Abstract
Opioid-induced constipation is a major side effect that persists with long-term opioid use. Previous studies demonstrated that nicotine-induced contractions are enhanced after long-term morphine exposure in guinea pig ileum. In the present study, we examined whether the increased sensitivity to nicotine could be observed in single enteric neurons after long-term morphine exposure, determined the subunits in mouse enteric neurons, and examined the effect of nicotine in reversing opioid-induced constipation. Nicotine (0.03–1 mM) dose-dependently induced inward currents from a holding potential of −60 mV in isolated single enteric neurons from the mouse ileum. The amplitude of the currents, but not the potency to nicotine, was significantly increased in neurons receiving long-term (16–24 h) but not short-term (10 min) exposure to morphine. Quantitative mRNA analysis showed that nicotinic acetylcholine receptor (nAChR) subunit expression in the mouse ileum was α3 ≥ β2 > β4 > α5 > α4 > β3 > α6. Nicotine-induced currents were obtained in neurons from α7, β2, α5, and α6 knockout mice. The currents were, however, inhibited by mecamylamine (10 μM) and the α3β4 blocker α-conotoxin AuIB (3 μM), suggesting that nicotine-induced currents were mediated by the α3β4 subtype of nAChRs on enteric neurons. Conversely, NS3861, a partial agonist at α3β4 nAChR, enhanced fecal pellet expulsion in a dose-dependent manner in mice that received long-term, but not short-term, morphine treatment. Overall, our findings suggest that the efficacy of nAChR agonists on enteric neurons is enhanced after long-term morphine exposure, and activation of the α3β4 subtype of nAChR reverses chronic, but not acute, morphine-induced constipation.
Introduction
Opioids are commonly used for the treatment of pain. Despite their highly efficacious analgesic properties, side effects such as tolerance, addiction, and constipation significantly limit their long-term use. Many patients tend to discontinue the use of opioids due to the discomfort caused by these side effects, particularly constipation. Whereas the analgesic effects of opioids are mediated through central actions, constipation is predominantly mediated through direct action on the peripheral µ-opioid receptors in the enteric nervous system (ENS). Morphine decreases gastrointestinal (GI) transit, largely as a result of diminishing the excitability of enteric neurons (North and Williams, 1977), resulting in decreased excitatory neurotransmitter release (Paton, 1957). Tolerance to the analgesic effects develops upon repeated administration of morphine but does not occur in the colon. Thus, long-term morphine treatment continues to induce constipation. It is therefore of considerable interest to identify potential mechanisms that allow for the reversal or prevention of chronic opioid-induced constipation.
Inhibition of cholinergic transmission is a major mechanism of opioid action in the ENS. The µ-opioid receptors are localized at presynaptic nerve terminals as well as on the cell bodies of both motor and interneurons in the myenteric plexus (Wood and Galligan, 2004). Activation of the µ-opioid receptors results in decreased excitability of neurons due to activation of K+ channels, and/or inhibition of Ca2+ and Na+ channels (Wood and Galligan, 2004; Smith et al., 2012). Thus, endogenous excitatory neurotransmitters such as acetylcholine have reduced activity during peristaltic reflex. Acetylcholine activates nicotinic receptors within the myenteric ganglia, stimulating both interneurons and motor neurons, thereby facilitating peristalsis. Nicotinic acetylcholine receptors (nAChRs) are pentameric ligand-gated ion channels that are highly expressed in the ENS (Galligan et al., 2000; Galligan, 2002) and allow for fast synaptic transmission. The five subunits comprising the nAChR form as homomeric or heteromeric units. There are 12 separate subunits, α2–α10 and β2–β4. Immunohistochemical, electrophysiological, and pharmacological studies indicate that the predominant nAChR subunits in myenteric neurons are the α3, α5, β2, and β4 subunits, which generally form the heteromeric receptor (Zhou et al., 2002). A significant implication for the role of the nAChR is that patients with anti-α3*-nAChR antibodies in the serum have autoimmune GI dysmotility (Meeusen et al., 2013).
In the context of opioid-induced constipation, previous studies have reported enhanced sensitivity to nicotine and other agonists after long-term morphine treatment in the guinea pig ileum. These studies from the early 1970s demonstrated the development of supersensitivity to several agonists in the morphine-tolerant guinea pig ileum, including nicotine (Goldstein and Schulz, 1973; Johnson et al., 1978). The mechanism for the supersensitivity is unclear but has been suggested to involve increased depolarization of the enteric neurons. In view of the enhanced sensitivity to nicotine, we surmised that nAChRs provide a potential target for stimulating GI motility in chronic opioid-induced constipation.
In this study, we have investigated the effects of nicotine on isolated enteric neurons from mice that received long-term morphine treatment. We show that nicotine-induced currents in myenteric neurons from adult mouse small intestine are enhanced after long-term, but not short-term, treatment with morphine. In addition, we demonstrate that the nAChR subunits α3 and β4 are predominant on the enteric neurons, and that both nicotine and NS3681, a α3β4* partial agonist, reverse chronic opioid-induced constipation in vivo.
