Abstract
Calorie restriction or a low-carbohydrate diet (LCD) can increase life span in normal cells while inhibiting carcinogenesis. Various phytochemicals also have calorie restriction-mimetic anticancer properties. We investigated whether an isocaloric carbohydrate-restriction diet and AMP-activated protein kinase (AMPK)-activating phytochemicals induce synergic tumor suppression. We used a mixture of AMPK-activating phytochemical extracts including curcumin, quercetin, catechins, and resveratrol. Survival analysis was carried out in a B16F10 melanoma model fed a control diet (62.14% kcal carbohydrate, 24.65% kcal protein and 13.2% kcal fat), a control diet with multiple phytochemicals (MP), LCD (16.5, 55.2, and 28.3% kcal, respectively), LCD with multiple phytochemicals (LCDmp), a moderate-carbohydrate diet (MCD, 31.9, 62.4, and 5.7% kcal, respectively), or MCD with phytochemicals (MCDmp). Compared with the control group, MP, LCD, or MCD intervention did not produce survival benefit, but LCDmp (22.80±1.58 vs. 28.00±1.64 days, P=0.040) and MCDmp (23.80±1.08 vs. 30.13±2.29 days, P=0.008) increased the median survival time significantly. Suppression of the IGF-1R/PI3K/Akt/mTOR signaling, activation of the AMPK/SIRT1/LKB1pathway, and NF-κB suppression were the critical tumor-suppression mechanisms. In addition, SIRT1 suppressed proliferation of the B16F10 and A375SM cells under a low-glucose condition. Alterations in histone methylation within Pten and FoxO3a were observed after the MCDmp intervention. In the transgenic liver cancer model developed by hydrodynamic transfection of the HrasG12V and shp53, MCDmp and LCDmp interventions induced significant cancer-prevention effects. Microarray analysis showed that PPARα increased with decreased IL-6 and NF-κB within the hepatocytes after an MCDmp intervention. In conclusion, an isocaloric carbohydrate-restriction diet and natural AMPK-activating agents induce synergistic anticancer effects. SIRT1 acts as a tumor suppressor under a low-glucose condition.
Keywords: AMPK, carbohydrate restriction, chemoprevention, energy metabolism, liver cancer, melanoma, phytochemical, SIRT1
Introduction
Calorie restriction (CR), defined as 20–40% restriction of the daily energy intake, extends the longevity of normal cells, but suppresses tumor growth (Colman et al., 2009). Inhibition of the insulin-like growth factor-1 receptor (IGF-1R)/phosphatidylinositol-3-kinase (PI3K)/Akt and mitogen-activated protein kinase pathways have been proposed as underlying tumor-suppression mechanisms (Dunn et al., 1997; Mjiyad et al., 2011). In particular, activation of the AMP-activated protein kinase (AMPK) pathway plays a crucial role through increased cellular NAD+/NADH ratio, which in turn activates NAD+-dependent class III histone deacetylase, silent mating-type information regulation 2 homolog 1 (SIRT1), and liver kinase B1 (LKB1). This signaling pathway results in a closed-loop AMPK/SIRT1/LKB1 activation process with tumor growth suppression (Lan et al., 2008; Shackelford and Shaw, 2009; Ruderman et al., 2010).
However, CR has major limitations including weight loss, especially in patients with cancer in the advanced stage. Recently, an isocaloric carbohydrate-free ketogenic diet or a low-carbohydrate diet (LCD, carbohydrate intake <20% of the daily caloric intake) has been introduced as a supportive dietary intervention (Last and Wilson, 2006; Masko et al., 2010; Ho et al., 2011; Seyfried et al., 2012). These interventions might also produce metabolic disturbances such as ketoacidosis or even weight loss (Bravata et al., 2003). Alternatively, a synthetic antidiabetic AMPK agonist, metformin, has been proposed as a CR-mimetic anticancer agent (Bost et al., 2012).
Various plant-derived bioactive phytochemicals including curcumin, quercetin, (-)-epigallocatechin-3-gallate (EGCG) in green tea extract, and resveratrol are known to be CR-mimetic AMPK activators (Dasgupta and Milbrandt, 2007; Pan et al., 2008; Park et al., 2009; Jung et al., 2010) and SIRT1 activators as well (Chung et al., 2010). Nevertheless, the anticancer effects of a single phytochemical are minimal because of its poor bioavailability, poor intestinal absorption, and rapid metabolism despite strong anticancer activities in-vitro studies. A single agent is only effective at an extremely high-dose oral administration (Scott et al., 2009). In contrast, a mixture of bioactive compounds exerts biological effects at a much lower dose, with synergistic enhancement of each individual compound (Liu, 2004; Ghosh et al., 2009; Ricciardiello et al., 2011).
Previous studies have reported that metformin induced massive apoptosis of cancer cells with glucose restriction, whereas these anticancer effects were attenuated under a high-glucose condition. Synergic tumor growth suppression has also been observed in mice when metformin was used in combination with a glucose uptake inhibitor (Cheong et al., 2011; Menendez et al., 2012). These data suggest that energy restriction in combination with AMPK-activating agents could enhance tumor suppression synergistically.
In this study, we tested whether an isocaloric carbohydrate-restriction diet supplemented with natural AMPK-activating agents would extend the survival time in an animal model using C57BL6 mice bearing B16F10 melanoma. We also explored whether CR-induced SIRT1 activation is oncogenic or acts as a tumor suppressor as the role of SIRT1 in malignant tumors is still controversial (Liu et al., 2009; Herranz and Serrano, 2010; Roth and Chen, 2013). In addition, we evaluated the chemoprevention effects of our dietary modification in a transgenic liver cancer model.
