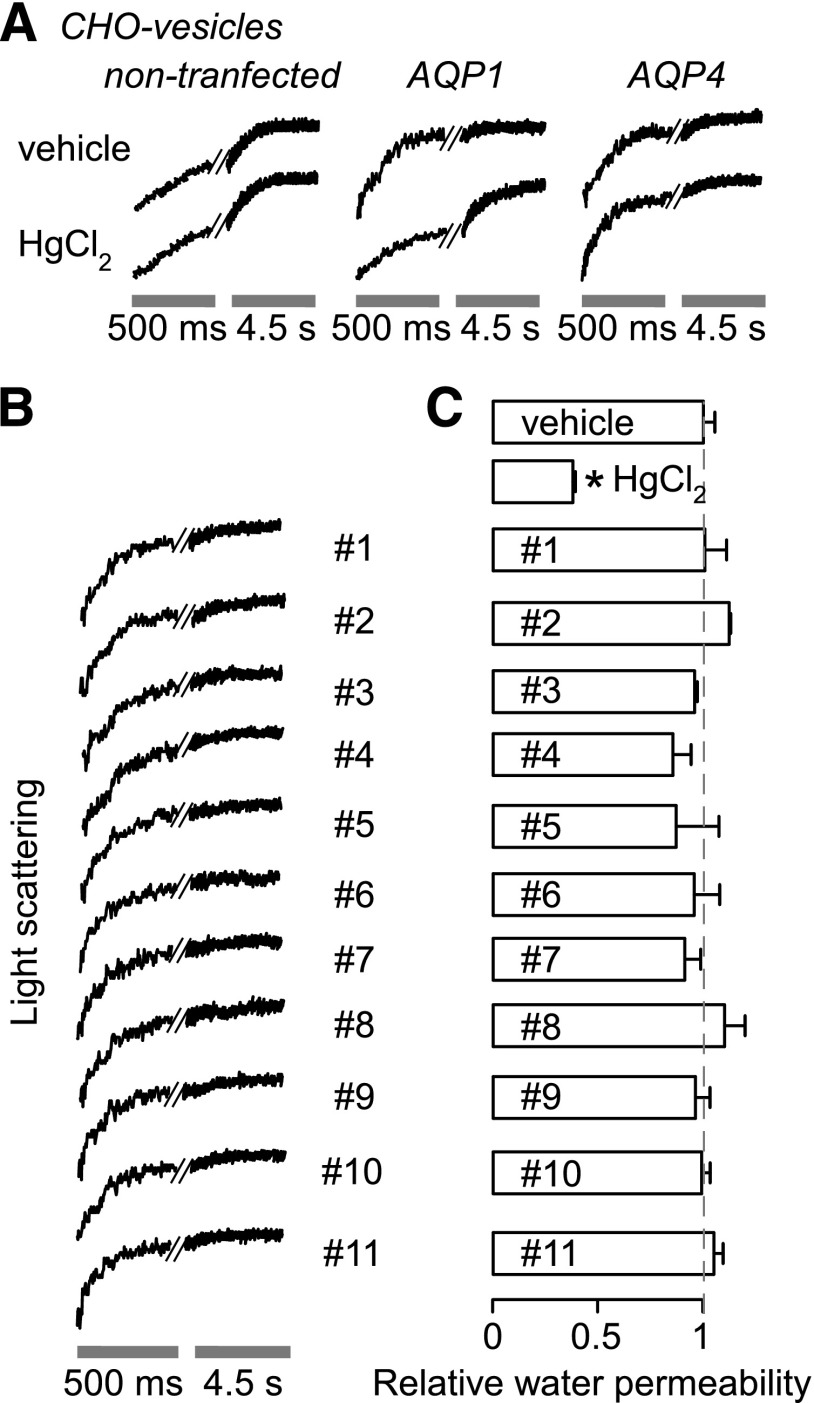
To test for AQP1 inhibition in a different cellular context, AQP1-enriched plasma membrane vesicles were isolated from AQP1-transfected (and control) CHO cells by homogenization and differential centrifugation. Osmotic water permeability was measured by stopped-flow light scattering as done for the erythrocytes and erythrocyte ghost membranes. Figure 7A shows that membrane vesicle shrinkage was ∼5-fold more rapid in vesicles prepared from AQP1-transfected CHO cells than control (nontransfected) CHO cells. Osmotic water permeability was inhibited by ∼85% by 0.3 mM HgCl2.