Methods
Sodium chloride (NaCl), magnesium chloride (MgCl2), calcium chloride (CaCl2), glucose, ATP disodium salt, HEPES, EGTA, nicotine hydrogen tartarate, mecamylamine, and hexamethonium were purchased from Sigma-Aldrich (St. Louis, MO). Potassium chloride (KCl) was purchased from Fisher Scientific (Waltham, MA), and collagenase was purchased from Worthington (Lakewood, NJ). Laminin and poly-D-lysine were purchased from BD Biosciences (Franklin lanes, NJ); glial cell line–derived neurotrophic factor was purchased from Neuromics (Edina, MN); fetal bovine serum was purchased from Gemini Bio-Products (West Sacramento, CA); and B-27, trypsin, and neurobasal A media were purchased from ThermoFisher Scientific (Waltham, MA). NS3861 [3-(3-bromo-2-thienyl)-8-methyl-8-azabicyclo[3.2.1]oct-2-ene fumarate] (Harpsoe et al., 2013), α-conotoxin MII (Harvey et al., 1997), and α-conotoxin AuIB (Luo et al., 1998) were purchased from Tocris Bioscience (Bristol, UK). Morphine sulfate and morphine pellets (75 mg) were obtained from the National Institutes of Drug Abuse (Bethesda, MD).
Animals.
Adult male Swiss-Webster mice (25-30 g) (Harlan Laboratories, Indianapolis, IN) housed in 12-hour light/dark cycle vivarium were used for the experiments. Mice null for the α5 and α7 (The Jackson Laboratory, Sacramento, CA), and α6 and β2 (Institut Pasteur, Paris, France) nAChR subunits and their wild-type littermates were bred in an animal care facility at Virginia Commonwealth University (Richmond, VA). These mice were backcrossed at least 12–15 generations to C57BL/6J mice (The Jackson Laboratory). Mutant/transgenic and wild-type littermates were obtained from crossing heterozygous mice. The animal protocols were approved by the Virginia Commonwealth University institutional animal care and use committee. Morphine or nicotine were administered in mice through the intraperitoneal route. Animals underwent long-term morphine exposure through surgical implantation of a 75-mg morphine pellet for 4 days.
Surgical Implantation of Pellets.
Pellets were implanted as previously described (Ross et al., 2008). Mice were anesthetized with 2.5% isoflurane, and the hair on the back of their neck was shaved. Lack of response to a pinch on the toe and the absence of righting reflex were used as signs to assess adequate anesthesia. Sterile surgical equipment was used for the process to minimize any potential contamination. Shaven skin was cleaned with povidone iodine (General Medical Corporation, Prichard, WV) and rinsed with alcohol. A 1-cm horizontal incision was made at the base of the skull, and the underlying subcutaneous space was moved toward the dorsal flanks using a glass rod. A placebo or morphine pellet was inserted in this space and closed using 9-mm wound clips (BD Biosciences). Iodine was applied again after closing the site, and the animals were allowed to recover in their home cages.
Enteric Neuron Cell Isolation.
Enteric neurons were isolated as previously described (Smith et al., 2012, 2013). Ileum tissue was obtained immediately from euthanized mice and placed in ice-cold Krebs solution [in mM: 118 NaCl, 4.6 KCl, 1.3 NaH2PO4, 1.2 MgSO4, 25 NaHCO3, 11 glucose, and 2.5 CaCl2) and bubbled with carbogen (95% O2/5% CO2)]. The luminal contents were flushed with ice-cold Krebs solution, and the tissues were threaded on a plastic rod through the lumen. The longitudinal muscle layer with the adherent myenteric plexus was gently stripped using a cotton-tip applicator. The isolated longitudinal–myenteric plexus (LMMP) was then minced and subjected to digestion with collagense for 1 hour and with trypsin for 7 minutes at 37°C in the water bath. Tissue was triturated and collected using centrifugation after each digestion step. The isolated cells were then washed and plated on laminin and poly-D-lysine coated cover slips in neurobasal A media containing 1% fetal bovine serum, 1× B-27 and 10 ng/ml glial cell line–derived neurotrophic factor and penicillin/streptomycin.
Electrical Recordings.
Standard whole-cell configuration was used for all recordings. An EPC 10 amplifier (HEKA, Bellmore, NY) was used for recordings. All patch-clamp recordings were performed in enteric neurons within 2 days after isolation. Coverslips with attached cells were placed in a recording chamber under an inverted microscope and continuously perfused with external solution containing the following (in mM): 135 NaCl, 5.4 KCl, 0.3 NaH2PO4, 1 MgCl2, 5 glucose, and 2 CaCl2 (pH adjusted to 7.4 using 1 M NaOH). The patch pipettes were prepared using a Flaming-Brown horizontal micropipette puller (P-87; Sutter Instrument, Novato, CA) and fire polished. Resistance of the pipettes used was 1.5–2.5 MΩ when filled with an internal solution containing the following (in mM): 100 K-aspartic acid, 30 KCl, 4.5 ATP, 1 MgCl2, 10 HEPES, and 0.1 EGTA. Series resistance was <10 MΩ and not compensated. The voltage-clamp recordings were performed at a holding potential of −60 mV. The currents were measured either by giving a series of voltage pulses or by recording using a gap-free protocol at −60 mV. Action potentials were induced in the neurons by a series of current injections in current-clamp mode at resting membrane potential. Nicotine and ATP were applied to the cell via bath perfusion. The cells were washed after each nicotine exposure for 3–5 minutes before exposing to the next concentration.
Polymerase Chain Reaction.
