Abstract
Activation of Gs-coupled receptors enhances engraftment of hematopoietic stem and progenitor cells (HSPCs). We tested the hypothesis that treprostinil, a prostacyclin analog approved for the treatment of pulmonary hypertension, can be repurposed to improve hematopoietic stem cell transplantation. Murine and human HSPCs were isolated from bone marrow and umbilical cord blood, respectively. Prostanoid receptor agonists and the combination thereof with forskolin were tested for their capacity to stimulate [3H]cAMP accumulation in HSPCs. Three independent approaches were employed to verify the ability of agonist-activated HSPCs to reconstitute the bone marrow in lethally irradiated recipient mice. The underlying mechanism was explored in cellular migration assays and by blocking C-X-C motif chemokine receptor 4 (CXCR4). Among several prostanoid agonists tested in combination with forskolin, treprostinil was most efficacious in raising intracellular cAMP levels in murine and human HPSCs. Injection of murine and human HSPCs, which had been pretreated with treprostinil and forskolin, enhanced survival of lethally irradiated recipient mice. Survival was further improved if recipient mice were subcutaneously administered treprostinil (0.15 mg kg−1 8 h−1) for 10 days. This regimen also reduced the number of HSPCs required to rescue lethally irradiated mice. Enhanced survival of recipient mice was causally related to treprostinil-enhanced CXCR4-dependent migration of HSPCs. Treprostinil stimulates the engraftment of human and murine hematopoietic stem cells without impairing their capacity for self-renewal. The investigated dose range corresponds to the dose approved for human use. Hence, these findings may be readily translated into a clinical application.
Introduction
The transplantation of hematopoietic stem cells (HSCs) is the only stem cell–based therapy routinely used in clinical medicine. HSC transplantation is employed for the treatment of leukemia and rare genetic defects in the blood cell compartment. Autologous bone marrow transplantation is a standard procedure to increase the therapeutic window of cytotoxic drugs (Aksentijevich and Flinn, 2002). It is of obvious therapeutic relevance if engraftment of hematopoietic stem cells can be stimulated. In vivo HSCs need a signal transduced via Gαs to populate the bone marrow niche (Adams et al., 2009). Gαs can be constitutively activated by cholera toxin: Cholera toxin ADP-ribosylates the catalytic arginine, which is required for GTP-hydrolysis and the resulting deactivation of Gαs (Freissmuth and Gilman, 1989). Enhanced engraftment was indeed observed after pretreatment of HSCs with cholera toxin (Adams et al., 2009). Murine and human hematopoietic stem and progenitor cells (HSPCs) express several types of the four G protein-coupled E prostanoid receptors (EP1–4). Pretreatment of HSPCs with 16,16-dimethyl-PGE (dmPGE2) enhances their subsequent engraftment (North et al., 2007; Hoggatt et al., 2009; Broxmeyer and Pelus, 2014). This effect is presumably mediated by canonical Gαs-dependent signaling: The cAMP-induced activation of protein kinase A synergizes with Wnt-dependent signals to stabilize β-catenin (Goessling et al., 2009). Early clinical data for dmPGE2 are promising (Cutler et al., 2013). However, de novo drug development is often impeded by unforeseeable issues of safety, pharmacokinetic variability, interactions, etc. Accordingly, the development of a new compound can encounter many obstacles (Zon, 2014), which drive up the costs and delay entry into late stage clinical phases. An alternative approach is to repurpose established drugs. The present study was designed to test whether treprostinil was suitable to enhance hematopoietic stem cell engraftment. Treprostinil is a stable analog of prostacyclin, it was approved for the treatment of pulmonary hypertension some 13 years ago under the brand name Remodulin. Thus, its human pharmacology and its safety profile are well known. In contrast to dmPGE2, treprostinil is only a low affinity agonist at inhibitory (i.e., Gi-coupled) EP3-receptors; furthermore it also stimulates EP2- and EP4-receptors in addition to I prostanoid receptor (IP receptor) (Whittle et al., 2012). In our description we adhere to the currently recommended receptor nomenclature, where e.g., EP2 and EP4 receptors are predominantly coupled to Gs (Alexander et al., 2013). Hence, by engaging multiple Gs-coupled receptors, treprostinil may be more efficacious than other prostanoids. Finally, treprostinil is metabolically stable; this allows for exploring whether in vivo administration of prostanoids offers an advantage over sole in vitro treatment with PGE2 or modified versions thereof (Pelus et al., 2011; Hoggatt et al., 2013a). It should therefore be feasible to repurpose treprostinil for improving hematopoietic stem cell transplantation. Our experiments confirmed this conjecture: When combined with forskolin, treprostinil caused a substantial increase in cAMP levels in (murine and human) HSPCs. This translated into enhanced bone marrow reconstitution by HSPCs, which had been pretreated with the combination of forskolin and treprostinil, in lethally irradiated recipient mice. Importantly, the beneficial effects were further augmented by an additional in vivo administration of treprostinil.
Materials and Methods
Materials.
Treprostinil (Remodulin; United Therapeutics Corporation, Durham, NC) was provided by SciPharm SàRL (L-2540 Luxembourg City, Luxembourg); dmPGE2, iloprost, and beraprost were purchased from Cayman Chemicals (Ann Arbor, MI). Ro-20-1724 was obtained from Calbiochem/EMD Millipore (Darmstadt, Germany). [3H]Adenine was from PerkinElmer (Boston, MA). The Lineage Cell Depletion Kit was from Miltenyi Biotec GmbH (Bergisch Gladbach, Germany); Human Cord Blood CD34 Positive Selection Kit, StemSpan serum-free expansion medium (SFEM), and MethoCult were from STEMCELL Technologies (Vancouver, BC, Canada); X-VIVO 15 from Lonza (Basel, Switzerland). Growth factors and cytokines required for cell culture were from PeproTech (Vienna, Austria). Chemicals and antibodies for flow cytometric analysis, FACSCanto II, and FACSDiva Software were obtained from BD Biosciences (Schwechat, Austria). The antibodies against human C-X-C motif chemokine receptor 4 (CXCR4) (12G5; allophycocyanin-labeled), human CD34 (4H11-antibody FITC-labeled), murine CD45.1 (A20; phycoerythrin-labeled), and murine CD45.2 (104; FITC-labeled) were from eBioscience (San Diego, CA); recombinant human stromal cell–derived factor-1 (rhSDF-1), and recombinant murine SDF-1 were purchased from R&D Systems, Inc. (Minneapolis, MN). AMD3100 was from Abcam (Cambridge, UK) and the two-chamber-Transwells (6.5-mm diameter, 5.0 μm pore) was obtained from Corning Life Sciences (Tewksbury MA). Isobutylmethylxanthine, forskolin, pertussis toxin, cholera toxin, and TRI Reagent, and all other chemicals were from Sigma-Aldrich (St. Louis, MO).
Isolation of Murine and Human HSPCs.
Bone marrow cells were flushed from the femora and tibiae of donor mice. Erythrocytes were lysed. Lineage Cell Depletion Kit was used to separate lin– and lin+ with magnetic beads. Human CD34+ cells were harvested from umbilical cord blood of healthy male and female donors (Scholz et al., 2004), using Human Cord Blood CD34 Positive Selection Kit.
Preincubation of HSPCs.
HSPCs were pretreated in vitro with the combination of 10 μM treprostinil and 30 μM forskolin in the presence of growth factors for 1 hour: The medium for murine HSPCs was StemSpan SFEM containing 0.5 mg l−1 benzylpenicillin and streptomycin, 50 ng ml−1 each murine stem cell factor, human FLT3, interleukin (IL)-11, and 150 ng ml−1 murine IL-3. The medium for human HSPCs was X-VIVO 15 medium supplemented by 50 ng ml−1 each of human FLT3, thrombopoetin, and stem cell factor. Unstimulated control cells were also kept in the respective media containing these growth factors. For determining cAMP formation, murine or human HSPCs were incubated with either treprostinil (10 μM) or dmPGE2 (10 μM), alone or in combination with forskolin or with iloprost or beraprost (30 μM each) alone or in combination with forskolin for 30 minutes. Cholera toxin (CTX; 10 μg ml−1) was used as positive control. Chemotaxis to SDF-1 (100 ng ml−1) was tested after in vitro pretreatment of HSPCs in the absence and presence of the combination of 10 μM treprostinil and 30 μM forskolin for 1 hour at 37°C followed by washing steps. AMD3100 was added at a final concentration of 10 μM.
Reverse Transcriptase and Polymerase Chain Reaction.
The mRNA from murine and human HSPCs, murine brain cells, human prostate cancer cell line PC3, and human colon cancer cells HCT116 (Fukuda et al., 2003; Jiang and Dingledine, 2013) was isolated using TRI Reagent. The mRNA was reverse transcribed to cDNA with RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Vienna, Austria). Polymerase chain reaction (PCR) amplification was performed with PerfectTaq DNA Polymerase (5 Prime, Gaithersburg, MD) in 45 cycles (denaturation for 30 seconds at 94°C, annealing for 30 seconds at 55°C, extension for 90 seconds at 72°C, followed by a final extension for 5 minutes at 72°C). Specific amplicons for IP, D prostanoid receptor isoform 1 (DP1), and EP1–4 receptors and CXCR4 were electrophoretically resolved on an agarose gel. Internal controls comprised amplicons of glyceraldehyde-3-phosphate dehydrogenase and β-actin, which were also amplified in 45 cycles under the same conditions. Quantitative PCR (qPCR) was performed using Maxima SYBR Green/ROX qPCR Master Mix (2X) (ThermoFisher Scientific, Vienna, Austria), equal amounts of cDNAs and primers (0.1 μM) in a final volume of 20 μl (15-second denaturation at 95°C, annealing at 60°C for 30 seconds, extension 72°C for 30 seconds). Human and murine hypoxanthine phosphoribosyltransferase-1 (HPRT1) (cycle thresholds at ∼29 and 33 cycles, respectively) were used as reference genes for qPCR. Primer efficiency was verified by serial dilution of the template. Each reaction was performed in triplicate. Relative abundance was calculated using the 2–ΔCt method (gene-specific expression level relative to that of the reference gene). Amplification was done for 45 cycles, cycle thresholds were reached after about 33/32, 31/36, 31/33, 30/33, and 30/36 for human/mouse EP1, EP2, EP3, EP4, and IP receptors, respectively, and after 30 and 31 cycles for the human and murine HRPT1, which were the reference transcripts. The specific primer pairs listed in Table 1 were purchased from Microsynth AG (Balgach, Switzerland).