Fig. 7.
Osmotic water permeability in plasma membrane vesicles from CHO cells. Osmotic water permeability was measured from the time course of scattered-light intensity in response to a 250 mM inwardly directed sucrose gradient. (A) Representative time course data for negative control (0.5% DMSO vehicle alone) and 0.3 mM HgCl2 in vesicles from nontransfected CHO cells expressing AQP1- and AQP4-M23. (B) Representative time course data for indicated compounds (each 50 μM). Vesicles were incubated with test compounds for ∼15 minutes before measurements. (C) Relative osmotic water permeability (S.E., n = 4). *P < 0.05 compared with control.
As another control, plasma membrane vesicles were prepared from CHO cells expressing AQP4, a water-selective channel that was originally named MIWC (mercurial-insensitive water channel) (Hasegawa et al., 1994b), whose water permeability is not inhibited by HgCl2 because of absence of a critical cysteine residue (Shi and Verkman, 1996). Osmotic water permeability in the AQP4-containing membrane vesicles was ∼5-fold more rapid than in control vesicles, and, in contrast to the AQP1-containing vesicles, it is not inhibited by HgCl2.
Figure 7B shows representative light-scattering data and averaged results for measurements of osmotic water permeability in the AQP1-containing plasma membrane vesicles. No significant effect was found for any of the test compounds.
Discussion
We tested 12 putative AQP1 inhibitors and one putative activator for their efficacy in reducing or increasing osmotic water permeability in rat and human erythrocytes and ghost membranes, and plasma membrane vesicles from AQP1-transfected CHO cells. At 50 µM, a concentration well above reported IC50 values for each of the compounds, no significant water transport inhibition or activation was found using stopped-flow assays with sensitivity to detect as small as a 5%–10% change in water permeability. The well-studied, albeit nonselective AQP1 inhibitor HgCl2 showed strong inhibition in all assay systems. The small apparent inhibition by compounds 9, 10, and 13 seen in the light-scattering swelling assay in human erythrocytes was likely an artifact, as these compounds were found to cause cell crenation and aggregation, and inhibition was not seen in the calcein fluorescence quenching assay. We conclude that, other than nonselective, sulfhydryl-reactive, heavy metal-containing compounds, no confirmed small-molecule AQP1 inhibitors have been reported to date. Albeit a negative study, the work here underscores the need to test putative AQP inhibitors using robust, sensitive assays, and, given the major potential clinical applications of AQP1 inhibitors, the need for continued screening and computational work to identify useful inhibitors.
It is not known why AQP1 appears to be refractory to identification of small-molecule inhibitors. Part of the reason may be its unique structure, in which small, relatively rigid monomers containing water-only pores are assembled in membranes as homotetramers. The narrow AQP1 water pore excludes small molecules; even if a small molecule could bind to the vestibule adjacent to the pore, there may remain many paths for water flow around the small molecule, as if trying to cork a wine bottle with a randomly shaped stone. However, though direct pore blockade may be difficult to achieve, allosteric closure of the pore from a distant site would seem possible. Perhaps the tight, relatively rigid structure of the AQP1 monomer, as well as its narrow pore region and small extracellular footprint, resists allosteric pore closure by externally bound molecules. In unpublished work (M.O. Anderson, C. Esteva-Font, and A.S. Verkman) we did not identify any useful AQP1 inhibitors from a screen of ∼150,000 synthetic small molecules with an erythrocyte lysis assay that was used successfully to identify nanomolar-potency urea transport inhibitors (Levin et al., 2007). We also did not identify any useful AQP1 inhibitors in a computational docking study of 106 commercially available compounds, followed by a water transport assay of 2000 compounds with the highest docking scores.