Quantitative real-time polymerase chain reaction (PCR) was performed on RNA extracted from the ileum LMMP. LMMP tissue was first collected in TRIzol, and RNA was extracted using the manufacturer protocol (Life Technologies, Carlsbad, CA). PCR experiments were performed following the Bio-Rad (Hercules, CA) iTaq Universal SYBR Green One-Step Kit. To quantify the mRNA of nAChRs from LMMP, a standard curve was first plotted using different concentrations of genomic DNA with each primer. The standard curve was plotted based on the Ct value obtained at each concentration of DNA. The quantity of mRNA in the LMMP sample was determined based on the position of the Ct value of the sample in the standard curve. Copy number was calculated using the online tool available at scienceprimer.com. The primers used were as follows: β2-forward, TGCTCCAACTCTATGGCGCT; β2-reverse, CACCAGCTCAGAGCCATTAG,; β3-forward, CAGGCTTCCTACGGGTCTTC; β3-reverse, GGGCGGACACATTTCTGATA; β4-forward, CCCTGCTCCTCGTCTCTCTGTT; β4-reverse, TGGAGATGAGCTGGGAGGAG; α3-forward, CCGCTGTCCATGCTGATGCT; α3-reverse: GCCACAGGTTGGTTTCCATG; α4-forward, TGCCGCTCCTGCTGCTCTTA; α4-reverse, GCGGACAAGGACCACATCTG; α5-forward, GTTGCCTGAGCTATCCTCTG; α5-reverse, CCACGTCCACTAACTGAGAT; α6-forward, GACCAGGGAAACCTGCACTC; and α6-reverse, GATCGGAGACATTCTCCACC. Experiments were performed in triplicate from three separate biologic samples. 18S ribosomal RNA was used as an internal control to quantify the normalized fold change.
Total Fecal Pellet Output.
Fecal pellet output was measured as an indication of the total GI motility. These studies were conducted in a blinded fashion with pellet counting performed by an observer oblivious to the group being tested. Mice were divided into the following groups: 1) control [placebo-pelleted (4 days)], 2) short-term morphine treatment (placebo pelleted plus given a single 10 mg kg−1 morphine injection), and 3) long-term morphine treatment [75 mg morphine pelleted (4 days)]. Mice in each group were given short-term treatment with a single saline injection or 0.175, 0.35, 0.525, and 1.050 mg kg−1 i.p. nicotine, and placed in an empty cage. The number of fecal pellets expelled between 30 and 60 minutes after the injection were counted. Short-term morphine injections were given 20 minutes prior to the saline or nicotine injections. A similar separate group of mice was tested for the effects of NS3681 (0.01, 0.05, 0.1, and 0.5 mg kg−1 i.p.). All the nicotine doses are calculated by multiplying the dose of salt by 0.35 (base/anhydrous salt), as described previously (Matta et al., 2007).
Data Analysis.
SigmaPlot 11.0 and GraphPad Prism 6 were used for data analysis. Data are presented as the mean ± SEM, and scatter plots used to show the distribution. P values <0.05 were considered to be significant. A two-tailed t test was used to compare the differences between two different groups; one-way analysis of variance (ANOVA), two-way ANOVA with Tukey-Kramer post hoc test, or Fisher’s least significant difference test were used for comparisons among multiple groups. Specific tests are provided under figure legends. The EC50 values were calculated using least square linear regression analysis followed by calculation of the confidence limits (Bliss, 1967).
Results
Long-Term Exposure to Morphine Enhanced the Nicotine-Induced Excitability.
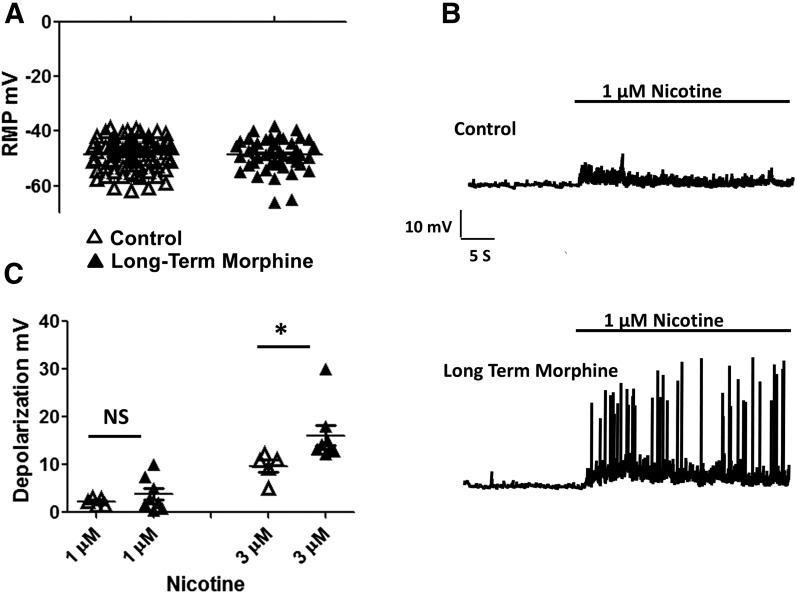
Nicotine-induced currents were examined in isolated neurons from the adult mouse myenteric plexus. In this primary culture preparation, both neurons and glia are isolated from the myenteric plexus (Smith et al., 2012, 2013). To record nicotinic currents from neurons, each cell was examined for its ability to elicit an action potential (Fig. 1A). Cells exhibiting action potentials also displayed inward currents when depolarized in voltage-clamp mode (Fig. 1B). The average resting membrane potential of enteric neurons from the mouse ileum was −47.6 ± 0.6 mV (n = 73), and long-term exposure to morphine (16–20 hours) (3 μM) did not significantly affect the resting potential −48.3 ± 1 mV (n = 42) (Fig. 2A). We have previously shown that treatment with morphine for 16–20 hours results in the development of tolerance and dependence in neurons, similar to that seen in neurons isolated from morphine-pelleted mice (Smith et al., 2014). In these cells, overnight treatment with morphine (3 µM) enhances excitability upon precipitated withdrawal (Smith et al., 2014). Previous reports (Leedham et al., 1992; Kong et al., 1997) have also suggested that the enhanced nicotine-induced contractions in guinea pigs given long-term treatment with morphine may be mediated due to depolarization of enteric neurons by long-term morphine treatment. Although long-term morphine treatment did not alter the resting potential, the enteric neurons were significantly more sensitive to nicotine after morphine treatment. As shown in Fig. 2B, nicotine at 1 µM slightly depolarized control neurons by 2.2 ± 0.3 mV; however, after long-term morphine treatment, the depolarization (3.8 ± 1.2 mV) resulted in spontaneous action potentials in 3 of 8 cells (Fig. 2B). At 3 µM nicotine concentration, the depolarization in control cells was 9.7 ± 1.2 mV, and in cells that received long-term morphine treatment it was 16.1 ± 2.2 mV (Fig. 2C). These data suggested that neurons were more sensitive to nicotine after long-term morphine treatment.