Materials and methods
Dietary formulas
The Picolab Rodent Diet 5053 formula (LabDiet, Brentwood, Missouri, USA) consisting of 62.14% kcal carbohydrate, 24.65% kcal protein, and 13.2% kcal fat with 3.07 kcal/g of metabolizable fuel value was used as the control formula. We purchased the TestDiet 9G1X formula (TestDiet, Brentwood, Missouri, USA) consisting of 16.5% kcal carbohydrate, 55.2% kcal protein, and 28.3% fat with a 4.13 kcal/g fuel value for the LCD intervention. We used green tea, curcumin, quercetin, and resveratrol extracts for CR-mimetic AMPK-activating agents. Approximately 5 mg of each phytochemical extract/g was added to the control and to the LCD formula to prepare the multiple phytochemical (MP) and LCDmp formulas, respectively. We designed a moderate-carbohydrate diet (MCD, <50% of the daily carbohydrate intake) formula consisting of 31.9% kcal carbohydrate, 62.4% kcal protein, and 5.7% kcal fat with 3.22 kcal/g of fuel value, and prepared an MCDmp formula by adding phytochemical extracts.
Survival analyses in the malignant melanoma model
B16F10 melanoma cells (1×106 cells) were injected subcutaneously into the back of 6-week-old C57BL6 male mice (Orient Bio Inc., Seongnam, Korea) after preliminary feeding of each dietary formula ad libitum for 1 week. We divided the mice randomly into six groups: control, MP, LCD, LCDmp, MCD, or MCDmp formula (n=15/group).
We carried out survival analysis using the Kaplan–Meier method, and diet-induced tumor suppression mechanisms were investigated using tumor tissues removed from the control (n=6) and MCDmp (n=5) groups.
Evaluation of energy-dependent signaling pathways
Western-blot analyses were carried out using antibodies against proteins involved in energy-dependent signaling pathways.
Evaluation of the role of SIRT1 under a carbohydrate restriction condition
B16F10 mouse or human A375SM melanoma cells were treated with various concentrations of SIRT1 Activator 3 (SA3), which is an allosteric SIRT1 activator (Nayagam et al., 2006), under standard glucose (11 mmol/l) or low-glucose (5.5 mmol/l) conditions in RPMI-1640 medium. A viability assay was performed and we evaluated the protein expression of p-AMPK, SIRT1, acetyl-p53 (Lys 382), acetyl-NF-κB, and nuclear fraction of the NF-κB p65 subunit in the B16F10 melanoma cells cultured in a standard medium or a low-glucose medium (LGM).
ChIP-on-chip microarray
Chromatin immunoprecipitation (ChIP) assay was performed with the EpiQuik TM ChIP Kit (Epigentek, Brooklyn, New York, USA) using randomly selected melanoma tissue samples (n=3/group from the control and MCDmp groups). Chromatin preparation was performed using an anti-H3K4me3 antibody as H3 methylation precedes acetylation (Wang et al., 2001). An Agilent microarray chip containing 97 651 oligonucleotide probes (Agilent Technology, Palo Alto, California, USA) was used for ChIP-on-chip analysis.
cDNA microarray
Total RNA fraction was extracted from the melanoma tissues removed from the control (n=7) and MCDmp groups (n=6), and cDNA microarray was performed.
Confocal microscopy
Frozen tissue sections from the control and MCDmp groups were prepared using a cryostat (micron HM-525; Thermo Scientific Inc., Pittsburgh, Pennsylvania, USA) at a 6-µm thickness. Immunohistochemistry to evaluate cellular polarity and tyrosinase-related protein-1 (TYRP1) was performed.
Cancer-prevention effects in a transgenic liver cancer model
We evaluated the cancer-prevention effects of each dietary intervention in a transgenic liver cancer model produced by transfection of the HrasG12V and shp53 into C57BL6 male mice as described previously (Ju et al., 2013).
This study was carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols of animal experiments were approved by our Institutional Animal Care and Use Committee (IACUC) (Number: 09-199-1 and 2012-0116).
Statistical analysis
Kaplan–Meier survival data were evaluated using the log-rank (Mantel–Cox) test, and a P value less than 0.05 was considered to be statistically significant. The statistical analysis of gene expression on cDNA microarray was carried out at the threshold of P less than 0.05 with more than two fold-change of gene expression. The protein or mRNA expression levels were evaluated as the percentage of control signals (% control).
Detailed materials and methods are described in Supplemental digital content 1.
Results
Survival analyses in the B16F10 melanoma model
Supplementation of a mixture of natural AMPK activators under a normal control diet (MP intervention) did not increase the survival time (Fig. 1a), although the mean tumor volume was slightly smaller than that of the control group (Supplemental digital content 2). Smaller tumor volume with a slower tumor growth rate was also observed in both LCD and LCDmp groups (Supplemental digital content 3, P<0.05). The median survival time was significantly increased in the LCDmp (Fig. 1b) and the MCDmp groups (Fig. 1c, Supplemental digital content 4) compared with the control group. The MCD intervention did not produce significant survival benefit (Fig. 1c). 18F-FDG MicroPET imaging showed a smaller tumor size with lower intratumoral 18F-FDG uptake in the MCDmp group compared with the control group (Fig. 2a). Protein expression of the hexokinase type 2 (HK2) and pyruvate kinase type M2 (PKM2) within tumor tissues was decreased in the MCDmp group (Fig. 2b).