It is also unclear why many putative AQP1 modulators have been reported in the literature, but water transport inhibition (or activation) cannot be confirmed, including the 13 small molecules studied here, and previously acetazolamide, tetraethylammonium, and dimethylsulfoxide (Yang et al., 2006; Tanimura et al., 2009). As discussed elsewhere (Verkman et al., 2014), part of the reason may be that the functional assays largely relied on the Xenopus oocyte expression system and calcein-loaded cell cultures, both of which are subject to artifacts. For example, compounds that affect cell size or shape, cell volume regulation, nonaquaporin ion or solute transporters, or calcein fluorescence quenching could appear to inhibit water permeability in these systems. Inhibitors of cellular proteins involved in major transport functions, such as bumetanide, acetazolamide, and tetraethylammonium, may affect resting cell volume and volume regulation. Artifacts in water transport measurements using the Xenopus oocyte expression system have also led to the conclusion that a wide variety of drugs, including many common, chemically unrelated antiepileptics and carbonic anhydrase inhibitors, inhibit brain water channel aquaporin-4 (AQP4) (Huber et al., 2009), with subsequent measurements failing to confirm inhibition (Yang et al., 2008). Given the apparent very low probability of identifying AQP inhibitors, it seems a priori unlikely that testing common drugs such as bumetanide, acetazolamide, antimigraines, and antiepileptics without large-scale screening would yield AQP inhibitors. It also seems unlikely that small molecules could activate AQP1, as its water permeability is constitutively high and not subject to regulation.
Compounds 1, 2, and 3 were identified by virtual (computational) screening of ∼106 compounds of the ZINC database and testing 14 compounds for inhibition of osmotic swelling in AQP1-expressing Xenopus laevis oocytes (Seeliger et al., 2013). The compounds were identified by molecular docking computations to a part of the extracellular surface of human AQP1. Compounds 1, 2, and 3 showed ∼80% reduced osmotic swelling of the oocytes with an IC50 of 8–20 µM. However, as found here, no inhibition was seen in light-scattering measurements on erythrocytes, suggesting an oocyte-specific action. A bona fide AQP1 inhibitor would be expected to produce inhibition in different assays and different cell systems.
Migliati et al. (2009) screened known channel blockers using the oocyte swelling assay. Although the identities and numbers of tested blockers were not mentioned, they focused on loop diuretic inhibitors of the NKCC cotransporter, reporting inhibition by bumetanide. Of 45 bumetanide scaffolds synthesized, compound 4 here (AqB013) was identified in oocyte assays as an inhibitor of AQP1 and AQP4 with IC50 ∼20 µM. In a follow-on study by the same investigators, compound 4 was tested in a brain injury model for reducing edema, though no beneficial effect was found (Oliva et al., 2011). The same group later found that an analog of the loop diuretic furosemide, compound 5 here (AqF026), activated AQP1, increasing its water permeability by ∼20% in the oocyte assay (Yool et al., 2013). The possibility that off-target actions of these compounds might be responsible for the apparent effects on oocyte water permeability, such as actions of the many NKCC-related ion transporters, was not considered.
Mola et al. (2009) screened approximately 3500 compounds using a calcein fluorescence assay in AQP1 and AQP4-expressing cells in a plate-reader assay in which cells were exposed to a 200 mM inwardly directed gradient of NaCl. Active compounds from the screen were retested using erythrocytes and vesicles derived from AQP4-expressing cells. Compounds 6, 7, 8, and 9 were reported to inhibit AQP1 with IC50 values of 25–50 µM. In our hands these compounds showed marked toxicity at 100 µM (data not shown). Compounds 6, 7, and 8 are non-drug-like; compounds 6 and 8 are organolead and organotin molecules, respectively, which as a general class of molecules are considered to be neurotoxins (Chang, 1990) and cause erythrocyte lysis (Kleszcyńska et al., 1997). Compound 7 (trichopolyn I) is a structurally complex 10-residue lipopeptide isolated from the fungus Trichoderma polysporum that belongs to the trichogin class of lipopeptaibols antibiotics whose mechanism of action is thought to be pore formation in bacterial cell membranes (de Zotti et al., 2009).
Recently, To et al. (2015) reported two compounds, 10 (an analog of 9) and 11, as AQP1 inhibitors using a yeast freeze-thaw assay performed in Escherichia coli expressing AQP1, in which cell viability was measured after two-cycles of freeze-thaw. The stated, though nonvalidated, rationale is that AQP1 permits water efflux during thawing and prevents cell bursting. Their study included light-scattering measurements on erythrocytes, though interpretation of possible inhibitory effects was confounded by the multiexponential kinetics of the light-scattering data. Very recently, Patil et al. (2016) reported compounds 12 and 13 as AQP1 inhibitors in a small screen. Apparent compound activities were quite variable in Xenopus oocyte, erythrocyte ghost, and AQP1 proteoliposome assays. Here, we found both compounds to be toxic to erythrocytes and vesicles.