Fig. 1.
(A) Current-clamp recording from the same cell showing action potentials upon giving a positive current injection of 60 pA. (B) Whole-cell voltage-clamp recordings from neurons showing inward sodium current induced upon giving a series of voltage steps from −120 to 0 mV.
Fig. 2.
Long-term exposure to morphine enhanced the nicotine-induced excitability. (A) Resting membrane potentials (RMP) of neurons from the control group (n = 73) and long-term morphine treatment group (n = 42) in a current-clamp mode. (B) Raw traces showing the depolarization induced by 1 µM nicotine in the control group and after long-term morphine treatment. (C) Amplitude of depolarization induced by 1 and 3 µM concentrations of nicotine in control cells and cells that received long-term treatment with morphine. Student’s t test, *P < 0.05.
Long-Term but not Short-Term Exposure to Morphine Enhances Nicotine-Induced Currents.
To study the short-term and long-term effects of morphine on nAChRs, enteric neurons were treated with 3 µM morphine for a period of 10 minutes in the bath or overnight for a period of 16–20 hours in culture. nAChR activity was then assessed in these cells using nicotine as an agonist in a whole-cell voltage-clamp mode (Fig. 3A). Cells were held at −60 mV, and the peak amplitudes of inward currents were plotted against the nicotine concentration. The peak amplitude of nicotine-induced currents was significantly greater in cells that received long-term morphine treatment. The dose-response curve showed significantly greater currents at nicotine concentrations of 300 µM and 1 mM in the cells exposed for a prolonged period to morphine but not in the group of cells exposed for a short period when compared with the control drug–naive group (Fig. 3A and B). Although the peak amplitudes were enhanced, there was no difference in the potency of nicotine. The EC50 values were 44 µM [95% confidence interval (CI) 27–61], 39 µM (95% CI 25 ± 53), and 42 µM (95% CI 28–56) in control cells, after short-term and prolonged exposure, respectively (Fig. 3C). We further tested the effect of nicotine upon a single application to ensure that desensitization is not a confounding factor. The amplitude of maximal currents induced by a single individual exposure to 1 mM nicotine was 139.4 ± 34.3 pA/pF in control neurons, 106.51 ± 11.1 pA/pF in neurons after short-term exposure to morphine, and 228 ± 14.7 pA/pF after long-term exposure to morphine (Fig. 3D). Pretreatment with the opioid-receptor antagonist naloxone (1 µM) blocked the long-term morphine exposure–induced nicotinic currents (Fig. 3D).
Fig. 3.
Short-term but not long-term exposure to morphine enhances nicotine-induced currents. (A) Raw traces of recording from neurons with different concentrations of nicotine. (B) Concentration-response curves of peak currents at different concentrations of nicotine. (C) Concentration-response curve plotted as a function of the maximum response in each group. (D) Scatter plot showing the distribution of data from currents induced by 1 mM nicotine in control cells (n = 15) and cells that received long-term morphine treatment (n = 17) in the presence of 1 µM naloxone (n = 5), and in cells that received long-term treatment with morphine in the presence of 1 µM naloxone (n = 5). Two-way ANOVA, Tukey-Kramer post hoc test, *P < 0.05.
Long-Term Exposure to Morphine Did not Alter ATP-Induced Current.
We next tested whether the effects of long-term morphine treatment are specific for nicotine-induced currents by examining another ligand-gated channel, the P2X receptor. ATP (1 mM), an agonist at the P2X receptor induced inward current at a holding potential of −60 mV. ATP-induced currents were examined in cells exposed to long-term morphine use. There were no significant differences seen among the currents induced by ATP in control cells versus those in cells that received long-term morphine treatment. The amplitude of currents induced by 1 mM ATP are 73.6 ± 17 pA/pF in control cells and 56.1 ± 12.4 pA/pF in cells that received long-term treatment with morphine (Fig. 4).
Fig. 4.
Long-term exposure to morphine did not alter the ATP-induced current. (A) Raw trace displaying the inward current induced by a 1 mM concentration of ATP. (B) Amplitude of current induced by 1 mM ATP in control cells (n = 7) and cells that received long-term morphine treatment (n = 5). Student’s t test, *P < 0.05.
α3 and β4 nAChR Subunits Are Highly Expressed in the Mouse Enteric Neurons.