Fig. 1.
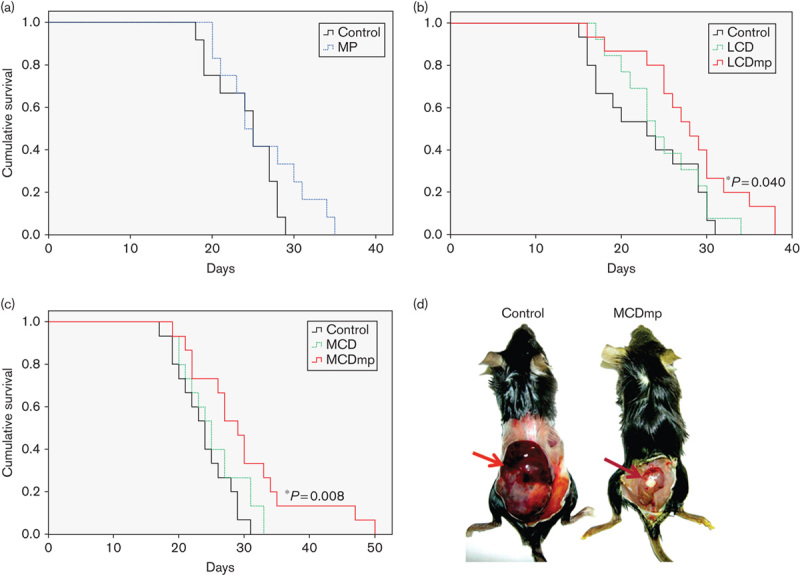
Dietary effects on survival. (a) Kaplan–Meier survival analysis showed that AMPK-activating phytochemicals alone do not produce survival benefit in a melanoma model. The median survival time was not significantly increased after the multiple phytochemical (MP) intervention. (b) Survival benefit was not observed after the low-carbohydrate diet (LCD) intervention either. However, LCD supplemented with a mixture of phytochemicals (LCDmp) significantly increased the median survival time. The median survival time of the control, LCD, and LCDmp groups was 22.80±1.58, 24.69±1.42, and 28.00±1.64 days after inoculation of melanoma cells, respectively. (c) The median survival time of the MCDmp group was also significantly increased compared with that of the MCD and control groups (30.13±2.29, 25.40±1.24, and 23.80±1.08 days, respectively). (d) Tumor size of the MCDmp group was smaller than that of the control group (arrow).
Fig. 2.
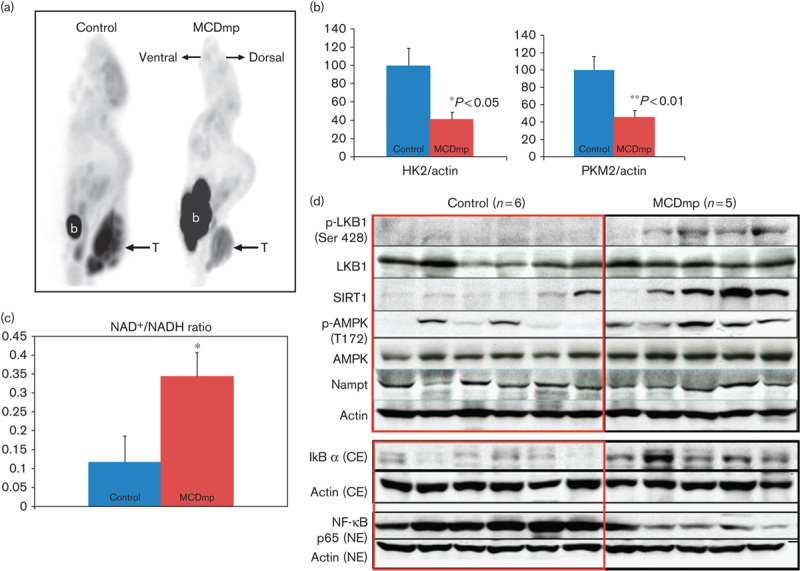
MCDmp-induced alterations in energy-dependent signaling pathways. (a) 18F-FDG PET scan shows decreased 18F-FDG uptake within tumors in the MCDmp group. T, tumor, b, urinary bladder. (b) The expression of the key enzymes for glycolysis (HK2 and PKM2) decreased significantly in the MCDmp group. (c) NAD+/NADH ratio was significantly increased (>3-fold) in melanoma tissues removed from the MCDmp group compared with the control group (*P=0.044, n=15 in each group). (d) Western-blot analysis showed activation of the LKB1/SIRT1/AMPK loop in the MCDmp group. There was no significant alteration in Nampt expression. Therefore, the NAD+/NADH ratio could be increased in the MCDmp group, independent of Nampt expression. In addition, the MCDmp intervention suppressed NF-κB p65 expression in the nuclear fraction of cells along with increased cytosolic IkB.
Alterations in energy-dependent signaling pathways
Serum glucose level was significantly lower in the MCDmp group (106.1±12.19 mg/dl) than in the control group (148±13.36 mg/dl) (P<0.04), as also the serum insulin level (0.10±0.02 and 0.18±0.02 ng/ml, respectively, P<0.02). Protein levels of the IGF-1R, PI3K, and p-Akt at Thr308, phospho-ribosomal protein S6 kinase (p-S6K) at Ser411, and the phospho-eukaryotic initiation factor 4E-binding protein 1 (p-4E-BP1) at Thr37/46 were decreased after the MCDmp intervention (Supplemental digital content 5).
