Abstract
Despite their efficacy, uptake of selective estrogen receptor modulators for breast cancer chemoprevention remains low. Exemestane, an aromatase inhibitor, has recently been identified as a potential chemopreventive option with fewer serious side effects compared with selective estrogen receptor modulators in postmenopausal women. The purpose of this study was to assess the uptake of exemestane in a breast cancer prevention clinic. A retrospective chart review was conducted to capture chemoprevention uptake by postmenopausal women presenting to the Yale Breast Cancer Prevention Clinic between November 2011 and November 2012. Descriptive statistics of the study population have been presented. Statistical analyses were carried out using SAS 9.3 (SAS Institute Inc., Cary, North Carolina, USA) between December 2012 and February 2013. Of 90 postmenopausal women, 56 were eligible for chemoprevention. Their mean age was 56.8 years. Among the women, 39% had osteopenia or osteoporosis. Thirteen women chose to start chemoprevention medication (23%). Although 31% of the chemopreventive medication administered included exemestane, only four of 56 postmenopausal women opted for exemestane (7%). Chemoprevention uptake rates of postmenopausal women in the setting of a breast cancer prevention clinic are higher than that reported in the general population; however, they remain low overall despite the inclusion of exemestane as an option. A significant proportion of postmenopausal women have decreased bone density, which is a potential barrier to exemestane uptake. The results provide practical implications suggesting that exemestane may have limited impact on breast cancer chemoprevention uptake. Further investigations should focus on understanding the factors that influence, predict, and increase chemoprevention uptake.
Keywords: aromatase inhibitors, breast cancer chemoprevention, chemoprevention uptake, exemestane, postmenopausal women
Introduction
Despite stabilization of breast cancer incidence rates and declining mortality in recent years, primary prevention of this disease remains a major public health issue, given that over 230 000 women are diagnosed with invasive breast cancer each year in the USA alone (Smigal et al., 2006; Reimers and Crew, 2012; Youlden et al., 2012). Approximately 75% of breast cancer patients are estrogen receptor (ER) positive, and this percentage increases with age (Anderson et al., 2011). Although ER-positive tumors have a better prognosis than ER-negative tumors, ER-positive tumors are responsible for most breast cancer deaths owing to their higher prevalence and therefore comprise an important focus for prevention efforts (Decensi et al., 2012).
The selective estrogen receptor modulators (SERMs) tamoxifen and raloxifene were the first drugs to gain recognition as effective agents for reducing the risk for ER-positive breast cancer in women at increased risk (Fisher et al., 1998; Cuzick et al., 2002, 2003; Powles et al., 2007). Several organizations including the US Preventative Task Force, the American Society of Clinical Oncology, and the National Comprehensive Cancer Network recommend breast cancer chemoprevention for individuals at increased risk (Bevers et al., 2010; Moyer and Force USPST, 2013; Visvanathan et al., 2013; http://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf). However, although SERMs have been shown to reduce breast cancer incidence by up to 50% in clinical trials (Fisher et al., 1998; Vogel et al., 2006), this impressive result has not translated well into clinical practice. The uptake of SERM chemoprevention among healthy US women of ages between 40 and 79 years has remained low in both the general (1%) and the high-risk populations (5%; Visvanathan et al., 2009; Ropka et al., 2010; Waters et al., 2010). One reason commonly cited for poor chemoprevention uptake is patient concern over potential side effects, including increased risk of vasomotor symptoms, endometrial cancer, thromboembolic events, and cataracts (Cummings et al., 1999; Martino et al., 2004; Fisher et al., 2005; Vogel et al., 2010). These data clearly indicate the need for alternative agents with less toxicity if breast cancer chemoprevention is to be successfully integrated into practice (Gail, 2011).