TABLE 1.
Primer pairs for PCR-mediated amplification of prostanoid and CXCR4 receptors
| Primer Forward (5′–3′) | Primer Reverse (5′–3′) | bp | |
|---|---|---|---|
| mEP1 | AGCAGGAGCCAAGTTCCAG | CATCCGCTAGGCTCAGGTTA | 106 |
| mEP2 | TTATGACCATCACCTTCGCC | TAAAAACCGAAGAGCTCGGA | 110 |
| mEP3 | TGGATCCCTGGGTTTATCTG | GGGAAACAGGTACTGCAATGA | 102 |
| mEP4 | TCTCTGGTGGTGCTCATCTG | TGCAAATCTGGGTTTCTGCT | 107 |
| mIP | GGGCACGAGAGGATGAAGT | GATGGCCTGAGTGAAGCCT | 107 |
| mDP1 | AAGGCTCCATAGTACGCACG | CTCAGACTACAGGCACGGGT | 129 |
| mHprt1 | TTTTCCAAATCCTCGGCATA | CTTCCTCCTCAGACCGCTTT | 146 |
| hEP1 | GAGAGCCAGGGCGCAGT | GCAAGGGCTCATGTCAGG | 101 |
| hEP2 | CCACCTCATTCTCCTGGCTA | TTTCCTTTCGGGAAGAGGTT | 101 |
| hEP3 | AGCGACCATTTGGAAAGATG | TGATGTGATCCTGGCAGAAA | 93 |
| hEP4 | TTACTCATTGCCACCTCCCT | CGCTCCAAACTTGGCTGATA | 95 |
| hIP | GTGTGCTCCCTGCCTCTC | GGGGTTGAAGGCGTAGAAG | 108 |
| hDP1 | CGGAGGTCTTCTGCTTCTTC | CACTATGTGTTCTCTGCCCG | 99 |
| hCXCR4 | AGGAAGCTGTTGGCTGAAAA | CTCACTGACGTTGGCAAAGA | 96 |
| hHprt1 | CACCCTTTCCAAATCCTCAG | CTCCGTTATGGCGACCC | 112 |
[3H]cAMP Accumulation.
The adenine nucleotide pool of murine or human HSPCs was metabolically labeled by incubation in medium containing [3H]adenine (1 μCi ml−1) for 16 hours. In some instances cells were also preincubated in the presence of pertussis toxin (100 ng ml−1). The cells were then pelleted and resuspended in medium containing phosphodiesterase inhibitors Ro 20-1724 and isobutylmethylxanthine at 100 μM and 125 μM, respectively. The accumulation of cAMP was stimulated by the addition of prostanoid receptor agonists and forskolin (see above). After 30 minutes, the cells were spun down and lysed in ice-cold 2.5% perchloric acid containing 0.1 mM cAMP for 1 hour at 4°C. After neutralization with 4.2 M potassium hydroxide, ATP and cAMP were separated by sequential chromatography (Johnson et al., 1994; Bergmayr et al., 2013). The accumulated [3H]cAMP was quantified by liquid scintillation counting. Each experiment was performed in triplicate.
Cell Viability, Cell Cycle Distribution, and Colony Formation.
Human or murine HSPCs were incubated in the presence of vehicle or the combination of 10 μM treprostinil and 30 μM forskolin at 37°C for 1 hour and 24 hours. After washing with phosphate-buffered saline at 4°C, cells were stained for externalized phosphatidylserine with the PE Annexin-V Apoptosis Detection Kit I according to the manufacturer’s protocol or for DNA content with propidium iodide (50 μg ml−1 in phosphate-buffered saline) for 40 minutes at 37°C. Data obtained by flow cytometry were analyzed with FACSDiva Software. Colony formation of murine HSPCs was determined as follows: After an incubation for 1 hour in the presence of 10 μM treprostinil and 30 μM forskolin, cells were resuspended in MethoCult containing granulocyte-macrophage colony-stimulating factor and IL-3 (10 ng ml−1 each) for the formation of granulo-monocytic colony–forming units and 3 IU ml−1 of erythropoietin and IL-3 for erythroid colony forming units and cultured at 37°C and 5% CO2 for 7–10 days. The number of colonies was counted under a light microscope.
Expression of CXCR4.
Human HSPCs were isolated from umbilical cord blood and subsequently incubated in the absence and presence of the combination of treprostinil (10 μM) and forskolin (30 μM) for 1–6 hours at 37°C. Subsequently, cells were washed and either lysed in TRIzol to isolate RNA or stained with the FITC-labeled 4H11-antibody against CD34 and the allophycocyanin-labeled 12G5-antibody against CXCR4/CD184. Dual-labeled cells were gated to quantify the surface expression of CXCR4 in a FACSCanto II (Becton, Dickinson and Company, Franklin Lakes, NJ).
Migration Assay.
Chemotaxis toward SDF-1 was determined using a two-chamber Transwell system. After in vitro stimulation and washing steps, cells were resuspended to obtain a suspension of about 2 × 106 cells ml−1 in StemSpan SFEM containing growth factors. The cell suspension (0.1 ml) was added to the upper chamber with or without 100 ng ml−1 SDF-1 in the lower chamber. After 4 hours at 37°C, the number cells that had migrated into the lower chamber was counted in a Luna automated cell counter (Logos Biosystems, Annandale, VA) and was expressed as percentage of the total cells originally added to the upper chamber. Assays were done in triplicate.
Transplantation of Murine and Human HSPCs.
BALB/c, C57BL/6J (CD45.2+), B6.SJL-PtrcAPep3B/BoyJ (CD45.1+), and NOD/SCID/IL-2Rg2/2 (NSG) mice were obtained from the Jackson Laboratory (Bar Harbor, ME) through Charles River Germany (Sulzfeld, Germany). Six-to-eight-week-old female and male mice with mean body weight of 20–25 g were used. Housing and husbandry was in accordance with the recommendations and requirements as defined by the Federation of Laboratory Animal Science Associations (FELASA) in Europe. Mice were kept in specific pathogen-free conditions in isolated ventilated cages [≤5 mice/cage; SmartFlow and EasyFlow (Tecniplast, Buguggiate, Italy)], the light/dark cycle was 12 hours, animal holding rooms were maintained at 21 ± 3°C, autoclaved food (pellets enriched with vitamins), and water was provided ad libitum. Animal welfare and health status were checked by animal technicians under the supervision of a veterinarian. Sample-size calculations were made on the basis of published reports (Adams et al., 2006; Adams et al., 2009; Hoggatt et al., 2009) and on the assumption that statistically significant (P < 0.05) differences were to be detected with a power of 95%. This condition was met with ten mice per group. The experimental protocol was reviewed by the animal ethics committee and approved by the Austrian Ministry of Science and Research (BMWF-66.009/0164-II/10b/2010) for compliance with the pertinent Austrian laws, which follow the 2010/63/EU Directive. The protocol involving human umbilical cord blood samples was approved by the Ethics Committee of the Medical University of Vienna.
All recipient mice received numbered ear tags. These numbers were randomly allocated to treatment groups (variation in cell numbers and drug regimen). The allocation was concealed from the person carrying out the tagging. The isogenic recipient mice (C57BL/6 or BALB/c) or NSG mice were subjected to lethal irradiation (9 Gy, split doses, 2 Gy min−1; Siemens Primus, 6MV, Siemens Austria). Mice were individually placed in chambers of an acrylic irradiation pie with 15 mice (alternating male and female) per pie. The radiation dose delivered was verified with a dosimeter. Thereafter, mice were allowed to recover from irradiation for 24 hours. Prior to transplantation, murine or human HSPCs were preincubated in the absence and presence of a combination of 10 μM treprostinil and 30 μM forskolin for 1 hour at 37°C. Subsequently, the incubation medium was removed by three cycles of centrifugation to avoid any carryover effects. The resuspension of HSPCs was injected via the tail vein. For transplantation of cells, syringes were filled with varying numbers of pretreated or control cells, or vehicle. Preliminary experiments defined the limiting number of HSPCs. The administered number of HSPCs ranged from 2.5 to 5 × 105 per mouse and is indicated in the respective figure legends. A second person, who was blinded to the syringe content, performed the tail vein injection. In some experiments, recipient mice were also subjected to an in vivo treatment: They were injected subcutaneously with treprostinil (0.015, 0.15, and 1.5 mg kg−1 8 h−1, starting immediately prior to transplantation of cells), or the combination of treprostinil (0.15 kg−1 8 h−1) and AMD3100 (3.3 mg kg−1 8 h−1) in a total volume of 80 μl per mouse, for 10 days. Untreated control mice received sham-injections of equal volume. The person who injected the drugs or vehicle did not prepare the syringes and was blinded to the content. The animal technicians were blinded to the treatment and monitored the well-being of the animals. On the basis of their training they assessed the outcome as death or imminent death by deciding whether a humane endpoint had been reached. The humane endpoints included emaciation (i.e., weight loss >30%), loss of activity (reduced mobility, prolonged crouching, etc.), loss of grooming/ruffled fur, or labored breathing. Mice meeting these criteria were killed by cervical dislocation. If not rescued by transplantation of murine or human HSPCs, irradiated mice succumbed to death within the first 2 weeks. For competitive transplantation assays, murine HSPCs were harvested from CD45.1-positive (CD45.1+) and CD45.2-positive (CD45.2+) donor mice and injected into the lethally irradiated recipient mice as outlined above. In the competitive setting, cells were also pretreated with 10 μg ml−1 cholera toxin as a positive control. After 16 weeks, the bone marrow of recipient mice was analyzed by flow cytometry according to CD45.1 and CD45.2 surface expression.
Secondary Transplants.
Bone marrow cell suspensions were obtained from primary recipient mice and transplanted into lethally irradiated secondary recipients. White blood cell counts and body weight were monitored over 12 months.