In the MCDmp group, the NAD+/NADH ratio was increased (>3-fold difference) (Fig. 2c). The phosphorylated-liver kinase B1 at Ser428 (p-LKB1-Ser428), phosphorylated-AMPK at Thr172 (p-AMPK-Thr172), and SIRT1 were increased independent of nicotinamide phosphoribosyltransferase (NAMPT) expression. NF-κB in the nuclear fraction was decreased (Fig. 2d). On confocal microscopy of melanoma tissues removed from mice, peripheral tumor cells showed restoration of cell polarity (Supplemental digital content 6).
Effects of SIRT1 on tumor cell proliferation
In-vitro MTT analyses showed that SA3 treatment did not affect cellular proliferation of both B16F10 and human melanoma cells in a standard glucose medium, but decreased proliferation under a low-glucose condition along with increasing p-AMPK in a dose-dependent manner (Fig. 3a, Supplemental digital contents 7 and 8). The level of acetyl-p53 was not decreased in the LGM despite increased SIRT1 expression (Fig. 3b). The NF-κB p65 subunit level in the nucleus fraction was decreased in the LGM and further suppressed after SA3 treatment (Fig. 3b and c).
Fig. 3.
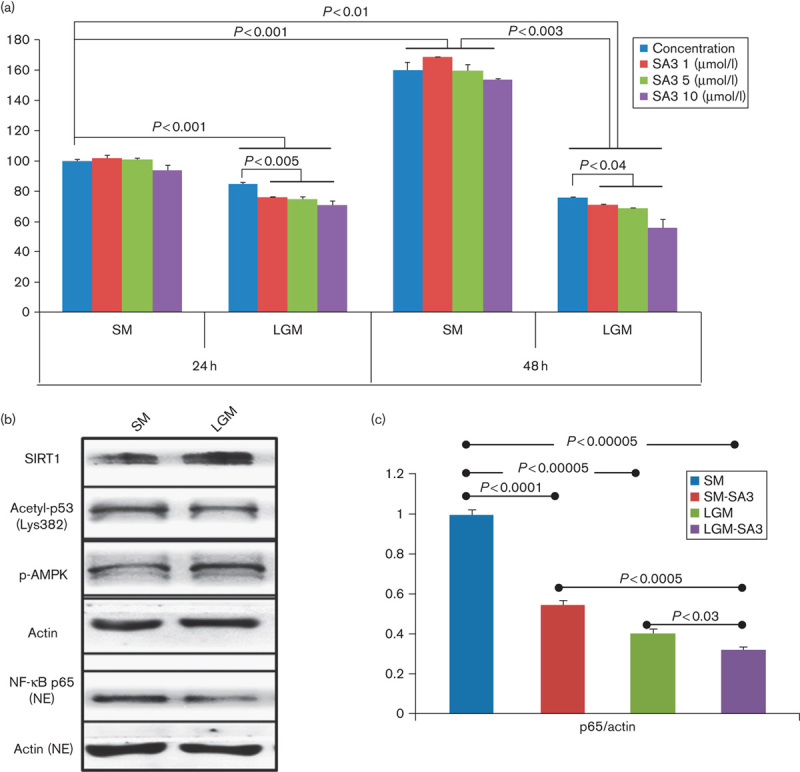
Tumor-suppressive role of SIRT1 under an energy restriction condition. (a) MTT assay of B16F10 melanoma cells cultured in a standard medium (SM, 11 mmol/l of glucose in RPMI-1640 medium) showed no significant cell proliferation at 24 or 48 h after treatment of SA3. However, cell proliferation was significantly suppressed after SA3 treatment when melanoma cells were culture in a low-glucose medium (LGM, 5.5 mmol/l of glucose). (b) SIRT1 and p-AMPK were increased in melanoma cells cultured in an LGM. However, the acetyl-p53 level was not significantly decreased despite an increased SIRT1 level. NF-κB p65 in the nuclear fraction was decreased under a low-glucose condition. (c) NF-κB level was decreased after SA3 treatment under both standard and low-glucose conditions.
Histone modification
The ChIP-on-chip study showed alterations in histone methylation (H3K4me3) in the MCDmp group. In particular, H3K4me3 was increased within the promoter region of the Pten and FoxO3a. ChIP-PCR analysis confirmed increased H3K4me3 within the Pten and FoxO3a promoter regions (Fig. 4a) and increased expression of these proteins as well as downstream Bim and BAX (Fig. 4b). PTEN, FOXO3a, and Bim were also increased in B16F10 melanoma cells cultured in a LGM (Fig. 4c).
Fig. 4.