Previous studies (Zhou et al., 2002) in the neonatal guinea pig ileum have suggested the functional expression of α3, β4, β2, and α5 subunits in the enteric neurons. Data from quantitative PCR quantifying the mRNA levels of nAChRs in the LMMP of ileum demonstrated a higher expression in the order of α3 > β2 > β4 > α5 > α4 > β3 > α6 subunits (Supplemental Fig. 1). However, the contribution of these subunits in nicotine-induced responses is not clear. To further examine the functional role of these subunits in the enteric neurons, we used a genetic and a pharmacological approach. Enteric neurons were isolated from C57BL/6J mice as these formed the background strain for all nicotinic knockouts. Figure 5A shows nicotine-induced inward currents from α7, α5, α6, and β2 knockout mice. Exposure to nicotine (3–300 μM) induced concentration-dependent inward currents in all knockout mice and were not significantly different in maximal amplitude to the C57BL/6J background (Fig. 5B and Supplemental Fig. 2). This indicates that these receptor subunits were not specifically involved in nicotine-induced currents in the enteric neurons. However, the nicotine-induced currents from α5 knockout enteric neurons showed a delayed desensitization compared with the knockout mice of other subtypes, suggesting that they may be involved in regulating the desensitization of the nAChR expressed in enteric neurons (Fig. 5A and B).
Fig. 5.
(A) Raw traces of currents induced by 0.3 mM nicotine from neurons isolated from LMMP of α7, c-57, α5, α6, and β2 knockout mice. (B) Concentration-response curves of nicotine-induced currents in cells from neurons isolated from LMMP of α7, c-57, α5, α6, and β2 knockout mice. Two-way ANOVA, Tukey-Kramer post hoc test, *P < 0.05.
To further define the composition of nAChRs mediating the effects of nicotine, we used α3β4 and α3β2/α6β2 nicotinic antagonists with different selectivity toward these nAChR subtypes. Mecamylamine (10 µM) and hexamethonium (10 µM) significantly blocked the nicotine-induced currents at concentrations previously reported to be more preferential for α3β4 receptors (Papke et al., 2010). This was further confirmed by inhibition of nicotine-induced currents by the α-conotoxin AuIB, which has been shown to be highly specific for α3β4 expressed receptor (Harvey et al., 1997; Luo et al., 1998). A submaximal dose of AuIB (3 µM) that is specific for α3β4 significantly blocked the nicotine-induced currents in enteric neurons. α-Conotoxin M-II (100 nM), a nAChR antagonist specific for α3β2/α6β2 subtypes, did not significantly block the nicotine-induced currents in the enteric neurons (Fig. 6A and B). These findings suggested that the nicotine-induced currents in the enteric neurons are primarily mediated through the α3β4* nAChR receptors, with possible coexpression of the α5 subunit modulating receptor desensitization.
Fig. 6.
Hexamethonium, mecamylamine, and α-conotoxin AuIB, but not M-II, block the nicotine-induced currents in neurons isolated from mouse LMMP. (A) Raw traces of nicotine-induced currents in control cells, and in the presence of 10 µM hexamethonium (n = 5), 10 µM mecamylamine (n = 5), 100 nM M-II (n = 9), and 3 µM AuIB (n = 8). (B) Scatter plot showing the distribution of the amplitude of currents in control cells, and in the presence of 10 µM hexamethonium,10 µM mecamylamine, 100 nM M-II, and 3 µM AuIB. *P < 0.05. Ctl, control.
Effects of Nicotine and NS3861 on GI Motility.
The above studies indicate that long-term morphine treatment leads to enhanced nicotine-induced effects on the myenteric neurons. The cellular studies in enteric neurons suggest that the nAChRs mediating nicotinic responses are likely to be α3β4. To determine the role of α3β4 nAChRs on the opioid-induced decrease in GI motility, we used nicotine and NS3861, a compound with high affinity and partial agonist properties in α3β4-expressed nAChRs (Harpsoe et al., 2013). As shown in Fig. 7, there was a mean of 5 ± 1.1 pellets (measured over a 30-minute interval) after saline injection in placebo-pelleted mice. Nicotine significantly decreased the number of fecal pellets expelled with increasing doses (0.175, 0.35, 0.525, and 1.050 mg kg−1) (Fig. 7A). In mice receiving short-term treatment with morphine (10 mg kg−1), there was complete inhibition of pellet expulsion during the first hour, and nicotine did not stimulate pellet expulsion (Fig. 7B). However, in morphine-pelleted animals, 0.175 mg kg−1 nicotine significantly increased the number of fecal pellets expelled to 3.3 ± 1. There were, however, no significant differences seen with other nicotine doses tested (0.35, 0.525, and 1.050 mg kg−1) (Fig. 7C).
Fig. 7.
Effects of nicotine on fecal output. Scatter plot displaying the number of fecal pellets expelled after a single intraperitoneal injection of saline or different concentrations of nicotine in placebo-pelleted mice (A), placebo-pelleted mice receiving 10 mg kg−1 morphine (B), and morphine-pelleted mice (C) (N = 5-10 in each group). One-way ANOVA, Fisher’s least significant difference test, *P < 0.05.
Similarly, NS3861 did not significantly alter the number of fecal pellets expelled at 0.01, 0.05, and 0.1 mg kg−1, and decreased the number of pellets with a dose of 0.5 mg kg−1 when given intraperitoneally. In mice that received short-term treatment with morphine (10 mg kg−1), there was complete inhibition of pellet expulsion, and NS3861 did not stimulate pellet expulsion. However, in the morphine-pelleted mice, the mean number of fecal pellets expelled was 1.2 ± 0.58, and the number of fecal pellets expelled increased in a dose-related manner, with a significant increase seen at 0.1 mg kg−1 NS3861 (Fig. 8).