In conclusion, we could not demonstrate modulation of AQP1 function by 12 reported inhibitors and one reported activator using several direct assays of osmotic water permeability and different assay conditions. In view of the multiple potential clinical applications of AQP1 inhibitors, the identification of AQP1 inhibitors remains a high priority.
Abbreviations
- AqB013
3-butylamino-4-phenoxy-N-pyridin-4-yl-5-sulfamoyl-benzamide
- AqF026
4-chloro-2-[(2-furanylmethyl) amino]-5-[[(phenylmethyl) amino] sulfonyl]-benzoic acid methyl ester
- AQP
aquaporin
- CHO
Chinese hamster ovary
- DMSO
dimethylsulfoxide
- HTS
high-throughput screening
- NSC164914
tributyl-(2,4,5-trichlorophenoxy) stannane
- NSC168597
tributyl lead chloride
- NSC301460
trichopolyn I
- NSC657298
(E)-1-[1-ethyl-4-hydroxy-4-[(E)-2-(4-methylphenyl) ethenyl] piperidin-3-yl]-3-(4-methylphenyl) prop-2-en-1-one
- NSC670226
2-[4-tert-butyl-1-[(4-fluorophenyl) methyl] cyclohexyl] oxy-N,N-dimethylethanamine
- NSC670229
2-[4-tert-butyl-1-[(4-methylphenyl) methyl] cyclohexyl] oxy-N,N-dimethylethanamine
- PBS
phosphate-buffered saline
Authorship Contributions
Participated in research design: Esteva-Font, Phuan, Anderson, Verkman.
Conducted experiments: Esteva-Font, Jin, Lee.
Wrote or contributed to the writing of the manuscript: Esteva-Font, Lee, Phuan, Anderson, Verkman.
Footnotes
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants DK101373, DK35124, DK72517, DK99803], the National Institute of Biomedical Imaging and Bioengineering [Grant EB00415], and the National Eye Institute [Grant EY13574].
References
- Beitz E, Golldack A, Rothert M, von Bülow J. (2015) Challenges and achievements in the therapeutic modulation of aquaporin functionality. Pharmacol Ther 155:22–35. [DOI] [PubMed] [Google Scholar]
- Brooks HL, Regan JW, Yool AJ. (2000) Inhibition of aquaporin-1 water permeability by tetraethylammonium: involvement of the loop E pore region. Mol Pharmacol 57:1021–1026. [PubMed] [Google Scholar]
- Carbrey JM, Agre P. (2009) Discovery of the aquaporins and development of the field. Handbook Exp Pharmacol 190:3–28. [DOI] [PubMed] [Google Scholar]
- Chang LW. (1990) The neurotoxicology and pathology of organomercury, organolead, and organotin. J Toxicol Sci 15 (Suppl 4):125–151. [DOI] [PubMed] [Google Scholar]
- de Groot BL, Engel A, Grubmüller H. (2001) A refined structure of human aquaporin-1. FEBS Lett 504:206–211. [DOI] [PubMed] [Google Scholar]
- De Zotti M, Biondi B, Formaggio F, Toniolo C, Stella L, Park Y, Hahm KS. (2009) Trichogin GA IV: an antibacterial and protease-resistant peptide. J Pept Sci 15:615–619. [DOI] [PubMed] [Google Scholar]
- Detmers FJ, de Groot BL, Müller EM, Hinton A, Konings IB, Sze M, Flitsch SL, Grubmüller H, Deen PM. (2006) Quaternary ammonium compounds as water channel blockers. Specificity, potency, and site of action. J Biol Chem 281:14207–14214. [DOI] [PubMed] [Google Scholar]
- Esteva-Font C, Jin BJ, Verkman AS. (2014) Aquaporin-1 gene deletion reduces breast tumor growth and lung metastasis in tumor-producing MMTV-PyVT mice. FASEB J 28:1446–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigeri A, Nicchia GP, Svelto M. (2007) Aquaporins as targets for drug discovery. Curr Pharm Des 13:2421–2427. [DOI] [PubMed] [Google Scholar]
- Gonen T, Walz T. (2006) The structure of aquaporins. Q Rev Biophys 39:361–396. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Lian SC, Finkbeiner WE, Verkman AS. (1994a) Extrarenal tissue distribution of CHIP28 water channels by in situ hybridization and antibody staining. Am J Physiol 266:C893–C903. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Ma T, Skach W, Matthay MA, Verkman AS. (1994b) Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J Biol Chem 269:5497–5500. [PubMed] [Google Scholar]