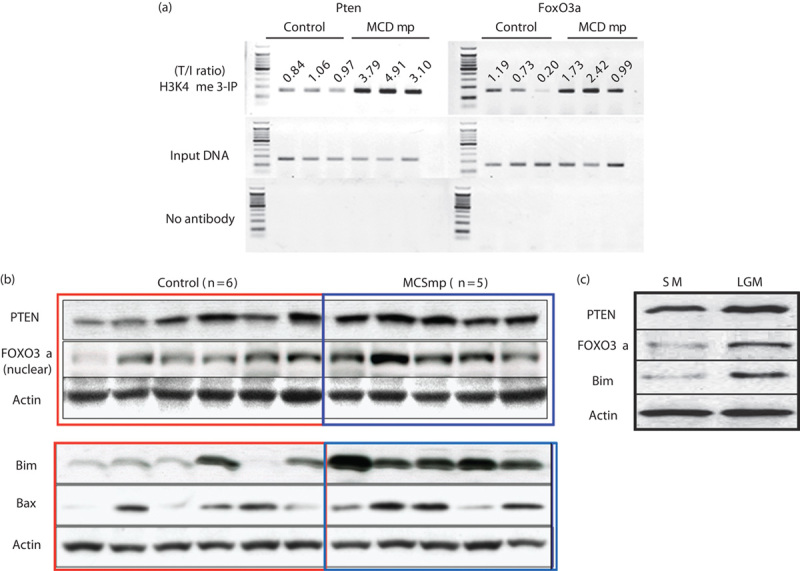
Epigenetic control of the MCDmp intervention. (a) ChIP-PCR assay of the Pten and FoxO3a using the anti-H3K4me3 antibody confirmed increased H3K4 methylation after the MCDmp intervention. The numbers indicate the target/input DNA ratios. (b) Western-blot analysis showed increased PTEN and FOXO3a as well as downstream target proteins, Bim and Bax, in the MCDmp group. (c) The low-glucose condition without AMPK-activating agents also induced increased expression of PTEN, FOXO3a, and downstream target Bim.
cDNA microarray results
Forty-three genes were downregulated and two genes were upregulated at the threshold of false discovery rate corrected (P<0.05) (Fig. 5a) in melanoma tumor tissues. In particular, Mitf and Tyrp1 were decreased, along with decreased vascular endothelial growth factor (VEGF) and DEK oncoprotein mRNAs. In contrast, expression of the serine peptidase inhibitor F member 1 (Serpinf1), which is known as the pigment epithelium derived factor was increased (Supplemental digital content 9). Western-blot analysis confirmed their protein expression patterns (Fig. 5b). Confocal microscopy of tumor tissue samples showed lower TYRP1 expression within the cytoplasm of tumor cells with less pigmentation in the MCDmp group (Fig. 5c and d). Compared with the MCD intervention, MCDmp produced stronger anticancer effects including suppression of the MITF, mTOR signaling, and mitogen-activated protein kinase pathways (Fig. 5e).
Fig. 5.
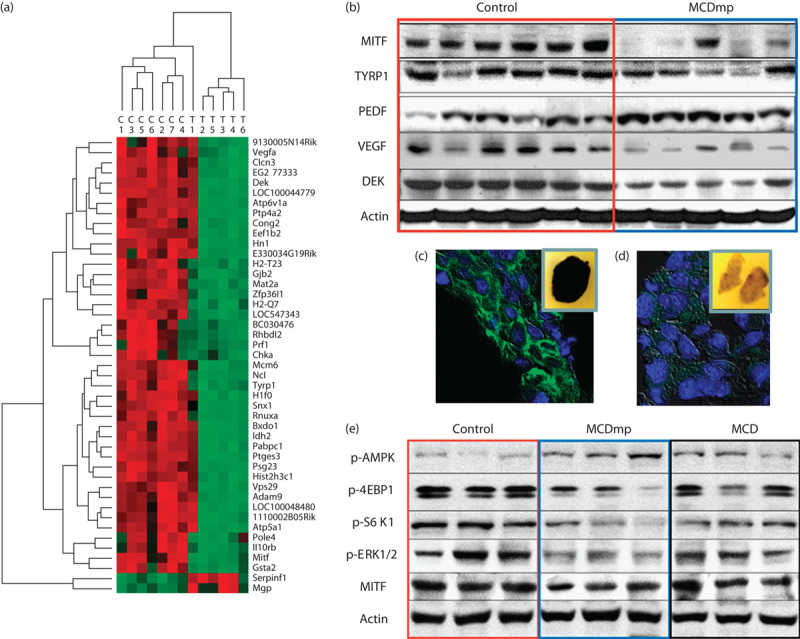
Diet-induced melanoma growth-suppression mechanism. (a) cDNA microarray analysis using melanoma tissues removed from the control and the MCDmp groups showed significant alterations in gene expression. Red represents upregulated mRNAs and green represents downregulated mRNAs. (b) The melanoma-specific proliferation markers, MITF and TYRP1, were decreased in the MCDmp group along with inhibition of VEGF, activation of the endogenous angiogenesis inhibitor (PEDF), and downregulation of the DEK oncogene. (c) Confocal analysis confirmed significantly decreased TYRP1 expression in the MCDmp group (green; anti-TYRP1, blue; DAPI). Inset images represent gross cut-sections of paraffin-embedded tumor tissue blocks showing decreased pigmentation in the MCDmp group. (d) Compared with MCD intervention, the MCDmp group showed higher expression of p-AMPK with suppression of the 4-EBP1, S6K1, ERK1/2, and MITF.