Results
Treprostinil Stimulates cAMP Accumulation in Murine and Human HSPCs.
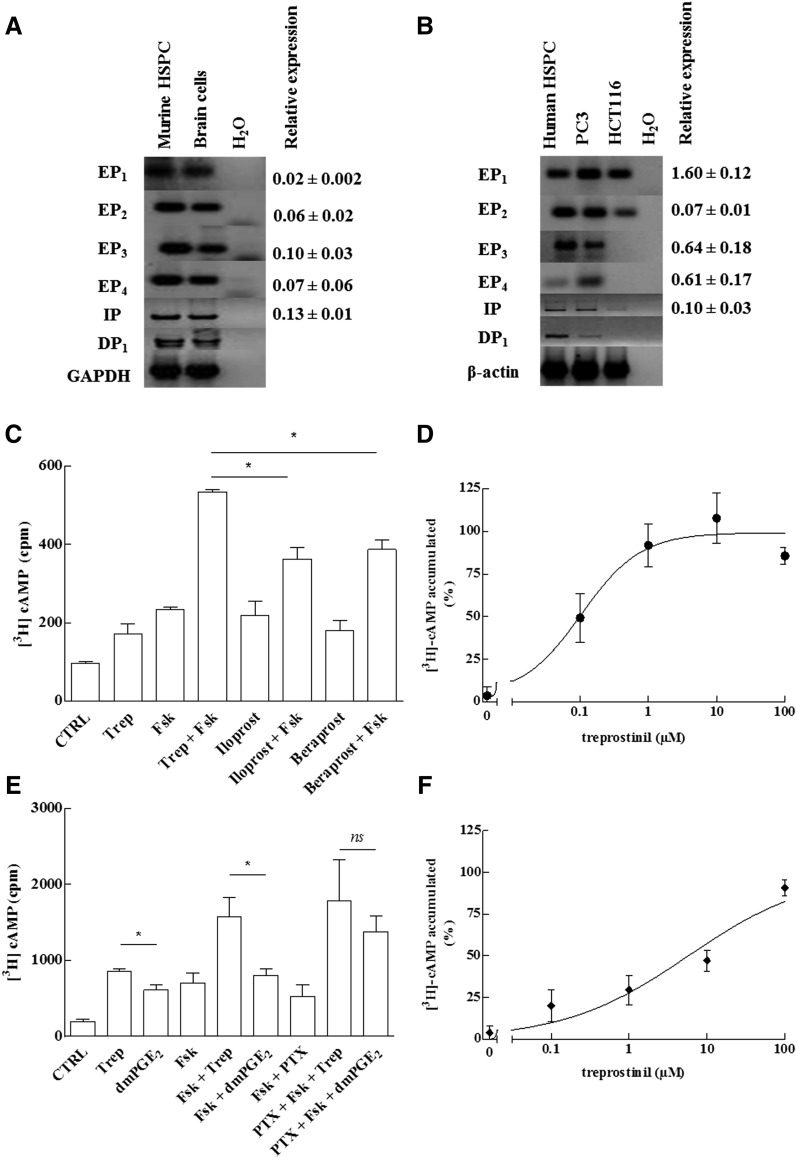
Previous work showed that engraftment of murine HSCs was enhanced by stimulating Gαs-coupled EP2-receptors with dmPGE2. We first documented that murine and human HSPCs expressed additional prostanoid receptors coupled to Gαs by PCR: Amplicons were identified for the prostanoid receptors (EP1, EP2, EP3, EP4, IP, and DP1) in both murine and human HSPCs (Fig. 1, A and B). The relative abundance of receptor transcripts was also estimated by quantitative PCR (qPCR; right hand numbers in Fig. 1, A and B). There was some interspecies variation: The level of mRNA encoding EP1 receptor was substantially higher in murine cells (i.e., ΔCt ≈ 0.67 versus reference gene murine HPRT1) than in human cells (i.e., ΔCt ≈ –5.7 versus reference gene human HPRT1); this was also true for the transcripts encoding murine EP3- and EP4-receptors. In contrast, transcripts for EP2 and IP-receptors were found at comparable relative levels in human and murine cells. The presence of the IP-receptor suggested that synthetic prostacyclin analogs ought to raise cAMP levels in HSPCs.
Fig. 1.
Prostanoid receptor expression (A, B) and stimulation of cAMP accumulation (C–F) in murine and human HSPCs. (A, B) RNA was isolated from murine (A) and human HSPCs (B) and reverse transcribed. RNA prepared from murine brain cells (mixed culture of neurons and glial cells) and the human prostate cancer cell lines PC3 and HCT116 served as positive controls. PCR-dependent amplification was done using primers listed in Table 1. Amplicons for all E prostanoid receptors (EP1–4, IP, and DP1) were electrophoretically resolved on an agarose gel and visualized by ethidium bromide staining. The lane labeled H2O denotes the control, where the amplification was done in the absence of prior reverse transcription. The mRNA encoding glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified as internal reference. Quantitative PCR was performed using Maxima SYBR Green/ROX qPCR Master Mix (2×) and equal amounts of cDNAs and primers. Human and murine HPRT1 was used as reference gene for normalization of qPCR experiments. Each reaction condition was performed in triplicate. Relative abundance was calculated using the 2–ΔCt method (gene-specific expression level relative to that of the endogenous reference gene HPRT1, which was set at 1). We failed to identify a primer pair capable of amplifying murine DP1 receptor transcripts with an efficiency close to 100%; accordingly, the quantification of DP1 receptor transcripts is not shown. (C–F) The cAMP response of murine (C, D) and CD34+ human HSPCs (E, F) was determined after metabolic labeling of the adenine nucleotide pool with [3H] adenine. In some instances (PTX), cells were concomitantly also preincubated with pertussis toxin (100 ng ml−1) for 16 hours. (C) Murine HSPCs were stimulated with treprostinil (10 μM; Trep), iloprost (30 μM), beraprost (30 μM), and forskolin (30 μM; Fsk) or the combination of indicated agonists and forskolin (30 μM). In the presence of 10 μM treprostinil, 30 μM forskolin was more efficacious than 30 μM iloprost and beraprost (p = 0.02, one-way ANOVA). (E) Human HSPCs were stimulated with treprostinil (10 μM), forskolin (30 μM), the combination thereof, or dmPGE2 (10 μM), or the combination thereof with forskolin (30 μM). Both, in the absence and presence of 30 μM forskolin, 10 μM treprostinil was more efficacious than 10 μM dmPGE2 (p = 0.03; Wilcoxon test). In contrast, in cells, which had been pretreated with pertussis toxin, stimulation with forskolin+treprostinil and forskolin+dmPGE2 did not result in any statistically significant difference in cAMP accumulation (ns). Panels D and F show the concentration-response curve for treprostinil-induced cAMP accumulation for murine (in the presence of 30 μM forskolin) and human HSPCs, respectively. The maximum levels of [3H]cAMP accumulation was 595 ± 60 cpm (D) and 1943 ± 262 cpm (F). Data are means ± S.D. (n = 3).
The adenine nucleotide pool of murine HSPCs was metabolically labeled with [3H]adenine and their response to treprostinil, iloprost, and beraprost was examined. Because these cells have a very modest cAMP response, the enzyme was sensitized by using forskolin. This diterpene binds in the pseudo substrate cleft between the catalytic C1 and C2 domains of adenylyl cyclase (Tesmer et al., 1997) and renders the various isoforms of the enzyme more responsive to the stimulatory G protein Gαs (Sunahara et al., 1996; Kudlacek et al., 2001). As can be seen from Fig. 1C, 10 μM treprostinil, 30 μM beraprost, and 30 μM iloprost per se caused a modest accumulation of cAMP in murine HSPCs that was comparable in magnitude to that elicited by 30 μM forskolin. However, when combined with 30 μM forskolin, 10 μM treprostinil caused an increase in cAMP levels that exceeded that caused by the IP receptor specific compounds iloprost and beraprost. In the presence of forskolin, treprostinil caused a concentration-dependent accumulation of cAMP in murine cells (Fig. 1D).
Similar experiments were performed with human HSPCs isolated from umbilical cord blood (Fig. 1, E and F). Treprostinil caused an increase in cAMP levels in human HSPCs, which was enhanced by 30 μM forskolin (Fig. 1E). The relative effects were reasonably comparable in human and murine HSPCs (cf. Fig. 1, C and E). Incubation of human HPCs with treprostinil resulted in cAMP accumulation that was robust enough to be determined in the absence of forskolin (Fig. 1E). The most conspicuous finding was the observation that the concentration-response curve of treprostinil (EC50 = ∼5 μM; Fig. 1F) spanned more than two orders of magnitude (Hill coefficient = 0.55). This indicates that more than one receptor subtype is involved. Finally, it was of interest to compare cAMP elevations elicited by dmPGE2 and by treprostinil: In the presence of 30 μM forskolin, stimulation by 10 μM treprostinil resulted in significantly higher cAMP than 10 μM dmPGE2 (Fig. 1E). The lower efficacy of dmPGE2 can be rationalized by taking into account that dmPGE2 is also a full agonist at the Gi-coupled EP3 receptor (Woodward et al., 2011) and thus causes both, a Gs-dependent stimulation of adenylyl cyclase via EP2 and EP4 receptors and a concomitant inhibition via Gi-coupled EP3-receptors. Pertussis toxin abolishes the interaction of Gi-coupled receptors with Gi (and related G proteins such as Go and Gt) by ADP-ribosylating a cysteine residue four amino acids removed from the C-terminus of the Gαi-subunit. Accordingly, HSPCs were preincubated for 16 hours in the presence of pertussis toxin. This pretreatment increased the response to dmPGE2 (cf. 6th and 9th bar in Fig. 1E) such that there was no significant difference between the cAMP response elicited by treprostinil + forskolin and that caused by dmPGE2 + forskolin (cf. 8th and 9th bar in Fig. 1E). Substantially lower EC50-values (i.e., in the nanomolar range) have been reported, if treprostinil was tested on cell lines overexpressing individual human prostanoid receptors. The reason for the discrepancies between our μ molar EC50 values and those reported by Whittle et al. (2012) is not clear, but we suspect that the very high receptor levels achieved by heterologous overexpression resulted in a substantial leftward shift of the concentration-response curve.