Aromatase inhibitors (AIs) have been proposed as safer alternatives with potentially better acceptance as chemopreventive agents for ER-positive breast cancer. AIs have long been recognized to reduce contralateral primary breast cancers at least as efficiently as tamoxifen therapy in postmenopausal women who received these drugs as adjuvant therapy for invasive cancer (Fisher et al., 1997; Cuzick et al., 2003; Atalay et al., 2004; Chow et al., 2008; Ellis et al., 2011; Goss et al., 2011). This observation, in addition to the expectation of a more favorable side-effect profile, stimulated interest in evaluating exemestane and anastrozole in chemoprevention trials (Johannessen et al., 1997; Goss et al., 2004, 2007, 2011; Cuzick, 2008). Published in 2010, the MAP.3 (Mammary Prevention 3) trial was the first double-blind, randomized, phase III trial demonstrating the use of an AI in a prevention setting (Moy et al., 2007). In this trial, 25 mg exemestane administered daily for 5 years compared with placebo reduced the risk of invasive breast cancer (primarily ER-positive breast cancer) by 73% in postmenopausal women at increased risk (Goss et al., 2011; Visvanathan et al., 2013). The most commonly observed side effects included vasomotor symptoms, arthralgia, and vaginal dryness (Moy et al., 2007). In addition, chemoprevention of breast cancer with exemestane in postmenopausal women worsened the age-related decrease in bone mineral density by approximately three times compared with placebo, despite adequate calcium and vitamin D supplementation (Cheung et al., 2012). Yet, the majority of women adhered to therapy (∼85%; Moy et al., 2007). Similar results were seen in the recently published IBIS-II (International Breast Cancer Intervention Study II) double-blind, randomized phase III trial that compared 1 mg anastrozole daily for 5 years with placebo in postmenopausal women at increased risk for breast cancer. Anastrozole reduced the risk for ER-positive breast cancers by 50%, with the most commonly reported side effects being an increase in musculoskeletal adverse events and vasomotor symptoms, with a confirmed increase in the frequency of hypertension, vaginal dryness, and dry eye. Full 5-year adherence to therapy was 70% in the anastrozole group (Cuzick, 2008).
These data support the efficacy of AIs as chemopreventive agents and indicate an acceptable safety profile; however, whether the acceptance of these agents will be better than that of SERMs remains to be seen. Although AIs have fewer serious side effects such as thrombosis or secondary cancers compared with SERMs, they have significant rates of vasomotor symptoms and arthralgia and may also contribute to osteoporosis (Crew et al., 2007; Walker et al., 2013). Uptake of AIs as chemopreventive agents in the clinic has not been reported to date. The goal of this study was to determine the overall uptake of exemestane among postmenopausal women at increased risk for breast cancer presenting to a breast cancer prevention clinic.
Methods
A retrospective chart review was conducted to capture patient characteristics, medical history, and chemoprevention uptake of all postmenopausal women presenting to the Yale Breast Cancer Prevention Center (YBCPC) between November 2011 and November 2012. Postmenopausal status was defined as follows: (i) self-reported natural menopause (12 months of amenorrhea in the absence of other biological or physiological causes) or (ii) self-reported surgical menopause (bilateral oophorectomy). The study protocol was approved by the Institutional Review Board at Yale University and a waiver was obtained for informed consent.
Study population
All women presenting to YBCPC were evaluated clinically and counseled about chemoprevention with either an SERM or exemestane by a single provider, trained as a breast medical oncologist (E.H.). Counseling with regard to chemopreventive options included evaluation of overall health, breast cancer risk, and bone density, and discussion of the benefits and potential risks of each treatment on the basis of available clinical chemoprevention guidelines (Bevers et al., 2010; Moyer and Force USPST, 2013; Visvanathan et al., 2013; http://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf). Breast cancer risk was assessed using the available literature and/or by risk modeling with the National Cancer Institute’s Breast Cancer Risk Assessment Tool (BCRAT; NCI). Risk assessment also included the use of the SERM risk/benefit indices published by Freedman et al. (2011) where applicable. Postmenopausal women deemed eligible for chemoprevention were those whose benefit from chemoprevention outweighed its known risks, those without prior or current SERM use, and those without any medical contraindications to chemoprevention. Women with known osteopenia or osteoporosis were considered eligible for chemoprevention but were offered an SERM rather than exemestane.