Cancer-prevention effects in a transgenic liver cancer model
Numerous tumor nodules developed throughout the entire liver at 4 weeks after transfection of both HrasG12V and shp53 DNAs. The nodules were proven to be poorly differentiated aggressive tumors on H&E staining. Fewer tumor nodules were observed only in the MCDmp and LCDmp groups compared with the control group (Supplemental digital content 10). There was no significant difference in cancer-prevention effects in the group in which MCDmp was started on the day of DNA transfection (Group 2) however, fewer and smaller tumor nodules developed when the MCDmp intervention was started 2 weeks before DNA transfection (Group 3) (Fig. 6a). Finally, postmortem liver tissues showed fewer tumor nodules in Group 3 (Fig. 6b and c). On Kaplan–Meier survival analysis, the overall survival was significantly prolonged only in Group 3 (P<3.5×10−7, Fig. 6d).
Fig. 6.
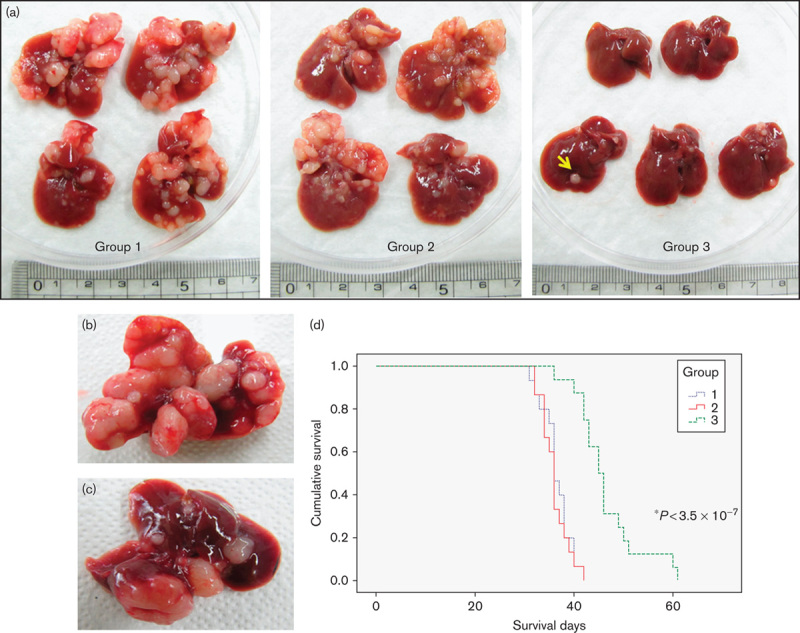
Dietary effects on cancer prevention. (a) Numerous tumor nodules developed within the liver after transfection of the HrasG12V and shP53 genes (Group 1). MCDmp intervention started after DNA transfection did not produce significant tumor-suppression activity (Group 2). However, fewer tumor nodules (arrow) developed when MCDmp was started 2 weeks before DNA transfection (Group 3). Post-mortem liver tissues also showed numerous tumor nodules throughout the liver in Group 1 (b)b, but fewer tumor nodules in Group 3 (c). (d) Kaplan–Meier survival analysis showed significant survival benefit only in Group 3. The median survival time of Group 1, Group 2, and Group 3 was 36±0.77, 36±0.45, and 45±1.20 days, respectively.
cDNA microarray analysis of the tumor-free liver tissues showed upregulation of the peroxisome proliferator-activated receptor α (PPARα) and downregulation of numerous genes that regulated energy metabolism, tumor initiation, and proliferation in Group 3 (Fig. 7a, Supplemental digital content 11). Among these, we confirmed decreased mRNA expression of the IL-6 and NF-κB in Group 3 compared with Group 1 using quantitative real-time PCR (Fig. 7b). H&E and TRITC–phalloidin immunostaining of the tumor-free liver tissues obtained 4 weeks after DNA transfection showed distorted lobular arrangement and bile canaliculi in Group 1, whereas normal liver architecture was grossly preserved in Group 3 (Fig. 7c). SIRT1 expression was increased in Group 3 compared with Group 1 (Supplemental digital content 12).
Fig. 7.
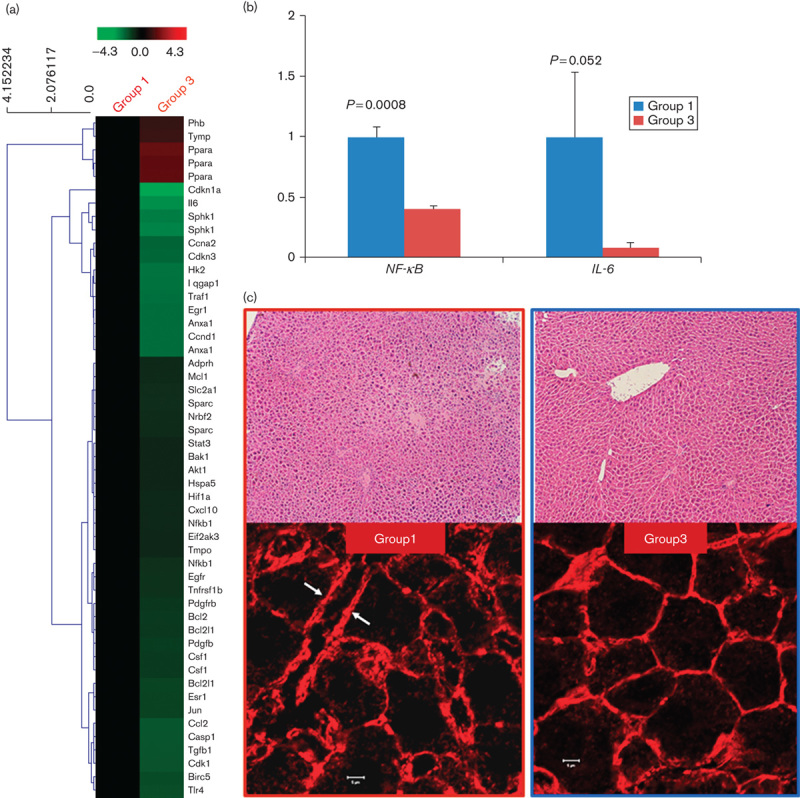
MCDmp-induced chemoprevention mechanism. (a) cDNA microarray analysis of the tumor-free liver tissues showed that numerous genes regulating glycolysis, tumorigenesis, and inflammation were downregulated in Group 3. (b) NF-κB and IL-6 expression was markedly decreased in Group 3 on real-time PCR. (c) H&E staining (×100, upper panel) of the tumor-free liver tissues obtained from Group 1 mice at 4 weeks after transfection shows a distorted lobular arrangement, but liver architecture is grossly preserved in Group 3. TRITC–phalloidin immunostaining (lower panel) shows severe dilatation and distortion of the bile canaliculi (arrows) in Group 1. However, the polyhedral architecture of the bile canaliculi is grossly preserved in Group 3. Scale bar=5 μm.