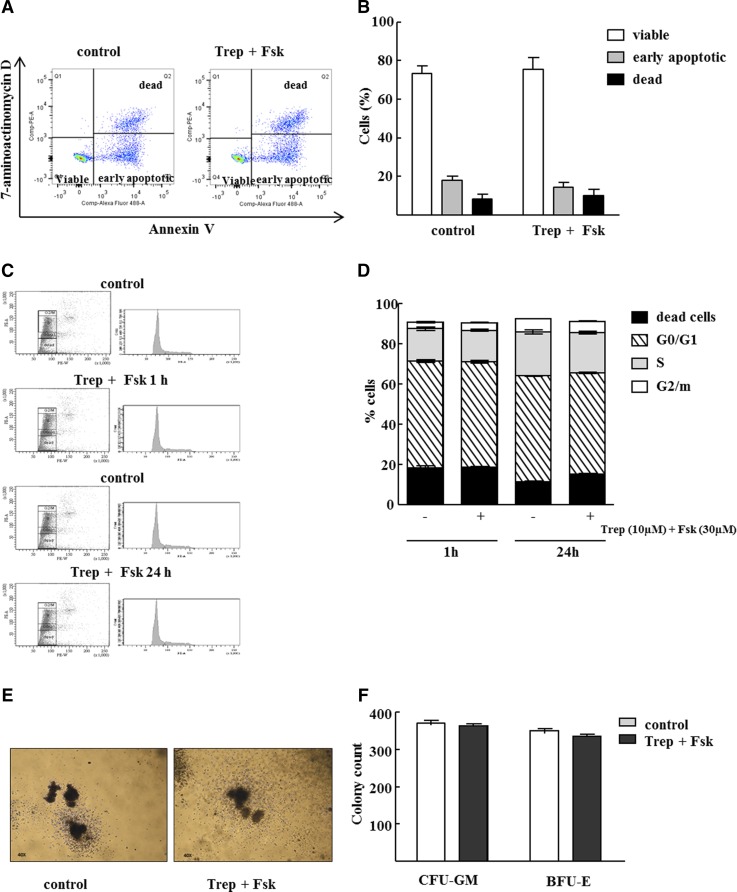
Pretreatment of HSPCs with Treprostinil and Forskolin Does Not Alter Cell Viability, Cell Cycle Progression, or Differentiation Potential.
A persistent elevation in cAMP may trigger apoptosis in hematopoietic cells (Insel et al., 2012). We thus examined whether in vitro pretreatment of human HSPCs with the combination of 30 μM forskolin and 10 μM treprostinil rendered cells more susceptible to apoptosis (Fig. 2, A and B) or impeded their entry into and progression through the cell cycle (Fig. 2, C and D) or altered the ability of murine HSPCs to give rise to specific lineages (Fig. 2, E and F). As illustrated by the original dot plots (Fig. 2A) and summarized in Fig. 2B, the presence of viable, early apoptotic and dead cells was comparable and the number of annexin-V positive cells was not increased upon in vitro treatment with 30 μM forskolin and 10 μM treprostinil. Likewise, the cell cycle distribution of asynchronously growing untreated human HSPCs and HSPCs maintained in the presence of treprostinil and forskolin was comparable, regardless of whether HSPCs were exposed for 1 hour or 24 hours (cf. Fig. 2C for representative original histograms and Fig. 2D for averaged data). Importantly we also failed to detect any effect of treprostinil and forskolin on the formation of myeloid and erythroid colonies: Murine HSPCs were isolated from bone marrow, incubated for 1 hour in the presence of treprostinil and forskolin, and resuspended in a methylcellulose-containing medium with growth factors required for supporting the differentiation and growth of colony-forming units of the CFU-GM and of the BFU-E. After 10 days, the morphology (Fig. 2E) and the number of colonies were comparable (Fig. 2F).
Fig. 2.
Pretreatment of murine and human HSPCs with treprostinil and forskolin does neither induce apoptosis nor alters cell cycle progression or differentiation potential. Human HSPCs were incubated with 10 μM treprostinil and 30 μM forskolin for 1h. Subsequently, (A, B) apoptosis induction and (C, D) cell cycle progression was assessed by flow cytometric analysis. Representative original pictures are depicted (A, C, left hand panel) and data obtained in three independent experiments was summarized (B, D, right hand panel). No difference in apoptotic cells or distribution of cells according to G0/1, S and G2 phase was detected between untreated and treated cells (one way ANOVA). (E, F) Murine HSPCs were isolated form bone marrow, pretreated and resuspended in a methylcellulose medium containing growth factors required for supporting the differentiation and growth of granulo-monocytic colony–forming units (CFU-GM) and of the erythrocyte lineage (BFU-E, burst-forming-unit erythroid). After 10 days the number of colonies was counted under a light microscope and shape and morphology of colonies was observed. Shown are representative photomicrographs and the quantification of three independent experiments. Data are means ± S.E.M. (n = 3).
Pretreatment of Murine HSPCs with Treprostinil and Forskolin Enhances Bone Marrow Reconstitution.
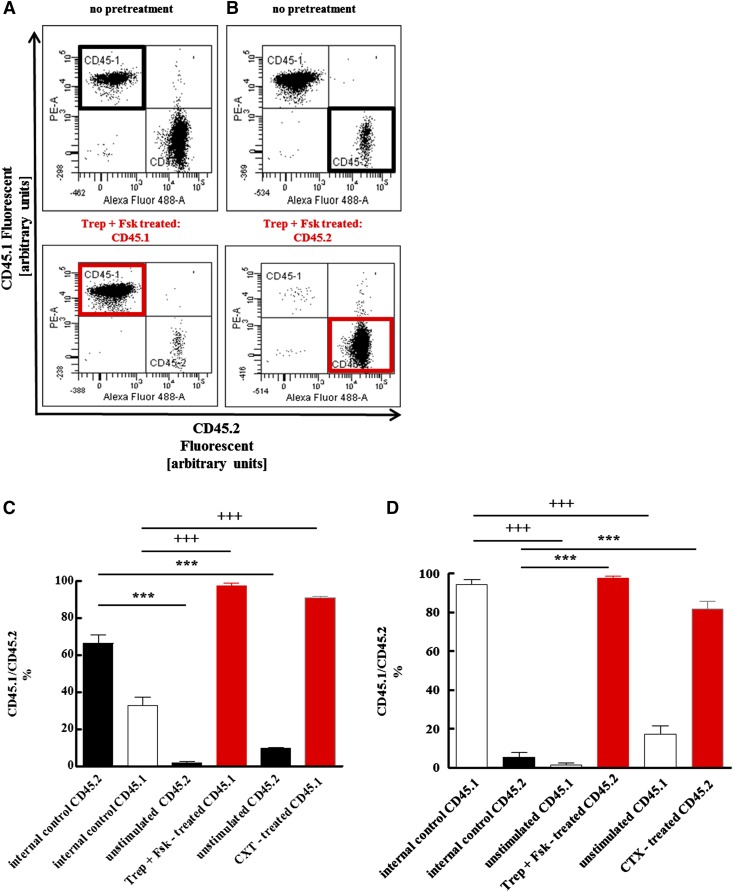
Activation of Gαs is predicted to enhance the ability of HSPCs to repopulate the bone marrow (Adams et al., 2009). Thus, upon pretreatment with treprostinil and forskolin, HSPCs ought to be more effective than unstimulated cells in reconstituting the bone marrow of lethally irradiated recipient mice. We verified this conjecture by isolating murine HSPCs either from C57BL/6 (CD45.2+) or B6.SJL-PtrcAPep3B/BoyJ (CD45.1+) mice. Prior to transplantation, donor cells were pretreated in vitro for 1 hour in the presence of treprostinil and forskolin. Standard medium alone was used as negative control (internal control cells in Fig. 3); CTX served as positive control. The use of CD45.1+ and CD45.2+ HSPCs allowed for differentiating the effects of the pretreatment on bone marrow reconstitution in a competitive setting: Either the CD45.1+ cells were pretreated and the CD45.2+ cells were incubated in medium as unstimulated control (Fig. 3, A and C) or vice versa (Fig. 3, B and D). Sixteen weeks after transplantation, the contribution of either donor cell type was assessed by analyzing surface expression of CD45.1 and CD45.2 on bone marrow cells of recipient mice by flow cytometry. Representative original data for each experiment are shown in Fig. 3A and Fig. 3B: The upper panels document the competitive bone marrow reconstitution without prestimulation of either donor cell type. In the corresponding lower panels, either CD45.1+ (Fig. 3A) or CD45.2+ (Fig. 3B) HSPCs were prestimulated by treprostinil and forskolin and allowed to compete with unstimulated CD45.2+ and CD45.1+ HSPCs. The outcome of HSPC transplantation is limited—at least in part—by the number of available cells (Purton and Scadden, 2007). Accordingly, we lowered the number of administered HSPCs from a total of 106 cells/mouse (CD45.1+/CD45.2+ = 1:1 in Fig. 3, A and C) to 5 × 105 cells/mouse and changed the ratio (CD45.1+/CD45.2+ = 10:1, Fig. 3, B and D). In the absence of any stimulation, the vast majority of reconstituted bone marrow cells originated from the injected untreated CD45.1+ HSPCs (left hand set of bars labeled “internal control” in Fig. 3 D). In contrast, if the CD45.2+ HSPCs were stimulated with treprostinil and forskolin (or with CTX) and then combined with the 10-fold excess of unstimulated CD45.1+ cells, they effectively competed for the bone marrow niche such that they gave rise to the vast majority of bone marrow cells in recipient animals (right hand set of bars in Fig. 3D). Thus, the effectiveness of pretreatment was most evident at low cell numbers. Of note, the effect size of treprostinil and forskolin was comparable in magnitude to that of CTX (left hand set of bars in Fig. 3, C and D).
Fig. 3.