Data collected for each patient included age, race, personal and family history of cancer, the presence of a known genetic mutation, history of precancerous lesions (e.g. atypia), history of breast biopsy, age of menarche, age at parity, BCRAT/Gail model score, hysterectomy status, medication history, smoking history, alcohol history, breast density, bone mineral density, and the presence of vasomotor symptoms.
Statistical analyses
The primary objective of this study was to compare the overall chemoprevention uptake of tamoxifen, raloxifene, and exemestane among a group of postmenopausal women presenting to a breast cancer prevention clinic. Basic descriptive statistics were applied to characterize the study population and uptake rates. Statistical analyses were carried out using SAS version 9.3 (SAS Institute Inc.) between December 2012 and February 2013.
Results
Study characteristics
A total of 215 women were seen at YBCPC in the defined time frame. Ninety women were postmenopausal and therefore selected for a detailed chart review.
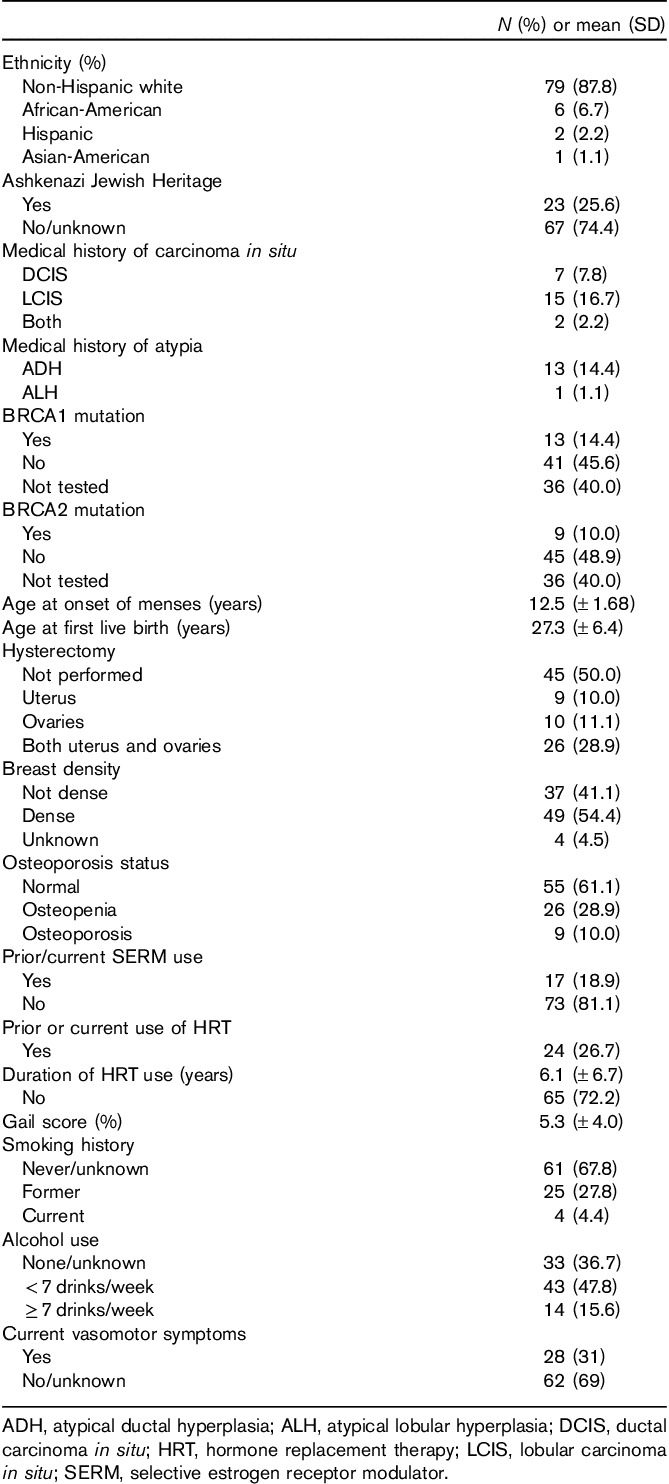
Characteristics of the study population are provided in Table 1. The mean age of the postmenopausal women presenting for evaluation was 56.8 years (range 41–79 years). The majority of women were non-Hispanic whites (87.8%), whereas African-Americans (6.7%), Hispanics (2.2%), and Asian-Americans (1.1%) constituted a minority. Categories of breast cancer risk included one or more of the following: breast atypia (15.5%), lobular carcinoma in situ (16.7%), prior ductal carcinoma in situ (7.8%), family history of breast cancer (in first, second, and/or third degree relatives; 45%), and/or a known deleterious BRCA 1 or 2 mutation (24.4%). The mean calculated 5-year breast cancer risk, on the basis of the BCRAT/Gail model, where applicable, was 5.3±4.0%. Approximately 38.9% of women had undergone a hysterectomy, and 54.4% had documented heterogeneous or extremely dense breast tissue. Thirty-one percent reported ongoing vasomotor symptoms at baseline, and 39% reported a known history of osteopenia or osteoporosis.