Discussion
Glucose is a major energy source in both normal and cancer cells. The glycolytic rate is increased in malignant tumors, and the normal metabolic control mechanisms are modified by numerous oncogenes to enhance the glycolytic rate (Levine and Puzio-Kuter, 2010). Our study confirmed that the inhibition of diet-induced hyperinsulinemia and the IGF-1R/PI3K/Akt pathway along with downregulation of the mTOR signaling could be important tumor-suppression mechanisms of the MCDmp intervention. HK2 and PKM2, which are the key enzymes that increase aerobic glycolysis in cancer cells (Sebastian and Kenkare, 1997; Sun et al., 2011), were also suppressed after the MCDmp intervention. This resulted in reduced glucose uptake with a lower intracellular glucose concentration (Fig. 2a and b), and this process could generate a vicious cycle in tumor cell-proliferation processes. Although amino acids, especially glutamine, can potentiate cellular proliferation, glucose restriction might inhibit glutamine uptake and cellular proliferation despite abundant amino acids (Lopez-Lazaro, 2008; Wellen et al., 2010).
AMPK/SIRT1/LKB1 closed-loop activation after energy restriction plays an important role in tumor suppression. SIRT1 activation might enhance tumor cell proliferation through deacetylation of p53; however, AMPK counteracts the deacetylase activity of SIRT1 on p53, but enhances p53 stability (Lee et al., 2012). Our study also showed that the level of acetyl-p53 in B16F10 melanoma cells did not decrease in a LGM even with increased SIRT1 expression. In-vitro study showed that melanoma cell proliferation was suppressed after treatment with an allosteric SIRT1 activator, SA3, along with AMPK activation in a dose-dependent manner when tumor cells were cultured in a LGM. Melanoma tissues from the MCDmp group showed activation of the AMPK/SIRT1/LKB1 signaling with apparent tumor growth suppression with restored cell polarity compared with the control group. As cell polarity is abolished during the carcinogenesis process (Lee and Vasioukhin, 2008; Luo et al., 2010), our data clearly show that our dietary modification inhibited tumor growth effectively through the activation of AMPK/SIRT1/LKB1 signaling, in particular, SIRT1 activation.
NF-κB is an important downstream target of SIRT1 that deacetylates the RelA/p65 subunit of NF-κB, thereby decreasing NF-κB-induced transcription of the genes encoding antiapoptotic proteins after SIRT1 activation (Yeung et al., 2004). Our study showed decreased NF-κB expression in tumor tissues removed from the MCDmp group as well as in B16F10 melanoma cells cultured in a LGM, especially after the treatment with SA3. Taken together, our data clearly show that energy restriction-induced SIRT1 activation is linked to tumor suppression.
FOXO3a is another target of SIRT1. Transcriptional activity of the FOXO3a can be activated within the nucleus by SIRT1-induced deacetylation of its lysine residues or AMPK-induced phosphorylation (Greer et al., 2007; Canto et al., 2009). Nutrient restriction could further enhance FOXO3a activity through enhanced translocation of the FOXO3a into the nucleus following inhibition of the PI3K/Akt signaling cascade (Calnan and Brunet, 2008). Nuclear FOXO3a can act as a tumor suppressor after binding to the promoters of various genes including Bim (Czabotar et al., 2009) or removing p53 from the SIRT1-binding sites (Nemoto et al., 2004). In fact, our study showed increased nuclear FOXO3a levels in both melanoma tissues from the MCDmp group and B16F10 melanoma cells cultured in a LGM.
Epigenetic modification of tumor-related genes could be involved in diet-induced tumor suppression. Li et al. (2010) have already reported increased longevity in normal cells after glucose restriction, but it resulted in growth inhibition of cancer cells by epigenetic modification. In this study, histone modification of the Pten and FoxO3a was observed in the MCDmp group. Although carbohydrate restriction alone can increase PTEN and FOXO3a expression, natural AMPK-activating phytochemicals could synergistically enhance tumor-suppression activities through various anticancer activities including histone modification, suppression of the energy-dependent signaling cascade, and cancer-related inflammation (Surh, 2003; Link et al., 2010).
cDNA microarray data showed final signaling pathways that are specific for melanoma growth suppression. In particular, MITF, which is the master transcription factor regulating melanocyte differentiation, cell cycle progression, and survival, was suppressed with decreased Tyrp1, which encodes tyrosinase interacting protein within melanoma tissue after the MCDmp intervention (Levy et al., 2006). Increased expression of pigment epithelium derived factor, which is an endogenous VEGF inhibitor, along with decreased VEGF, and DEK oncoprotein suppression could play critical roles in melanoma growth suppression by inhibiting angiogenesis and antiapoptotic activities, respectively (Khodadoust et al., 2009; Konson et al., 2010). Notably, the MCDmp intervention produced stronger anticancer effects than the MCD intervention. Therefore, the AMPK activation induced from energy restriction and natural AMPK-activating agents synergistically inhibit tumor growth, although carbohydrate restriction or phytochemical supplementation alone may not be effective in increasing the survival time.