Competitive advantage of HSPCs after pretreatment with the combination of treprostinil and forskolin or cholera toxin over unstimulated controls. Murine HSPCs were isolated from CD45.1+ and from CD45.2+ donor mice. Either CD45.1+ (A, C) or CD45.2+ (B, D) cells were incubated for 1 hour in medium in the absence of any additional stimulus, in the presence of the combination of 10 μM treprostinil and 30 μM forskolin (Trep + Fsk, pretreated) or of 10 μg ml−1 cholera toxin (as a positive control). First, cell suspensions were prepared that contained either equivalent amounts of both unstimulated CD45.1+ and CD45.2+ cells [top panel in (A); bars labeled “internal control” in (C)] or equivalent amounts of stimulated and untreated donor cells [bottom panel in (A)]. These suspensions containing a total of 106 cells were administered by tail vein injection to lethally irradiated recipients (A, C). Conversely, CD45.2+ cells, which were either not stimulated [top panel in (B); bars labeled “internal control” in (D)], or stimulated [bottom panel in (B)], and untreated CD45.1+ were mixed at a ratio of 1:10; a total of 5 × 105 cells were injected into recipient mice (B, D). The proportion of bone marrow cells arising from the two sources was determined by flow cytometry 16 weeks after transplantation. Representative original dot plots are shown in (A) and (B); bar diagrams in (C) and (D) represent the means ± S.E.M. (n = 5 animals per group) from two independent experiments. The indicated statistical comparisons of CD45.2- (+) and CD45.1-positive cells (*) were done by analysis of variance (ANOVA) followed by Tukey’s multiple comparison (P = 0.0001).
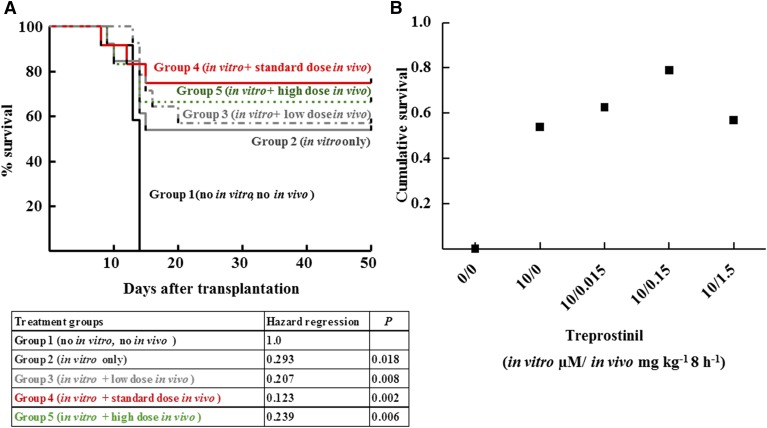
Defining the Optimal Regimen for Rescuing Lethally Irradiated Recipient Mice by Treprostinil-Treated Murine HSPCs.
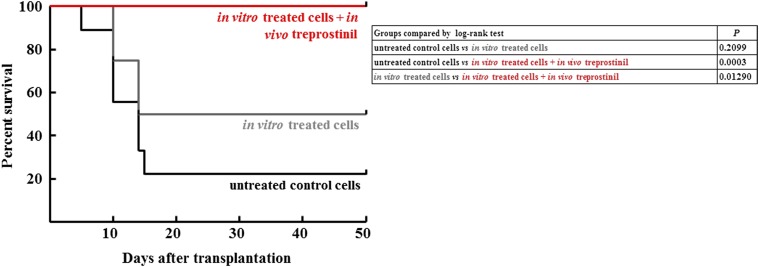
Rescue of lethally irradiated mice by transplantation depends on the cell number of transplanted HPSCs (Purton and Scadden, 2007). Under the conditions employed, a minimum of 5 × 105 was required: Six out of six transplanted animals survived, but survival decreased upon transplantation of lower cell numbers. If cell numbers were lowered to 3 × 105 HSPCs, the vast majority of these animals (i.e., seven out nine) did not survive (black line in Fig. 4). In contrast, seven of 13 recipient mice survived if the murine HSPCs had been stimulated with the combination of treprostinil and forskolin in vitro for 1 hour prior to injection (grey line in Fig. 4). We surmised that prolonged stimulation of HSPCs provided an additional benefit. Accordingly, recipient mice were administered 0.15 mg kg−1 treprostinil by subcutaneous injection immediately prior to transplantation of pretreated HSPCs and every 8 hours for the next 10 days. This dose was selected on the basis of its approved indication in pulmonary hypertension. The human dose was converted to its murine equivalent on the basis of the allometric relation (Reagan-Shaw et al., 2008). This treatment regimen gave the best outcome: All animals survived (red line in Fig. 4). The difference between the survival curves of group 2 and group 3 was statistically significant. Additional control experiments included sole in vivo administration of treprostinil (0.15 mg kg−1 8 h−1) to recipient mice did not rescue mice from lethal irradiation. This ruled out that treprostinil facilitated recovery from hematopoietic injury or protected mice from lethal irradiation (Hoggatt et al., 2013b). Sole in vivo administration of treprostinil (0.15 mg kg−1 8 h−1) did not rescue recipient mice transplanted with limited cell numbers (2 × 105 cells). All mice, which had been only treated in vivo (five of five without transplantation and five of five with a transplantation of untreated cells) died within the first 10 days after lethal irradiation (not shown). We explored the dose range required to elicit the additional beneficial effect of s.c. treprostinil by varying the administered dose over two orders of magnitude. Thus, the intermediate dose of 0.15 mg kg−1 8 h−1 (group 4, red line in Fig. 5) was compared with the low dose 0.015 mg kg−1 8 h−1 (group 3, gray line in Fig. 5) and to the high dose 1.5 mg kg−1 8 h−1 (group 5, green line in Fig. 5). The experiment was designed to investigate the dose range in which the in vivo administration of treprostinil was safe and advantageous when compared with sole in vitro incubation of hematopoietic progenitor cells with treprostinil and forskolin. Thus, the crucial parameter was the fate of those mice allocated to groups 3–5. These had been treated in vivo with treprostinil as opposed to recipient mice allocated to group 1 (untreated) and group 2 (which did not receive any in vivo treatment). It is obvious from Fig. 5A that the mice in groups 3–5 benefitted compared with untreated mice (group 1). The crucial outcome is survival after 60 days, because it reflects successful bone marrow engraftment. We therefore replotted the data to visualize the effect of treprostinil on outcome in Fig. 5B. The data obtained with the high dose (1.5 mg kg−1 8 h−1) allowed for the definition of a safety margin of 10 for treprostinil: Whereas this dose was less effective than the standard dose, it was not inferior to sole in vitro treatment (group 2) and there was no evidence for overt toxicity.
Fig. 4.
Survival curves for lethally irradiated BALB/c mice after transplantation with murine HSPCs, which had been left untreated, treated with the combination of treprostinil and forskolin and further stimulated by in vivo administration of treprostinil. Murine HSPCs were incubated in the absence (no treatment; n = 9) or presence of the combination of 10 μM treprostinil and 30 μM forskolin (in vitro pretreatment); subsequently, the cell suspension (3 × 105 cells per mouse) was administered by tail vein injection to lethally irradiated recipient mice. Some mice, which received pretreated cells, were also treated in vivo with treprostinil (in vitro and in vivo treatment; n = 10; 0.15 mg kg−1 s.c for 10 days), whereas others received no further treatment (in vitro pretreatment; n = 13). The differences between the survival curves are statistically significant: in vitro pretreatment versus in vitro/in vivo treatment, P = 0.01290; untreated control versus in vitro/in vivo treatment, P = 0.0003 (log-rank test).
Fig. 5.
In vivo administration of treprostinil enhances survival of recipient mice. Kaplan-Meier plots for survival of lethally irradiated recipient mice receiving 2 × 105 untreated murine HSPCs (group 1, n = 12), HSPCs that had been solely pretreated in vitro with the combination of treprostinil and forskolin (group 2, n = 13), or mice that were transplanted with pretreated murine HSPC and administered—in addition—treprostinil by subcutaneous injection (group 3: 0.015 mg kg−1, n = 12; group 4: 0.15 mg kg−1, n = 14; group 5: 1.5 mg kg−1, n = 14). The experiment was performed as outlined in the legend to Fig. 4 and in Materials and Methods. In panel (B), survival is replotted as a function of the administered treprostinil dose to illustrate the bell-shaped nature of the concentration-response curve. The statistical comparison was done by a Cox proportional hazard regression analysis: There was a dose-dependent effect of treprostinil on survival; however, the relation was bell-shaped: at the highest dose (1.5 mg kg−1 8 h−1), survival was lower than at the intermediate dose (0.15 mg kg−1 8 h−1). The Cox proportional hazard regression analysis identified the effect of different treatment regimens on survival. Treatment groups were modeled as a categorical predictor variable. Regression was performed using IBM SPSS Statistics 20.0. Omnibus Test revealed that the inclusion of the covariate “treatment group” was of high statistical significance: The decrease in hazard ratio displayed treatment dependence (see tabulated values). Compared with the “no treatment” control (group 1), the risk of fatal events decreased progressively from group 2 (sole in vitro treatment) to group 4 (additional in vivo treatment with the standard treprostinil dose). This trend leveled off and declined in animals exposed to a high treprostinil dose (group 5) as expected from a bell shaped dose-response relationship.
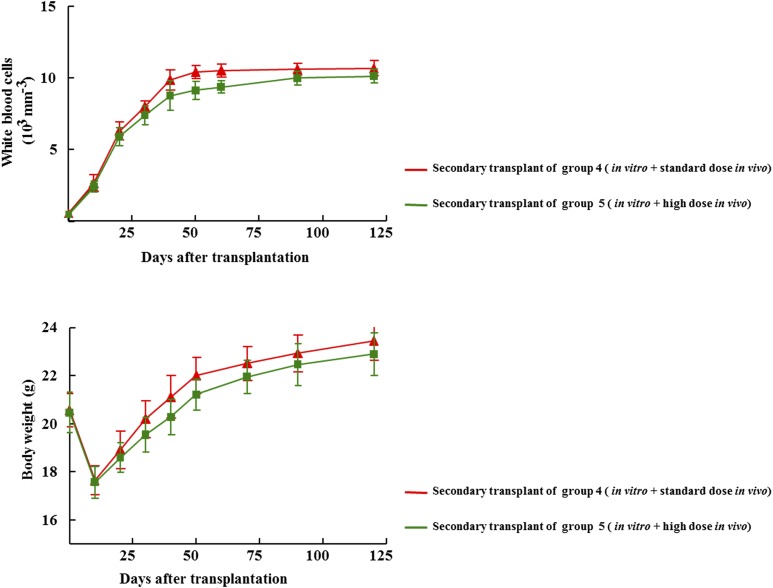
Secondary Transplants of Treprostinil-Treated Bone Marrow Provide Formal Proof For Safety.