Table 1.
Summary of postmenopausal women attending high-risk breast clinic (n=90)
Upon clinical assessment, 34 of the 90 postmenopausal women were determined not to be candidates for chemoprevention because of significant medical comorbidities (e.g. active cancer diagnosis, n=19), prior and/or current use of SERMs (n=11), current hormone replacement therapy use (n=2), and advanced age (age>75 years, n=2), leaving 56 women for analysis of chemoprevention uptake.
Thirty-four women (61%) of the 56 eligible candidates for chemoprevention had a normal or unknown bone mineral density, whereas 22 (39%) of them had documented osteopenia and/or osteoporosis (Table 2).
Table 2.
Bone density status of eligible women (n=56)

Decisions about exemestane treatment
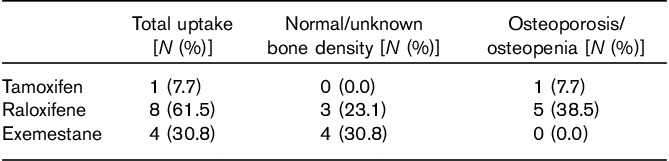
Of the 56 postmenopausal women eligible for chemoprevention, 13 (23%) opted to start administering chemopreventive medication (Fig. 1). Of these, seven had normal bone density, whereas six had either osteopenia or osteoporosis. Eight women accepted raloxifene, one accepted tamoxifen, and four accepted exemestane (Table 3). Although 31% of the women who accepted chemoprevention uptake opted for exemestane, only four (7%) of the 56 postmenopausal women eligible for breast cancer chemoprevention were ultimately started on exemestane in a clinical setting. The reasons commonly mentioned by patients to their provider (E.H.) for deciding against chemoprevention were primarily related to concerns over potential side effects such as development and/or worsening of vasomotor symptoms, worsening of baseline arthritic discomfort, secondary cancer risks, thrombosis risks, and/or potential worsening of bone density.
Fig. 1.
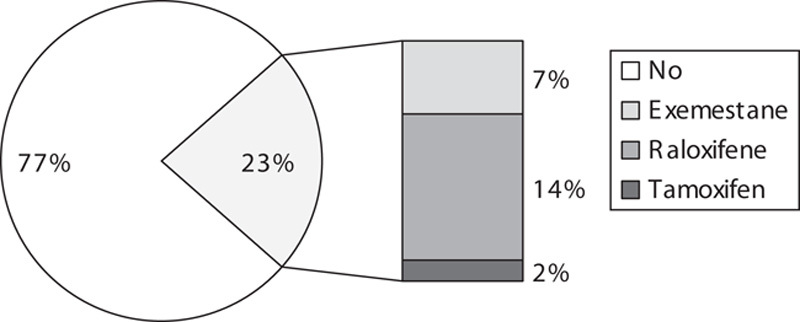
Chemoprevention uptake among postmenopausal women at Yale Breast Cancer Prevention Clinic.