- Hub JS, de Groot BL. (2008) Mechanism of selectivity in aquaporins and aquaglyceroporins. Proc Natl Acad Sci USA 105:1198–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber VJ, Tsujita M, Kwee IL, Nakada T. (2009) Inhibition of aquaporin 4 by antiepileptic drugs. Bioorg Med Chem 17:418–424. [DOI] [PubMed] [Google Scholar]
- Jeyaseelan K, Sepramaniam S, Armugam A, Wintour EM. (2006) Aquaporins: a promising target for drug development. Expert Opin Ther Targets 10:889–909. [DOI] [PubMed] [Google Scholar]
- Jin BJ, Esteva-Font C, Verkman AS. (2015) Droplet-based microfluidic platform for measurement of rapid erythrocyte water transport. Lab Chip 15:3380–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleszcyńska H, Hładyszowski J, Pruchnik H, Przestalski S. (1997) Erythrocyte hemolysis by organic tin and lead compounds. Z Naturforsch C 52:65–69. [PubMed] [Google Scholar]
- Kato M, Pisliakov AV, Warshel A. (2006) The barrier for proton transport in aquaporins as a challenge for electrostatic models: the role of protein relaxation in mutational calculations. Proteins 64:829–844. [DOI] [PubMed] [Google Scholar]
- Levin MH, de la Fuente R, Verkman AS. (2007) Urearetics: a small molecule screen yields nanomolar potency inhibitors of urea transporter UT-B. FASEB J 21:551–563. [DOI] [PubMed] [Google Scholar]
- Ma T, Frigeri A, Tsai ST, Verbavatz JM, Verkman AS. (1993) Localization and functional analysis of CHIP28k water channels in stably transfected Chinese hamster ovary cells. J Biol Chem 268:22756–22764. [PubMed] [Google Scholar]
- Migliati E, Meurice N, DuBois P, Fang JS, Somasekharan S, Beckett E, Flynn G, Yool AJ. (2009) Inhibition of aquaporin-1 and aquaporin-4 water permeability by a derivative of the loop diuretic bumetanide acting at an internal pore-occluding binding site. Mol Pharmacol 76:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobasheri A, Marples D. (2004) Expression of the AQP-1 water channel in normal human tissues: a semiquantitative study using tissue microarray technology. Am J Physiol Cell Physiol 286:C529–C537. [DOI] [PubMed] [Google Scholar]
- Mola MG, Nicchia GP, Svelto M, Spray DC, Frigeri A. (2009) Automated cell-based assay for screening of aquaporin inhibitors. Anal Chem 81:8219–8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Pallone T, Smith BL, Christensen EI, Agre P, Maunsbach AB. (1995) Aquaporin-1 water channels in short and long loop descending thin limbs and in descending vasa recta in rat kidney. Am J Physiol 268:F1023–F1037. [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jr, Kang Y, Truettner JS, Sanchez-Molano J, Furones C, Yool AJ, Atkins CM. (2011) Fluid-percussion brain injury induces changes in aquaporin channel expression. Neuroscience 180:272–279. [DOI] [PubMed] [Google Scholar]
- Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT. (2005) Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J 19:76–78. [DOI] [PubMed] [Google Scholar]
- Patil RV, Xu S, van Hoek AN, Rusinko A, Feng Z, May J, Hellberg M, Sharif NA, Wax MB, Irigoyen M, et al. (2016) Rapid identification of novel inhibitors of the human aquaporin-1 water channel. Chem Biol Drug Des DOI: 10.1111/cbdd.12713 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuan PW, Ratelade J, Rossi A, Tradtrantip L, Verkman AS. (2012) Complement-dependent cytotoxicity in neuromyelitis optica requires aquaporin-4 protein assembly in orthogonal arrays. J Biol Chem 287:13829–13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston GM, Jung JS, Guggino WB, Agre P. (1993) The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J Biol Chem 268:17–20. [PubMed] [Google Scholar]