HSPCs have a (quasi-infinite) capacity for self-renewal. Secondary transplants provide formal proof that murine HSPCs repopulated the bone marrow of primary recipients. In addition, secondary transplants allow for detecting detrimental effects: e.g., ex vivo manipulations of HSPCs may limit their capacity for long-term self-renewal. Accordingly, we collected bone marrow cells 160 days after transplantation from primary recipient mice of groups 4 and 5 of the experiment depicted in Fig. 5, because they had received HSPCs, which were pretreated in vitro and had also been exposed in vivo to the standard dose (0.15 mg kg−1 8 h−1) and the high dose of treprostinil (1.5 mg kg−1 8 h−1). If bone marrow cells isolated from those first recipients were administered to lethally irradiated mice, these secondary recipients were rescued and all mice survived more than 150 days: Hematopoiesis was restored; white blood cell counts reached normal levels within 30 days and were maintained for the entire observation period (Fig. 6, upper panel). Likewise, the animals thrived after the initial radiation sickness-induced drop in body weight (Fig. 6, lower panel) and we did not find any signs of overt pathology over an observation period of more than 400 days.
Fig. 6.
Time-dependent change in peripheral white blood cell counts (upper graph) and body weight (lower graph) after transplantation of bone marrow cells obtained from primary recipients. One-hundred, sixty days after transplantation four mice originating from treatment group 4 and group 5 respectively, were sacrificed, their femurs were flushed with phosphate-buffered saline to recover bone marrow cells. Subsequently, 2 × 106 bone marrow cells were transplanted into lethally irradiated secondary recipient mice. All mice that received this secondary transplant survived. At the indicated time points, blood was drawn, the white blood cell count was determined, and the weight of the mice recorded. Data are means ± S.E.M.
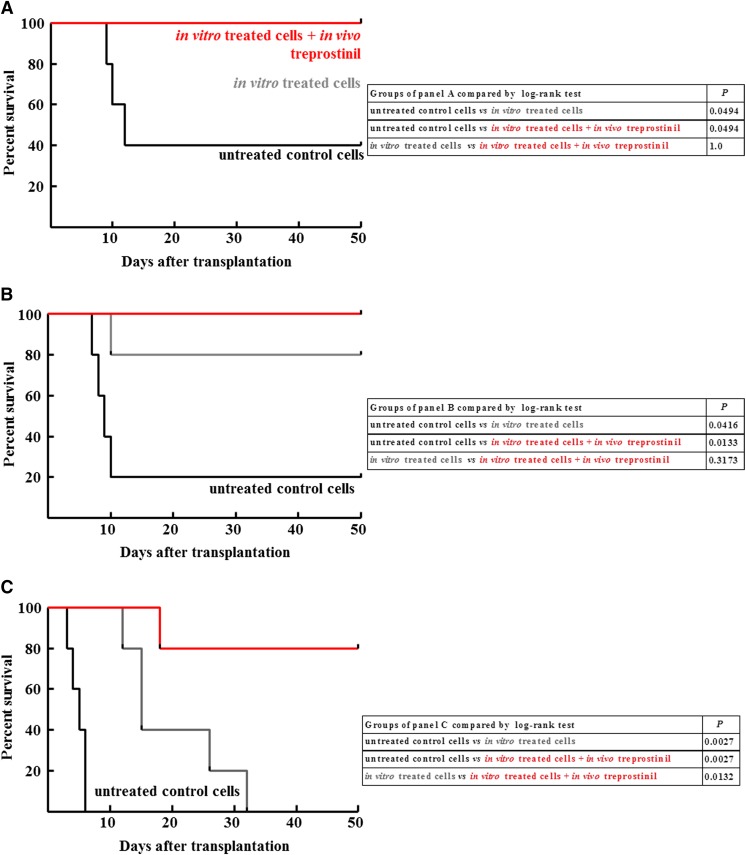
Rescue of Lethally Irradiated NSG Mice by Treprostinil-Stimulated Human HSPCs.
Human HSPCs also express several Gs-coupled prostanoid receptors and treprostinil treatment increases cAMP levels in these cells (cf. Fig. 1, E and F). We therefore hypothesized that treprostinil also stimulated the engraftment of human HSPCs. Accordingly, CD34+ human HPSCs were isolated from umbilical cord blood and injected into lethally irradiated NSG mice. Their bone marrow niche is permissive to engraftment of human HSPCs (Shultz et al., 2005). The experimental design recapitulated that outlined for murine HSPCs in Fig. 4: The number of CD34+ was titrated from 2.5 × 105 (Fig. 7A) or from 2 × 105 (Fig. 7B) to 1.5 × 105 per mouse (Fig. 7C). Three conditions were examined, namely transplantation of human CD34+ cells, which had been incubated in vitro in the presence of vehicle (group 1, black lines in Fig. 6) or stimulated in vitro with treprostinil and forskolin (group 2, gray lines in Fig. 6) and where the recipient NSG mice were subsequently administered s.c. 0.15 mg kg−1 8 h−1 treprostinil for 10 days (group 3, red lines in Fig. 7). The fate of recipient mice, which were injected with untreated human HSPCs (group 1 in Fig. 6), depended on the number of cells: 40% and 20% survived upon injection of 2.5 × 105 (Fig. 7A) and 2 × 105 cells (Fig. 7B), respectively, but all died within the first ten days if they were injected 1.5 × 105 cells (Fig. 7C). This dependence on cell number was also seen, if the human HSPCs had been pretreated in vitro (group 2, gray curves in Fig. 7), but it was less evident for those mice administered s.c. 0.15 mg kg−1 8 h−1 treprostinil over ten days (group 3, red curves in Fig. 7). Importantly, even at the lowest cell number, 4 out 5 mice survived, if they received treprostinil in vivo, whereas all group 2 mice died (Fig. 7 C). Conversely, at the high cell number, sole in vitro pretreatment rescued all animals (Fig. 7 A); it is obvious that under these conditions, the additional in vivo administration of treprostinil cannot provide any further benefit. The statistical comparisons (tabulated in Fig. 7) also document that the difference between the three conditions is most readily appreciated at low cell numbers.
Fig. 7.
Survival curves for lethally irradiated NSG mice after transplantation with different amounts of human HSPCs. CD34+ human hematopoietic progenitor cells were isolated from umbilical cord blood and incubated in the absence (no treatment, group 1) or presence of the combination of treprostinil and 30 μM forskolin (in vitro pretreatment, groups 2 and 3); subsequently, the cell suspension (2.5 × 105, 2 × 105, and 1.5 × 105 cells per mouse in the (A), (B), and (C) panels, respectively) was administered by tail vein injection to lethally irradiated recipient NSG mice. Some mice, which received pretreated cells, were also treated in vivo with treprostinil (in vitro and in vivo treatment, group 3; 0.15 mg kg−1 8 h−1, s.c. and for 10 days), whereas others received no further treatment (in vitro pretreatment – group 2). Each group was comprised of 5 animals. The tables summarize the comparisons of the individual Kaplan-Maier plots by log-rank test.
The Beneficial Action of Treprostinil Is Contingent on SDF-1.
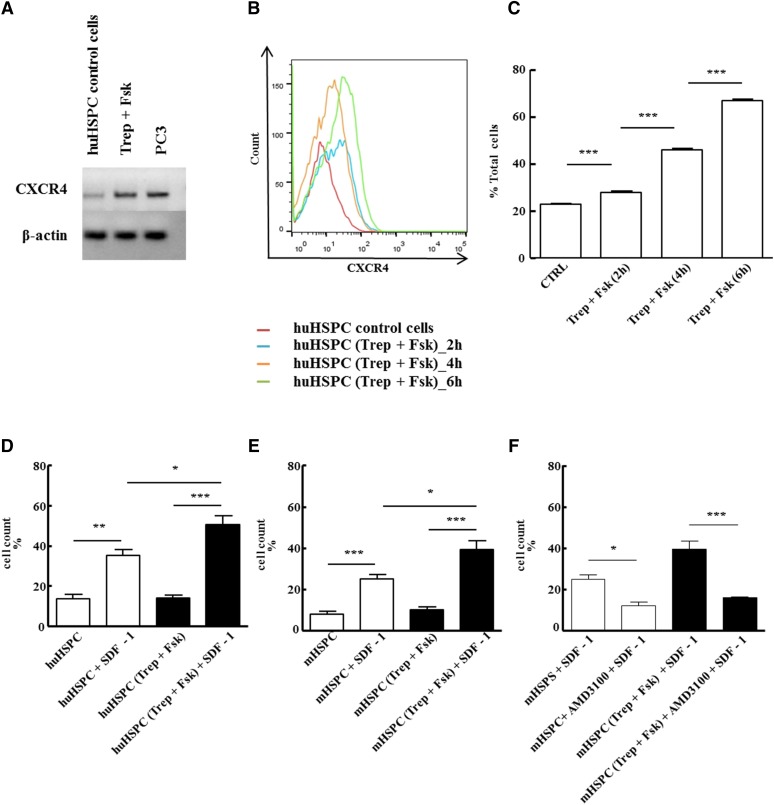
Enhanced engraftment of dmPGE2-treated HSCs was attributed to effects on cell survival, proliferation, and homing (Hoggatt et al., 2009). The SDF-1/CXCR4 axis plays a major role in homing of HSCPs to the bone marrow niche. Under our experimental conditions, in vitro pretreatment of murine and human HSPCs with treprostinil and forskolin enhanced bone marrow reconstitution but did not alter cell viability or cell cycle progression in vitro (cf. Fig. 3). Thus we surmised that the beneficial action of treprostinil resulted from enhanced engraftment of HSPCs through SDF-1/CXCR4–mediated effects. This conjecture was examined as follows: Prestimulation of human HSPCs with treprostinil and forskolin raised mRNA levels (Fig. 8A) and surface levels of CXCR4 (Fig. 8, B and C). The upregulation of CXCR4 was translated into enhanced migration of human (Fig. 8D) and murine (Fig. 8E) HSPCs toward SDF-1. This directed migration was specific, because it was blocked by the selective CXCR4-antagonist plerixafor/AMD3100 (Hatse et al., 2002) (Fig. 8F). Likewise, basal migration (i.e., random migration in the absence of SDF-1) was not enhanced by pretreating HSPCs with treprostinil and forskolin (cf. first and third bar in Fig. 8, B and C). The antagonism by AMD3100 was recapitulated in vivo: The bone marrow of lethally irradiated recipient mice was reconstituted with treprostinil- and forskolin-pretreated HSPCs, and the mice were subsequently administered subcutaneously the optimum dose of treprostinil for 10 days. The concomitant administration of AMD3100 (3.3 mg kg−1 8 h−1) blunted the beneficial action of treprostinil so that all recipient mice eventually succumbed to bone marrow failure (Fig. 9). Thus, taken together, the observations indicate a mechanistic link between treprostinil-induced cAMP accumulation and enhanced CXCR4-signaling in HSPCs.