Table 3.
Selection of chemopreventive drug type among those patients opting for chemoprevention (n=13)
Discussion
To our knowledge, this is the first report that examines the application of exemestane as a chemopreventive agent in a breast cancer prevention clinic. Although the overall prevention uptake rate was found to be 23% and is higher than the generally reported prevention uptake rate in the community, it still remains low. Among those who were eligible for chemoprevention, only 7% started on exemestane. Our findings suggest that AIs will have limited impact on the prevention of breast cancer, despite their impressive efficacy results from clinical trials.
We identified three potential barriers to the acceptance of AI chemoprevention in clinical practice. First, a large proportion (39%) of postmenopausal women otherwise eligible for chemoprevention had documented bone loss on bone density measurements. While the MAP.3 and IBIS-II trials included women with bone mineral density of up to 2.0, many practicing clinicians may be reluctant to offer exemestane solely for breast cancer prevention purposes in this setting, and many patients may be reluctant to take on this risk. While these women may be the ideal candidates for an SERM, as they have a favorable effect on bone density, with studies demonstrating up to a 32% reduction in fracture incidence, other potentially frightening side effects (e.g. thromboembolic complications, endometrial cancer) limit their acceptance (Anon, 1998; Fisher et al., 1998; Smigal et al., 2006). Although exemestane may have less effect on bone density compared with other AIs, 2 years of treatment with exemestane in the MAP.3 trial was found to be associated with a three-fold worsening of bone density loss in postmenopausal women compared with placebo, despite calcium and vitamin D supplementation (Cheung et al., 2012). With nearly 50 000 deaths each year ultimately attributed to hip fractures (Deprey, 2009; Stevens and Rudd, 2013; http://www.cdc.gov/homeandrecreationalsafety/falls/adulthipfx.html), osteoporosis represents a major competing threat to health in postmenopausal women and cannot be ignored when considering chemoprevention options, including AIs. Given that osteopenia is not an absolute contraindication to use of an AI in the prevention setting, how an individual provider frames these competing risks to the patient will certainly influence AI uptake rates.
A second major barrier to AI uptake in the clinical prevention setting is the potential for significant side effects, including vasomotor symptoms and arthralgia. Although most of the toxicity of exemestane in the MAP.3 trial was reported as grade 2 or less, symptoms including hot flashes, musculoskeletal arthritis, and joint pain had mean grade 3 toxicity scores, which may impair the uptake of and adherence to long-term preventive use of exemestane by healthy women (Decensi et al., 2012). Interestingly, overall health-related quality of life with exemestane did not show a significant difference as compared with the control arm (Decensi et al., 2012). IBIS-II showed similar results, with significant musculoskeletal and vasomotor symptoms with anastrozole but 75% adherence at 3 years (Cuzick, 2008; Ropka et al., 2010). Yet, the clinical experience with poor SERM chemoprevention uptake demonstrates that, despite encouraging clinical trial results showing no significant impact on the quality of life, women are reluctant to administer medication for breast cancer prevention that carries the potential for side effects (Ropka et al., 2010). In addition, the absence of experience and hesitation in controlling the side effects of available SERM chemopreventive agents among internists, gynecologists, and family medicine practitioners have been observed in clinical trials (Rondanina et al., 2008), and often, it is the strength of the physician’s recommendation that appears to influence uptake in the high-risk population (McKay et al., 2005).