- Preston GM, Agre P. (1991) Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA 88:11110–11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Baumgart F, van Hoek AN, Verkman AS. (2012) Post-Golgi supramolecular assembly of aquaporin-4 in orthogonal arrays. Traffic 13:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. (2005) Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 434:786–792. [DOI] [PubMed] [Google Scholar]
- Schnermann J, Chou CL, Ma T, Traynor T, Knepper MA, Verkman AS. (1998) Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proc Natl Acad Sci USA 95:9660–9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeliger D, Zapater C, Krenc D, Haddoub R, Flitsch S, Beitz E, Cerdà J, de Groot BL. (2013) Discovery of novel human aquaporin-1 blockers. ACS Chem Biol 8:249–256. [DOI] [PubMed] [Google Scholar]
- Shi LB, Verkman AS. (1996) Selected cysteine point mutations confer mercurial sensitivity to the mercurial-insensitive water channel MIWC/AQP-4. Biochemistry 35:538–544. [DOI] [PubMed] [Google Scholar]
- Søgaard R, Zeuthen T. (2008) Test of blockers of AQP1 water permeability by a high-resolution method: no effects of tetraethylammonium ions or acetazolamide. Pflugers Arch 456:285–292. [DOI] [PubMed] [Google Scholar]
- Sui H, Han BG, Lee JK, Walian P, Jap BK. (2001) Structural basis of water-specific transport through the AQP1 water channel. Nature 414:872–878. [DOI] [PubMed] [Google Scholar]
- Tanimura Y, Hiroaki Y, Fujiyoshi Y. (2009) Acetazolamide reversibly inhibits water conduction by aquaporin-4. J Struct Biol 166:16–21. [DOI] [PubMed] [Google Scholar]
- To J, Yeo CY, Soon CH, Torres J. (2015) A generic high-throughput assay to detect aquaporin functional mutants: potential application to discovery of aquaporin inhibitors. Biochim Biophys Acta 1850:1869–1876. [DOI] [PubMed] [Google Scholar]
- Verbavatz JM, Brown D, Sabolić I, Valenti G, Ausiello DA, Van Hoek AN, Ma T, Verkman AS. (1993) Tetrameric assembly of CHIP28 water channels in liposomes and cell membranes: a freeze-fracture study. J Cell Biol 123:605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS. (2012) Aquaporins in clinical medicine. Annu Rev Med 63:303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS, Anderson MO, Papadopoulos MC. (2014) Aquaporins: important but elusive drug targets. Nat Rev Drug Discov 13:259–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Feng XC, Li YM, Yang H, Ma TH. (2006) Aquaporins as potential drug targets. Acta Pharmacol Sin 27:395–401. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Iwata Y, Miura S, Kawada K. (2012) Reinvestigation of drugs and chemicals as aquaporin-1 inhibitors using pressure-induced hemolysis in human erythrocytes. Biol Pharm Bull 35:2088–2091. [DOI] [PubMed] [Google Scholar]
- Yang B, Kim JK, Verkman AS. (2006) Comparative efficacy of HgCl2 with candidate aquaporin-1 inhibitors DMSO, gold, TEA+ and acetazolamide. FEBS Lett 580:6679–6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Zhang H, Verkman AS. (2008) Lack of aquaporin-4 water transport inhibition by antiepileptics and arylsulfonamides. Bioorg Med Chem 16:7489–7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yool AJ, Morelle J, Cnops Y, Verbavatz JM, Campbell EM, Beckett EA, Booker GW, Flynn G, Devuyst O. (2013) AqF026 is a pharmacologic agonist of the water channel aquaporin-1. J Am Soc Nephrol 24:1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidel ML, Ambudkar SV, Smith BL, Agre P. (1992) Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry 31:7436–7440. [DOI] [PubMed] [Google Scholar]
- Zhang D, Vetrivel L, Verkman AS. (2002) Aquaporin deletion in mice reduces intraocular pressure and aqueous fluid production. J Gen Physiol 119:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, van Hoek AN, Biwersi J, Verkman AS. (1993) A point mutation at cysteine 189 blocks the water permeability of rat kidney water channel CHIP28k. Biochemistry 32:2938–2941. [DOI] [PubMed] [Google Scholar]