Fig. 8.
In vitro pretreatment with treprostinil and forskolin enhances the action of SDF-1 via CXCR4. (A) RNA was isolated from human HSPCs that had been incubated in the absence (control) or presence of the combination of 10 μM treprostinil and 30 μM forskolin (Trep + Fsk) for 1 hour. RNA prepared from the human prostate cancer cell line PC3 served as positive control. After reverse transcription, PCR-dependent amplification was done using primers listed in Table 1. Amplicons for CXCR4 were electrophoretically resolved on an agarose gel and visualized by ethidium bromide staining. The mRNA encoding β-actin was amplified as internal control. The data are representative of two additional experiments with similar results. (B, C) Human HSPCs were incubated in the absence (red trace = vehicle control) or in the presence of the combination of treprostinil (10 μM) and forskolin (30 μM) for 2 hours (blue trace), 4 hours (orange trace), and 6 hours (green trace) at 37°C and stained for CXCR4 with an allophycocyanin-labeled antibody. (B) FACS histograms from a representative experiment. (C) Bar diagram shows means ± S.E.M. from three independent experiments. Statistically significant differences were examined using repeated-measures ANOVA followed by Bonferroni’s multiple comparison. (D–F) Freshly isolated murine and human HPSC were pretreated in vitro with either vehicle (open bars) or 10 μM treprostinil and 30 μM forskolin (closed bars) for 1 hour at 37°C followed by washing steps. A suspension (2 × 105 cells in 0.1 ml of medium containing growth factors) of human (D) or murine HSPCs (E, F) was added to the upper chamber of a Transwell and allowed to migrate toward SDF-1 (100 ng ml−1 in the lower chamber) for 4 hours. Cells that had migrated through the 5-μm filter were counted. HSPCs were also incubated in the absence and presence of 10 μM AMD3100 to confirm that the effects arose from a stimulation of CXCR4 (F). Data represent ± S.E.M. from three independent experiments carried out in triplicate. The statistical comparison was done by repeated-measures ANOVA followed by Tukey’s multiple comparison (*P < 0.05, **P < 0. 01, ***P < 0.001).
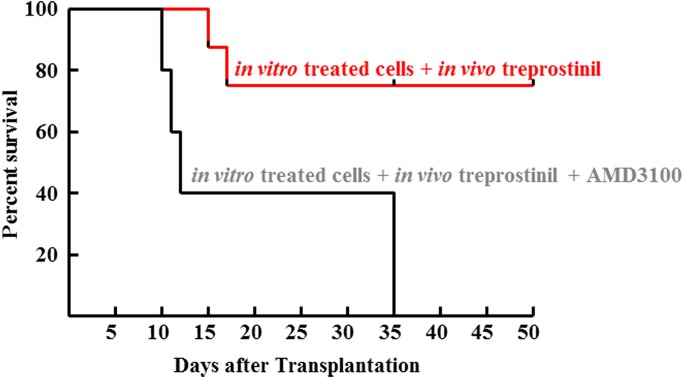
Fig. 9.
In vivo administration of CXCR4-antagonist (AMD3100) abrogates the beneficial effect of treprostinil on the survival of recipient mice. Murine HPSCs were pretreated in vitro with treprostinil and forskolin as outlined in the legend to Fig. 4 and injected (2 × 105 per mouse) into lethally irradiated recipient mice. These were subsequently divided into two groups. Mice allocated to group 1 (n = 10) were further subjected to in vivo treatment with treprostinil (0.15 mg kg−1), and mice in group 2 (n = 10) received both treprostinil (0.15 mg kg−1 8 h−1) and AMD3100 (3.3 mg kg−1 8 h−1) by subcutaneous injection every 8 hours for 10 days. The difference between the two survival curves was statistically significant (P = 0.007, log-rank test).
Discussion
Patients undergoing HSC transplantation incur a high risk of life-threatening infections as long as their white blood cell count remains low (Wingard et al., 2011; Chi et al., 2013). Thus, there is an unmet medical need to accelerate bone marrow recovery. In theory, this can be achieved by increasing the number of transplanted stem cells, because this augments the likelihood of their engraftment. In practice, the supply of HSCs is limited. Hence, pharmacological manipulations are of interest, if they safely enhance bone marrow engraftment. Several strategies can be envisioned, but their translation into clinical application would probably require several years (Piccoli et al., 2013; Speth et al., 2014; Popat et al., 2015). Our approach assumes that, among several candidate pathways, Gαs-stimulated cAMP accumulation would most probably identify a safe drug suitable for translation into clinical application for the following reasons: 1) The pathway can be stimulated via Gs-coupled receptors, which can be identified by cataloging their expression and by verifying that their stimulation raises cAMP in HSPCs (Alexander et al., 2013). Agonists can be selected on the basis of their clinical safety profile. 2) In vitro, the action of agonists can be greatly augmented by using forskolin (Sunahara et al., 1996; Kudlacek et al., 2001). Thus, in the presence of forskolin, agonists can raise cAMP to levels comparable to or exceeding those achieved by cholera toxin. Cholera toxin provides a reference point for gauging drug efficacy, because it was shown to enhance the capacity of HSPCs to reconstitute hematopoiesis (Adams et al., 2009). With this strategy, we identified treprostinil as a candidate drug suitable for improving human HSC transplantation on the basis of the following observations: Murine and human HSPCs expressed mRNAs encoding several Gs-coupled prostanoid receptors (i.e., DP1-, IP-, EP2-, and EP4-receptor). Treprostinil is an agonist at all these receptors (Aronoff et al., 2007; Whittle et al., 2012). Consistent with its broad pharmacological profile, treprostinil was more efficacious in stimulating cAMP accumulation than the IP receptor–selective agonists beraprost and iloprost (Alexander et al., 2013). Murine and human HSPCs tolerated treprostinil and forskolin without any detectable effect on their viability and their ability to undergo asymmetric cell division and lineage-specific differentiation. 3) Pretreating murine and human HSPCs with treprostinil and forskolin enhanced their engraftment in lethally irradiated recipient mice. The combination was as efficacious as the pretreatment with cholera toxin. 4) The additional treatment of recipient mice with treprostinil in vivo further enhanced bone marrow engraftment.
Prostanoids play a crucial role in regulating vertebrate HSCs; this was first appreciated in zebra fish (North et al., 2007). Accordingly, dmPGE2 promotes engraftment of HSCs (Frisch et al., 2009; Hoggatt et al., 2009; Goessling et al., 2011). In fact, in a phase I trial, the period of neutropenia was shortened by some 3.5 days, if human cord blood cells were preincubated with dmPGE2 prior to their administration to recipients (Cutler et al., 2013). On the basis of our observations, we propose treprostinil as an alternative suitable for promoting bone marrow engraftment in human recipients for the following reasons: the beneficial effect resulting from in vitro pretreatment of HSPCs with the combination of treprostinil and forskolin was further augmented by administering treprostinil to recipients over several days; and treprostinil was approved for the treatment of pulmonary hypertension in 2002. Thus, the clinical experience with treprostinil covers many thousands of patient years, and its safety profile is well understood. This also includes long-term data in both adults (Sadushi-Kolici et al., 2012; Benza et al., 2013) and children (Siehr et al., 2013). It is therefore reasonable to posit that treprostinil can be repurposed and safely administered to patients undergoing HSC transplantation. It is worth noting that, in the optimal regimen, the HSPCs were first preincubated ex vivo with the combination of forskolin and treprostinil prior to the treatment of the recipient with treprostinil. We consider it improbable that this pretreatment with the combination of forskolin and treprostinil will be a major regulatory impediment, because forskolin is removed prior to administration of the donor HSPCs and only treprostinil is administered to the recipient. In contrast, dmPGE2 is not yet an approved drug, thus the dose-response relation that governs side effects in man is poorly understood. In human HSPCs, treprostinil was substantially more efficacious in raising cAMP than dmPGE2. This can be rationalized by taking into account that dmPGE2 is also a potent agonist of EP3-receptors (Woodward et al., 2011). Concomitant stimulation of Gi-coupled EP3-receptors by dmPGE2 is predicted to limit cAMP accumulation elicited via EP2- and/or EP4-receptors. In contrast, treprostinil is a low affinity agonist at EP3-receptors (Whittle et al., 2012). It is also worth considering that prostanoids have multiple sites of actions in the bone marrow: Our data unequivocally showed that the additional in vivo treatment of recipient mice with treprostinil was more efficacious than sole in vitro preincubation of the HSPCs, but the dose-response curve was bell-shaped. The mechanistic basis for the bell-shaped curve is not clear. However, it is not surprising that the action of treprostinil in vivo is complex. Gs-coupled E prostanoid receptors that are relevant to engraftment of HPSCs are not only expressed in the stem cell compartment. In fact, EP4 receptors are for instance, also found on the cells lining the endosteal niche (Hoggatt et al., 2013a). At the very least, our observations indicate that dmPGE2, which acts at EP2, EP3, and EP4-receptors (Woodward et al., 2011), probably has several additional sites of action, which limit its application in vivo.