A third barrier to AI breast cancer chemoprevention uptake is the inherent difficulty in communicating accurate risk/benefit profiles to women at increased risk of breast cancer. For a patient and provider to decide to pursue any chemoprevention recommendation, the woman’s risk of breast cancer must first be assessed and then weighed against the potential risks and benefits of chemoprevention. Although two models for breast cancer risk assessment, namely, the BCRAT/Gail Model and the IBIS model, are free and available online, only 18% of primary care physicians use them to calculate breast cancer risk (Guerra et al., 2009). Lack of confidence in primary prevention of breast cancer and time restriction during clinical visits have been cited as potential reasons for the software not being frequently utilized (Sabatino et al., 2007). There are no similar tools to personalize predictions of risks for side effects beyond the SERM Benefit/Risk Indices published in 2011 (Freedman et al., 2011). However, even if an accurate risk/benefit assessment is obtained, explanation of absolute versus relative risk reduction with the use of chemoprevention, in comparison with side effects and risks from the medications, can be time-consuming and challenging. Unfortunately, decision aids and reading material have had limited success in increasing SERM chemoprevention uptake, ranging from 0.5 to 5.6% (Port et al., 2001; Taylor and Taguchi, 2005; Fagerlin et al., 2010, 2011; Loehberg et al., 2010). It seems unlikely that the introduction of AIs will simplify risk assessment and decision making.
For breast cancer chemoprevention to succeed, the significant clinical trial results seen in P-1, STAR, MAP.3, and IBIS-II are not enough. Indeed, two approaches must be considered to move the breast cancer prevention field forward. One approach could be to focus efforts on identifying new agents with even more favorable toxicity profiles; even if such agents had lesser efficacy, their overall impact on breast cancer incidence rates would be large if they were broadly used. Alternatively, a similar large impact on breast cancer incidence would be seen if we are able to accurately identify the smaller population of women at highest risk for breast cancer, among whom the risk/benefit profiles are the most favorable. Ultimately, for SERMs and AIs to play a major role in breast cancer prevention, the latter approach must be pursued, with attention focused on improving risk modeling and identifying accurate and reliable biomarkers of breast cancer risk.
We believe our study to have several strengths. All patients were seen in a clinic setting specifically dedicated to breast cancer prevention. Breast cancer risk was universally defined by available risk modeling when appropriate, and patients were seen by a single provider, ensuring uniformity of approach. Limitations of the study include the relatively small sample size, the retrospective nature of the study, and the inability to determine details of patient decision making around AI and chemoprevention uptake from chart review. The fact that patients were seen by a single provider at a single site could also introduce bias; however, if present, it would potentially bias results toward chemoprevention uptake in general.
In conclusion, the uptake of the AI exemestane into the clinical breast cancer prevention setting was found to be low, with only 7% of eligible postmenopausal women pursuing exemestane treatment. A significant proportion of postmenopausal women at increased risk for breast cancer have decreased bone density, which appears to potentially limit the population in which AI could otherwise be utilized. Further research must be undertaken for AIs to successfully impact breast cancer incidence rates, with efforts focused on accurate identification of those women at highest risk for breast cancer.
Acknowledgements
The authors thank all the participants presenting to the Yale Breast Cancer Prevention Center (YBCPC) between November 2011 and November 2012.
Conflicts of interest
There are no conflicts of interest.