Fig. 8.
Effects of NS3861 on fecal output. Scatter plot displaying the number of fecal pellets expelled after a single intraperitoneal injection of saline or different concentrations of NS3861 in placebo-pelleted mice (A), placebo-pelleted mice receiving 10 mg kg−1 morphine (B), and morphine-pelleted mice (C) (N = 5 in each group). One-way ANOVA, Tukey-Kramer post hoc test, *P < 0.05.
Enhanced Responses to Nicotine Are not Associated with Dependence in Enteric Neurons.
Long-term exposure to morphine was previously shown to induce tolerance and dependence in single enteric neurons from ileum but not from the colon (Ross et al., 2008; Smith et al., 2014). To identify whether the enhanced responses to nicotine are associated with morphine dependence, we studied the effects of long-term morphine exposure on the response to nicotine in enteric neurons from the colon. Nicotine-induced currents were significantly enhanced in colonic cells that received long-term morphine treatment. The amplitudes of currents induced by 1 mM nicotine were 148 ± 28.5 pA/pF in control cells and 222 ± 24.4 pA/pF in cells that received long-term treatment with morphine. The EC50 values of nicotine were 18.4 µM (95% CI 11.6–29.1) in control cells and 7.1 µM (95% CI 3.2–15.7) cells that received long-term treatment with morphine (Fig. 9).
Fig. 9.
Nicotine-induced currents in neurons isolated from mouse colon. (A) Concentration-response curves of nicotine-induced currents in neurons isolated from mouse colon. (B) Scatter plot showing the distribution of data at the peak of inward currents induced by 1 mM nicotine in control cells (n = 6) and cells that received long-term treatment with morphine (n = 5). Two-way ANOVA, Tukey-Kramer post hoc test, *P < 0.05.
α3β4 mRNA Expression Is not Altered after Prolonged Exposure to Morphine.
To test whether the enhanced responses to nicotine seen after prolonged exposure to morphine are mediated through an increase in transcription of the α3β4 nAChR receptor, we examined the α3β4 mRNA expression in LMMP from mice pelleted with either placebo or 75 mg morphine for 4 days. The normalized fold changes in mRNA were not significantly different among the two tested groups. (Fig. 10).
Fig. 10.
α3β4 mRNA expression is not altered after prolonged exposure to morphine. The normalized fold changes in the mRNA of β4 (A) and α3 (B) subunits in placebo-pelleted and morphine-pelleted animals (N = 5). Student’s t test, *P < 0.05.
Discussion
In the present study, we show that: 1) long-term morphine treatment increases the efficacy for nicotine in isolated single neurons; 2) the α3β4 subtype of nAChRs mediates the nicotine-induced currents in the adult mouse enteric neurons; 3) NS3861, a partial agonist at the α3β4 nAChR, reversed morphine-induced constipation in mice that received long-term, but not short-term, morphine treatment; 4) the effects of morphine were specific for nAChRs and were not seen in P2X receptors expressed in the enteric neurons; and 5) the enhanced efficacy in nAChRs is not due to the development of opioid dependence, because similar findings have also been seen in neurons isolated from the colon.
Morphine-induced constipation is a major problem limiting its clinical utility. Peripheral μ-opioid antagonists have recently become available as an option for this treatment; however, there are limitations to their use, including potential cardiovascular side effects. In this study, we have found that the nAChR α3β4 sensitivity is significantly enhanced after prolonged opioid treatment. nAChR subtypes are highly expressed in the enteric neurons, and an α3β4 nicotinic agonist provides a prokinetic effect after long-term, but not short-term, opioid exposure.
Previous reports have suggested that the increase in the potency of nicotine after prolonged exposure to morphine (Goldstein and Schulz, 1973; Johnson et al., 1978) may derive from the depolarization of enteric neurons by long-term morphine treatment due to reduced sodium potassium pump activity (Leedham et al., 1992; Kong et al., 1997). However, in isolated neurons that were treated with morphine overnight and demonstrated an enhanced response to nicotine, no significant difference in the resting membrane potential was observed. Furthermore, the responses to ATP remained unchanged, although ATP and acetylcholine are generally cotransmitters in the myenteric plexus. This suggests that long-term morphine-induced enhancement of the nAChR is specific and may involve either a membrane-delimited pathway or alterations in intracellular signaling. It is noteworthy that α3β4 nicotinic antagonists and partial agonists also reverse morphine withdrawal symptoms, including diarrhea in the mouse (Muldoon et al., 2014).
We further examined the specific receptor subtype affected by long-term opioid treatment. In the present study, we approached this question by examining the mRNA expression levels and by using transgenic models along with a pharmacological approach in mouse enteric neurons. nAChRs from the LMMP of ileum displayed mRNA expression in the order α3 > β2 > β4 > α5 > α4 > β3 > α6 subunits (Supplemental Fig. 1), which is in agreement with previous reports on neonatal guinea pig ileum (Zhou et al., 2002). However, the contribution and function of each of these subunits in the enteric neurons is not clear. The amplitude of nicotine-induced currents measured from enteric neurons of α7, α6, α5, and β2 subunit knockout mice were not significantly different from control mice, suggesting that they are not the subunits mediating nicotine-induced currents in the enteric neurons. However, nicotine-induced currents from α5 knockout mice displayed a delayed desensitization pattern, suggesting that α5 also may be a potential subunit modifying the inactivation of the nicotine-induced currents. The current profile of α5 knockout enteric neurons is similar to that seen by Gerzanich et al. (1998) in α3 expressing oocytes. Although further studies are needed to examine α3 and β4 knockout mice, the main limitation is that α3-null mutant mice do not survive to adulthood (Xu et al., 1999). Mecamylamine, a nicotinic antagonist with a preferential selectivity for α3β4, completely abolished the nicotine-induced currents at a concentration of 10 µM. Similarly, hexamethonium (10 µM), a nonselective antagonist, significantly decreased the nicotine-induced currents. α-Conotoxin AuIB (3 µM), at a concentration highly specific for α3β4, also significantly reduced nicotine-induced currents (Harvey et al., 1997; Luo et al., 1998; Zhou et al., 2002). Collectively, these data suggest that α3 and β4 subunits mediate the nicotine-induced currents in enteric neurons. The α5 subunit may be coexpressed with this nicotinic subtype and modulate its desensitization properties.