Acknowledgments
The authors thank Peter Husslein, Harald Zeiser, Christa Hauser, and their teams for access to and support of human samples. We thank Patrick Thurner for statistical advice, Christian Nanoff, Richard Moriggl, Dagmar Stoiber, Martin Hohenegger and Sonja Sucic for helpful discussions, and Christian Balasz and the staff of the animal facility for experimental help and animal care.
Abbreviations
- AMD3100
plerixafor, 1,1′-[1,4-phenylenebis(methylene)]bis[1,4,8,11-tetraazacyclotetradecane]
- CTX
cholera toxin
- CXCR4
C-X-C motif chemokine receptor 4
- dmPGE2
16,16-dimethyl-PGE2
- DP1
isoform of D prostanoid receptor
- EP1-4
four isoforms of E prostanoid receptors
- HPRT1
hypoxanthine phosphoribosyltransferase-1
- HSCs
hematopoietic stem cells
- HSPCs
hematopoietic stem and progenitor cells
- IP receptor
I prostanoid receptor
- IL
interleuken
- NSG
NOD/SCID/IL-2Rg2/2
- PCR
polymerase chain reaction
- qPCR
quantitative PCR
- SDF-1
stromal cell–derived factor-1
- SFEM
serum-free expansion medium
Authorship Contributions
Participated in research design: Freissmuth, Sexl, Zebedin-Brandl.
Conducted experiments: Kazemi, Bergmayr, Zebedin-Brandl.
Performed data analysis: Kazemi, Prchal-Murphy, Zebedin-Brandl.
Contributed new reagents or analytic tools: Javaheri, Pham, Themanns, Prchal-Murphy, Strohmaier, Sexl.
Wrote or contributed to the writing of manuscript: Freissmuth, Kazemi, Sexl, Zebedin-Brandl.
Footnotes
References
- Adams GB, Alley IR, Chung UI, Chabner KT, Jeanson NT, Lo Celso C, Marsters ES, Chen M, Weinstein LS, Lin CP, et al. (2009) Haematopoietic stem cells depend on Galpha(s)-mediated signalling to engraft bone marrow. Nature 459:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. (2006) Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439:599–603. [DOI] [PubMed] [Google Scholar]
- Aksentijevich I, Flinn I. (2002) Chemotherapy and bone marrow reserve: lessons learned from autologous stem cell transplantation. Cancer Biother Radiopharm 17:399–403. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ, CGTP Collaborators (2013) The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol 170:1459–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff DM, Peres CM, Serezani CH, Ballinger MN, Carstens JK, Coleman N, Moore BB, Peebles RS, Faccioli LH, Peters-Golden M. (2007) Synthetic prostacyclin analogs differentially regulate macrophage function via distinct analog-receptor binding specificities. J Immunol 178:1628–1634. [DOI] [PubMed] [Google Scholar]
- Benza RL, Tapson VF, Gomberg-Maitland M, Poms A, Barst RJ, McLaughlin VV. (2013) One-year experience with intravenous treprostinil for pulmonary arterial hypertension. J Heart Lung Transplant 32:889–896. [DOI] [PubMed] [Google Scholar]
- Bergmayr C, Thurner P, Keuerleber S, Kudlacek O, Nanoff C, Freissmuth M, Gruber CW. (2013) Recruitment of a cytoplasmic chaperone relay by the A2A adenosine receptor. J Biol Chem 288:28831–28844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer HE, Pelus LM. (2014) Inhibition of DPP4/CD26 and dmPGE₂ treatment enhances engraftment of mouse bone marrow hematopoietic stem cells. Blood Cells Mol Dis 53:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi AK, Soubani AO, White AC, Miller KB. (2013) An update on pulmonary complications of hematopoietic stem cell transplantation. Chest 144:1913–1922. [DOI] [PubMed] [Google Scholar]
- Cutler C, Multani P, Robbins D, Kim HT, Le T, Hoggatt J, Pelus LM, Desponts C, Chen YB, Rezner B, et al. (2013) Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood 122:3074–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freissmuth M, Gilman AG. (1989) Mutations of GS alpha designed to alter the reactivity of the protein with bacterial toxins. Substitutions at ARG187 result in loss of GTPase activity. J Biol Chem 264:21907–21914. [PubMed] [Google Scholar]
- Frisch BJ, Porter RL, Gigliotti BJ, Olm-Shipman AJ, Weber JM, O’Keefe RJ, Jordan CT, Calvi LM. (2009) In vivo prostaglandin E2 treatment alters the bone marrow microenvironment and preferentially expands short-term hematopoietic stem cells. Blood 114:4054–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R, Kelly B, Semenza GL. (2003) Vascular endothelial growth factor gene expression in colon cancer cells exposed to prostaglandin E2 is mediated by hypoxia-inducible factor 1. Cancer Res 63:2330–2334. [PubMed] [Google Scholar]
- Goessling W, Allen RS, Guan X, Jin P, Uchida N, Dovey M, Harris JM, Metzger ME, Bonifacino AC, Stroncek D, et al. (2011) Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell 8:445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT, et al. (2009) Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136:1136–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatse S, Princen K, Bridger G, De Clercq E, Schols D. (2002) Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett 527:255–262. [DOI] [PubMed] [Google Scholar]
- Hoggatt J, Mohammad KS, Singh P, Hoggatt AF, Chitteti BR, Speth JM, Hu P, Poteat BA, Stilger KN, Ferraro F, et al. (2013a) Differential stem- and progenitor-cell trafficking by prostaglandin E2. Nature 495:365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggatt J, Singh P, Sampath J, Pelus LM. (2009) Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood 113:5444–5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggatt J, Singh P, Stilger KN, Plett PA, Sampson CH, Chua HL, Orschell CM, Pelus LM. (2013b) Recovery from hematopoietic injury by modulating prostaglandin E(2) signaling post-irradiation. Blood Cells Mol Dis 50:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel PA, Zhang L, Murray F, Yokouchi H, Zambon AC. (2012) Cyclic AMP is both a pro-apoptotic and anti-apoptotic second messenger. Acta Physiol (Oxf) 204:277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Dingledine R. (2013) Role of prostaglandin receptor EP2 in the regulations of cancer cell proliferation, invasion, and inflammation. J Pharmacol Exp Ther 344:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Alvarez R, Salomon Y. (1994) Determination of adenylyl cyclase catalytic activity using single and double column procedures. Methods Enzymol 238:31–56. [DOI] [PubMed] [Google Scholar]
- Kudlacek O, Mitterauer T, Nanoff C, Hohenegger M, Tang WJ, Freissmuth M, Kleuss C. (2001) Inhibition of adenylyl and guanylyl cyclase isoforms by the antiviral drug foscarnet. J Biol Chem 276:3010–3016. [DOI] [PubMed] [Google Scholar]
- North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH, Grosser T, et al. (2007) Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447:1007–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelus LM, Hoggatt J, Singh P. (2011) Pulse exposure of haematopoietic grafts to prostaglandin E2 in vitro facilitates engraftment and recovery. Cell Prolif 44 (Suppl 1):22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli C, Agriesti F, Scrima R, Falzetti F, Di Ianni M, Capitanio N. (2013) To breathe or not to breathe: the haematopoietic stem/progenitor cells dilemma. Br J Pharmacol 169:1652–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popat U, Mehta RS, Rezvani K, Fox P, Kondo K, Marin D, McNiece I, Oran B, Hosing C, Olson A, et al. (2015) Enforced fucosylation of cord blood hematopoietic cells accelerates neutrophil and platelet engraftment after transplantation. Blood 125:2885–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purton LE, Scadden DT. (2007) Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell 1:263–270. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. (2008) Dose translation from animal to human studies revisited. FASEB J 22:659–661. [DOI] [PubMed] [Google Scholar]
- Sadushi-Koliçi R, Skoro-Sajer N, Zimmer D, Bonderman D, Schemper M, Klepetko W, Glatz J, Jakowitsch J, Lang IM. (2012) Long-term treatment, tolerability, and survival with sub-cutaneous treprostinil for severe pulmonary hypertension. J Heart Lung Transplant 31:735–743. [DOI] [PubMed] [Google Scholar]
- Scholz W, Platzer B, Schumich A, Höcher B, Fritsch G, Knapp W, Strobl H. (2004) Initial human myeloid/dendritic cell progenitors identified by absence of myeloperoxidase protein expression. Exp Hematol 32:270–276. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, et al. (2005) Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 174:6477–6489. [DOI] [PubMed] [Google Scholar]
- Siehr SL, Ivy DD, Miller-Reed K, Ogawa M, Rosenthal DN, Feinstein JA. (2013) Children with pulmonary arterial hypertension and prostanoid therapy: long-term hemodynamics. J Heart Lung Transplant 32:546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth JM, Hoggatt J, Singh P, Pelus LM. (2014) Pharmacologic increase in HIF1α enhances hematopoietic stem and progenitor homing and engraftment. Blood 123:203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunahara RK, Dessauer CW, Gilman AG. (1996) Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol 36:461–480. [DOI] [PubMed] [Google Scholar]
- Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. (1997) Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha.GTPgammaS. Science 278:1907–1916. [DOI] [PubMed] [Google Scholar]
- Whittle BJ, Silverstein AM, Mottola DM, Clapp LH. (2012) Binding and activity of the prostacyclin receptor (IP) agonists, treprostinil and iloprost, at human prostanoid receptors: treprostinil is a potent DP1 and EP2 agonist. Biochem Pharmacol 84:68–75. [DOI] [PubMed] [Google Scholar]
- Wingard JR, Hsu J, Hiemenz JW. (2011) Hematopoietic stem cell transplantation: an overview of infection risks and epidemiology. Hematol Oncol Clin North Am 25:101–116. [DOI] [PubMed] [Google Scholar]
- Woodward DF, Jones RL, Narumiya S. (2011) International Union of Basic and Clinical Pharmacology. LXXXIII: classification of prostanoid receptors, updating 15 years of progress. Pharmacol Rev 63:471–538. [DOI] [PubMed] [Google Scholar]
- Zon L. (2014) Translational research: the path for bringing discovery to patients. Cell Stem Cell 14:146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]