References
- [No authors listed] (1998). Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 351:1451–1467. [PubMed] [Google Scholar]
- Anderson WF, Katki HA, Rosenberg PS. (2011). Incidence of breast cancer in the United States: current and future trends. J Natl Cancer Inst 103:1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atalay G, Dirix L, Biganzoli L, Beex L, Nooij M, Cameron D, et al. (2004). The effect of exemestane on serum lipid profile in postmenopausal women with metastatic breast cancer: a companion study to EORTC Trial 10951, ’Randomized phase II study in first line hormonal treatment for metastatic breast cancer with exemestane or tamoxifen in postmenopausal patients’. Ann Oncol 15:211–217. [DOI] [PubMed] [Google Scholar]
- Bevers TB, Armstrong DK, Arun B, Carlson RW, Cowan KH, Daly MB, et al. (2010). Breast cancer risk reduction. J Natl Comp Canc Netw 8:1112–1146. [DOI] [PubMed] [Google Scholar]
- Cheung AM, Tile L, Cardew S, Pruthi S, Robbins J, Tomlinson G, et al. (2012). Bone density and structure in healthy postmenopausal women treated with exemestane for the primary prevention of breast cancer: a nested substudy of the MAP.3 randomised controlled trial. Lancet Oncol 13:275–284. [DOI] [PubMed] [Google Scholar]
- Chow LW, Yip AY, Loo WT, Lam CK, Toi M. (2008). Celecoxib anti-aromatase neoadjuvant (CAAN) trial for locally advanced breast cancer. J Steroid Biochem Mol Biol 111:13–17. [DOI] [PubMed] [Google Scholar]
- Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, et al. (2007). Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol 25:3877–3883. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, et al. (1999). The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 281:2189–2197. [DOI] [PubMed] [Google Scholar]
- Cuzick J. (2008). IBIS II: a breast cancer prevention trial in postmenopausal women using the aromatase inhibitor anastrozole. Expert Rev Anticancer Ther 8:1377–1385. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Forbes J, Edwards R, Baum M, Cawthorn S, Coates A, et al. , IBIS investigators (2002). First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet 360:817–824. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Powles T, Veronesi U, Forbes J, Edwards R, Ashley S, Boyle P. (2003). Overview of the main outcomes in breast-cancer prevention trials. Lancet 361:296–300. [DOI] [PubMed] [Google Scholar]
- Decensi A, Dunn BK, Puntoni M, Gennari A, Ford LG. (2012). Exemestane for breast cancer prevention: a critical shift? Cancer Discov 2:25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprey SM. (2009). Descriptive analysis of fatal falls of older adults in a Midwestern county in the year 2005. J Geriatr Phys Ther 32:67–72. [PubMed] [Google Scholar]
- Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, et al. (2011). Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype – ACOSOG Z1031. J Clin Oncol 29:2342–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerlin A, Zikmund-Fisher BJ, Nair V, Derry HA, McClure JB, Greene S, et al. (2010). Women’s decisions regarding tamoxifen for breast cancer prevention: responses to a tailored decision aid. Breast Cancer Res Treat 119:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerlin A, Dillard AJ, Smith DM, Zikmund-Fisher BJ, Pitsch R, McClure JB, et al. (2011). Women’s interest in taking tamoxifen and raloxifene for breast cancer prevention: response to a tailored decision aid. Breast Cancer Res Treat 127:681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. (1997). Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 15:2483–2493. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. (1998). Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90:1371–1388. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. (2005). Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 97:1652–1662. [DOI] [PubMed] [Google Scholar]
- Freedman AN, Yu B, Gail MH, Costantino JP, Graubard BI, Vogel VG, et al. (2011). Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clinical Oncol 29:2327–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gail MH. (2011). Personalized estimates of breast cancer risk in clinical practice and public health. Stat Med 30:1090–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss PE, Qi S, Josse RG, Pritzker KP, Mendes M, Hu H, et al. (2004). The steroidal aromatase inhibitor exemestane prevents bone loss in ovariectomized rats. Bone 34:384–392. [DOI] [PubMed] [Google Scholar]
- Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. (2011). Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 364:2381–2391. [DOI] [PubMed] [Google Scholar]
- Goss PE, Hadji P, Subar M, Abreu P, Thomsen T, Banke-Bochita J. (2007). Effects of steroidal and nonsteroidal aromatase inhibitors on markers of bone turnover in healthy postmenopausal women. Breast Cancer Res 9:R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra CE, Sherman M, Armstrong K. (2009). Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med 22:272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen DC, Engan T, Di Salle E, Zurlo MG, Paolini J, Ornati G, et al. (1997). Endocrine and clinical effects of exemestane (PNU 155971), a novel steroidal aromatase inhibitor, in postmenopausal breast cancer patients: a phase I study. Clin Cancer Res 3:1101–1108. [PubMed] [Google Scholar]
- Loehberg CR, Jud SM, Haeberle L, Heusinger K, Dilbat G, Hein A, et al. (2010). Breast cancer risk assessment in a mammography screening program and participation in the IBIS-II chemoprevention trial. Breast Cancer Res Treat 121:101–110. [DOI] [PubMed] [Google Scholar]
- Martino S, Cauley JA, Barrett-Connor E, Powles TJ, Mershon J, Disch D, et al. (2004). Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst 96:1751–1761. [DOI] [PubMed] [Google Scholar]
- McKay A, Latosinsky S, Martin W. (2005). Acceptance of tamoxifen chemoprevention by physicians and women at risk. Cancer 103:209–210. [DOI] [PubMed] [Google Scholar]
- Moy B, Richardson H, Johnston D, Pater JL, Chlebowski R, Alés-Martinez JE, et al. (2007). NCIC CTG MAP.3: enrollment and study drug adherence of ethnic minority women in a breast cancer prevention trial. Breast Cancer Res Treat 106:141–142. [Google Scholar]
- Moyer VA, U.S. Preventive Services Task Force (2013). Medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 159:698–708. [DOI] [PubMed] [Google Scholar]
- NCI. Breast Cancer Risk Tool. Available at: http://www.cancer.gov/bcrisktool/. [Accessed on 20 July 2014].