The fecal pellet output assay performed using nicotine showed an increase in fecal pellet output with a dose of 0.175 mg kg−1. However, an inverted U–shaped response was seen with increasing doses of nicotine. The inverted U–shaped dose response to nicotine is typically also seen in many behavioral assays (Picciotto, 2003) that may involve the activation of other subunits. NS3861, a partial agonist with the highest affinity at α3β4 compared with other subtypes of nAChRs, displayed a dose-dependent enhancement in the number of fecal pellets expelled in mice receiving long-term, but not short-term, morphine treatment. However, at higher doses of NS3861, there was a decrease in the transit. This may be due to the desensitization of the receptor or off-target effects at α3β2 receptors. This suggests that the α3β4 subtype of nAChRs provides a potential target to reverse chronic morphine-induced constipation.
Long-term exposure to morphine leads to tolerance and dependence (North et al., 1978; Smith et al., 2014). To examine whether the enhanced response to nicotine is associated with morphine dependence in a single isolated cell, we tested the nicotine-induced currents after prolonged exposure to morphine in enteric neurons from the colon. We have previously shown that overnight exposure to morphine induces dependence in enteric neurons in the ileum, but not in the colon (Smith et al., 2014). Enhanced responses to nicotine were seen in cells isolated from the colon after long-term morphine treatment (Fig. 9). This suggested that the enhanced response to nicotine may not be associated with dependence.
An increase in the nicotine-induced currents may be due to an increase in the receptor number or a post-translational modification leading to changes in biophysical properties of the receptor. An increase in the receptor number can be mediated through an increase in the transcription of the receptor gene or alteration in membrane trafficking of the receptor. mRNA levels of α3β4 were not significantly changed in the LMMP of ileum from morphine-pelleted mice (for 4 days), suggesting that the change in the transcription of α3β4 is not the cause behind the enhanced response to nicotine. However, it is possible that protein expression is altered. Further studies will be required to determine the receptor protein expression of these subunits after long-term morphine treatment. Previous reports have shown that nAChR activity may be enhanced by phosphorylating or altering membrane trafficking (Walsh et al., 2008; Govind et al., 2009, 2012; Wecker et al., 2010). Govind et al. (2012) demonstrated that nicotine-induced upregulation of α4β2 nAChR occurs by multiple processes, which include a fast transient component and a slower component that involve the inhibition of proteosomal degradation of the β2 subunit resulting in increased nAChR assembly. It remains to be established whether similar processes occurs due to long-term morphine exposure in enteric neurons. Increased nAChR numbers have been reported in the brains of morphine-dependent mice (Neugebauer et al., 2013).
Since constipation persists with long-term morphine treatment due to the lack of tolerance development in the colon (Ross et al., 2008), our present findings suggest that identifying α3β4 nAChR agonists with peripheral selectivity may be a useful strategy in the treatment of opioid-induced constipation in patients who have received long-term opioid treatment.
Supplementary Material
Abbreviations
- ANOVA
analysis of variance
- ENS
enteric nervous system
- GI
gastrointestinal
- LMMP
longitudinal muscle myenteric plexus
- nAChR
nicotinic acetylcholine receptor
- PCR
polymerase chain reaction
Authorship Contributions:
Participated in research design: Gade, Kang, Damaj, Dewey, and Akbarali .
Conducted experiments: Gade, Khan, and Kang.
Contributed transgenic mice: Damaj.
Performed data analysis: Gade, Khan, Kang, and Akbarali
Wrote or contributed to the writing of the manuscript: Gade, Grider, Damaj, Dewey, and Akbarali.