- Port ER, Montgomery LL, Heerdt AS, Borgen PI. (2001). Patient reluctance toward tamoxifen use for breast cancer primary prevention. Ann Surg Oncol 8:580–585. [DOI] [PubMed] [Google Scholar]
- Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. (2007). Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst 99:283–290. [DOI] [PubMed] [Google Scholar]
- Reimers L, Crew KD. (2012). Tamoxifen vs. raloxifene vs. exemestane for chemoprevention. Curr Breast Cancer Rep 4:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondanina G, Puntoni M, Severi G, Varricchio C, Zunino A, Feroce I, et al. (2008). Psychological and clinical factors implicated in decision making about a trial of low-dose tamoxifen in hormone replacement therapy users. J Clin Oncol 26:1537–1543. [DOI] [PubMed] [Google Scholar]
- Ropka ME, Keim J, Philbrick JT. (2010). Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol 28:3090–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino SA, McCarthy EP, Phillips RS, Burns RB. (2007). Breast cancer risk assessment and management in primary care: provider attitudes, practices, and barriers. Cancer Detect Prev 31:375–383. [DOI] [PubMed] [Google Scholar]
- Smigal C, Jemal A, Ward E, Cokkinides V, Smith R, Howe HL, Thun M. (2006). Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin 56:168–183. [DOI] [PubMed] [Google Scholar]
- Stevens JA, Rudd RA. (2013). The impact of decreasing U.S. hip fracture rates on future hip fracture estimates. Osteoporos Int 24:2725–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R, Taguchi K. (2005). Tamoxifen for breast cancer chemoprevention: low uptake by high-risk women after evaluation of a breast lump. Ann Fam Med 3:242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvanathan K, Chlebowski RT, Hurley P, Col NF, Ropka M, Collyar D, et al. (2009). American Society of Clinical Oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol 27:3235–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvanathan K, Hurley P, Bantug E, Brown P, Col NF, Cuzick J, et al. (2013). Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 31:2942–2962. [DOI] [PubMed] [Google Scholar]
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. , National Surgical Adjuvant Breast and Bowel Project (NSABP) (2006). Effects of tamoxifen vs. raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295:2727–2741. [DOI] [PubMed] [Google Scholar]
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. , National Surgical Adjuvant Breast and Bowel Project (2010). Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila) 3:696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G, Xenophontos M, Chen L, Cheung K. (2013). Long-term efficacy and safety of exemestane in the treatment of breast cancer. Patient Prefer Adherence 7:245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EA, Cronin KA, Graubard BI, Han PK, Freedman AN. (2010). Prevalence of tamoxifen use for breast cancer chemoprevention among US women. Cancer Epidemiol Biomarkers Prev 19:443–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD. (2012). The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol 36:237–248. [DOI] [PubMed] [Google Scholar]