Footnotes
This research was supported by National Institutes of Health grants R01-DA-024009, R01-DA-036975, and P30-DA-033934.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Bliss CI. (1967) Statistics in Biology: Statistical Methods for Research in the Natural Sciences, McGraw-Hill, New York. [Google Scholar]
- Galligan JJ. (2002) Pharmacology of synaptic transmission in the enteric nervous system. Curr Opin Pharmacol 2:623–629. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, LePard KJ, Schneider DA, Zhou X. (2000) Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst 81:97–103. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J. (1998) alpha 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J Pharmacol Exp Ther 286:311–320. [PubMed] [Google Scholar]
- Goldstein A, Schulz R. (1973) Morphine-tolerant longitudinal muscle strip from guinea-pig ileum. Br J Pharmacol 48:655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind AP, Vezina P, Green WN. (2009) Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol 78:756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind AP, Walsh H, Green WN. (2012) Nicotine-induced upregulation of native neuronal nicotinic receptors is caused by multiple mechanisms. J Neurosci 32:2227–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpsøe K, Hald H, Timmermann DB, Jensen ML, Dyhring T, Nielsen EO, Peters D, Balle T, Gajhede M, Kastrup JS, et al. (2013) Molecular determinants of subtype-selective efficacies of cytisine and the novel compound NS3861 at heteromeric nicotinic acetylcholine receptors. J Biol Chem 288:2559–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, McIntosh JM, Cartier GE, Maddox FN, Luetje CW. (1997) Determinants of specificity for alpha-conotoxin MII on alpha3beta2 neuronal nicotinic receptors. Mol Pharmacol 51:336–342. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Westfall DP, Howard SA, Fleming WW. (1978) Sensitivities of the isolated ileal longitudinal smooth muscle-myenteric plexus and hypogastric nerve-vas deferens of the guinea pig after chronic morphine pellet implantation. J Pharmacol Exp Ther 204:54–66. [PubMed] [Google Scholar]
- Kong JQ, Leedham JA, Taylor DA, Fleming WW. (1997) Evidence that tolerance and dependence of guinea pig myenteric neurons to opioids is a function of altered electrogenic sodium-potassium pumping. J Pharmacol Exp Ther 280:593–599. [PubMed] [Google Scholar]
- Leedham JA, Kong JQ, Taylor DA, Johnson SM, Fleming WW. (1992) Membrane potential in myenteric neurons associated with tolerance and dependence to morphine. J Pharmacol Exp Ther 263:15–19. [PubMed] [Google Scholar]
- Luo S, Kulak JM, Cartier GE, Jacobsen RB, Yoshikami D, Olivera BM, McIntosh JM. (1998) alpha-conotoxin AuIB selectively blocks alpha3 beta4 nicotinic acetylcholine receptors and nicotine-evoked norepinephrine release. J Neurosci 18:8571–8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, et al. (2007) Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 190:269–319. [DOI] [PubMed] [Google Scholar]
- Meeusen JW, Haselkorn KE, Fryer JP, Kryzer TJ, Gibbons SJ, Xiao Y, Lennon VA. (2013) Gastrointestinal hypomotility with loss of enteric nicotinic acetylcholine receptors: active immunization model in mice. Neurogastroenterol Motil 25:84–88.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon PP, Jackson KJ, Perez E, Harenza JL, Molas S, Rais B, Anwar H, Zaveri NT, Maldonado R, Maskos U, et al. (2014) The α3β4* nicotinic ACh receptor subtype mediates physical dependence to morphine: mouse and human studies. Br J Pharmacol 171:3845–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer NM, Einstein EB, Lopez MB, McClure-Begley TD, Mineur YS, Picciotto MR. (2013) Morphine dependence and withdrawal induced changes in cholinergic signaling. Pharmacol Biochem Behav 109:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Karras PJ. (1978) Opiate tolerance and dependence induced in vitro in single myenteric neurones. Nature 272:73–75. [DOI] [PubMed] [Google Scholar]
- North RA, Williams JT. (1977) Extracellular recording from the guinea-pig myenteric plexus and the action of morphine. Eur J Pharmacol 45:23–33. [DOI] [PubMed] [Google Scholar]
- Papke RL, Wecker L, Stitzel JA. (2010) Activation and inhibition of mouse muscle and neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther 333:501–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton WD. (1957) The action of morphine and related substances on contraction and on acetylcholine output of coaxially stimulated guinea-pig ileum. Br Pharmacol Chemother 12:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR. (2003) Nicotine as a modulator of behavior: beyond the inverted U. Trends Pharmacol Sci 24:493–499. [DOI] [PubMed] [Google Scholar]
- Ross GR, Gabra BH, Dewey WL, Akbarali HI. (2008) Morphine tolerance in the mouse ileum and colon. J Pharmacol Exp Ther 327:561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TH, Grider JR, Dewey WL, Akbarali HI. (2012) Morphine decreases enteric neuron excitability via inhibition of sodium channels. PLoS One 7:e45251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TH, Ngwainmbi J, Grider JR, Dewey WL, Akbarali HI. (2013) An in-vitro preparation of isolated enteric neurons and glia from the myenteric plexus of the adult mouse. J Vis Exp ▾▵: 50688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TH, Ngwainmbi J, Hashimoto A, Dewey WL, Akbarali HI. (2014) Morphine dependence in single enteric neurons from the mouse colon requires deletion of β-arrestin2. Physiol Rep 2:e12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh H, Govind AP, Mastro R, Hoda JC, Bertrand D, Vallejo Y, Green WN. (2008) Up-regulation of nicotinic receptors by nicotine varies with receptor subtype. J Biol Chem 283:6022–6032. [DOI] [PubMed] [Google Scholar]
- Wecker L, Pollock VV, Pacheco MA, Pastoor T. (2010) Nicotine-induced up regulation of α4β2 neuronal nicotinic receptors is mediated by the protein kinase C-dependent phosphorylation of α4 subunits. Neuroscience 171:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JD, Galligan JJ. (2004) Function of opioids in the enteric nervous system. Neurogastroenterol Motil 16:17–28. [DOI] [PubMed] [Google Scholar]
- Xu W, Gelber S, Orr-Urtreger A, Armstrong D, Lewis RA, Ou CN, Patrick J, Role L, De Biasi M, Beaudet AL. (1999) Megacystis, mydriasis, and ion channel defect in mice lacking the alpha3 neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A 96:5746–5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Ren J, Brown E, Schneider D, Caraballo-Lopez Y, Galligan JJ. (2002) Pharmacological properties of nicotinic acetylcholine receptors expressed by guinea pig small intestinal myenteric neurons. J Pharmacol Exp Ther 302:889–897. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.