Dietary modification not only suppresses tumor cell proliferation but also inhibits the development of cancer. In our transgenic hepatic tumor model, fewer tumor nodules developed after LCDmp and MCDmp interventions. Our data suggest that carbohydrate restriction with AMPK-activating phytochemicals controls malignant transformation of cells synergistically despite cells already acquired oncogenic properties through the suppression of the cancer-related inflammatory process including inhibition of the NF-κB/IL-6/STAT3 pathway (Yang et al., 2010). Increased PPARα expression could also enhance chemoprevention effects as CR or CR-mimetic phytochemicals might induce PPARα expression directly or indirectly by AMPK activation and inhibits various transcription factors, particularly NF-κB (Seymour et al., 2010; Peters et al., 2012). Increased SIRT1 activity could negatively regulate NF-κB-dependent STAT3 expression (Bernier et al., 2011). However, carbohydrate restriction or supplementation of AMPK-activating agents alone did not produce significant cancer-prevention effects. Notably, cancer-prevention effects were not apparent when dietary intervention was started after DNA transfection. This suggests that a diet-induced metabolic reprogramming period is necessary before malignant transformation of cells for cancer prevention. Indeed, previous data showed that the incidence of cancer was significantly low in rhesus monkeys when CR was started at a young age (Mattison et al., 2012).
In conclusion, we confirmed that isocaloric carbohydrate restriction in combination with a mixture of natural AMPK-activating agents could be an effective anticancer approach through synergic activities of each dietary intervention in animal models. SIRT1 enhances tumor suppression under an energy restriction condition.
Acknowledgements
The authors thank Dr Brian Hong, Jong Woo Kim, and Yong Jae Hwang for the preparation of the MCD and MCDmp formulas for animal use. This study was carried out in part at the Yonsei-Carl Zeiss Advanced Imaging Center, Yonsei University College of Medicine.
This research was supported by Brain Korea 21 project (7-2007-0352) at the Yonsei Medical College of Medicine and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2013-31-0342).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Jong Doo Lee, Min-Ah Choi and Simon Weonsang Ro contributed equally to the writing of this article.
All supplementary digital content is available directly from the corresponding author.
References
- Bernier M, Paul RK, Martin-Montalvo A, Scheibye-Knudsen M, Song S, He HJ, et al. (2011). Negative regulation of STAT3 protein-mediated cellular respiration by SIRT1 protein. J Biol Chem 286:19270–19279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost F, Sahra IB, Marchand-Brustel YL, Tanti JF. (2012). Metformin and cancer therapy. Curr Opin Oncol 24:103–108. [DOI] [PubMed] [Google Scholar]
- Bravata DM, Sanders L, Huang J, Krumholz HM, Olkin I, Gardner CD, et al. (2003). Efficacy and safety of low-carbohydrate diets. A systemic review. JAMA 289:1837–1850. [DOI] [PubMed] [Google Scholar]
- Calnan DR, Brunet A. (2008). The FoxO code. Oncogene 27:2276–2288. [DOI] [PubMed] [Google Scholar]
- Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. (2009). AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458:1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong JH, Park ES, Liang J, Dennison JB, Tsavachidou D, Nguyen-Charles C, et al. (2011). Dual inhibition of tumor energy pathway by 2-deoxyglucose and metformin is effective against a broad spectrum of preclinical cancer models. Mol Cancer Ther 10:2350–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I. (2010). Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys 501:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. (2009). Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czabotar PE, Colman PM, Huang DCS. (2009). Bax activation by Bim? Cell Death Differ 16:1187–1191. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. (2007). Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A 104:7217–7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, Barrett JC. (1997). Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res 57:4667–4672. [PubMed] [Google Scholar]
- El Mjiyad N, Caro-Maldonado A, Ramírez-Peinado S, Muñoz-Pinedo C. (2011). Sugar-free approaches to cancer cell killing. Oncogene 30:253–264. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Kay NE, Secreto CR, Shanafelt TD. (2009). Curcumin inhibits prosurvival pathways in chronic lymphocytic leukemia B cells and may overcome their stromal protection in combination with EGCG. Clin Cancer Res 15:1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. (2007). The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 282:30107–30119. [DOI] [PubMed] [Google Scholar]
- Herranz D, Serrano M. (2010). SIRT1: recent lessons from mouse models. Nat Rev Cancer 10:819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho VW, Leung K, Hsu A, Luk B, Lai J, Shen SY, et al. (2011). A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation. Cancer Res 71:4484–4493. [DOI] [PubMed] [Google Scholar]
- Ju HL, Ahn SH, Kim do Y, Baek S, Chung SI, Seong J, et al. (2013). Investigation of oncogenic cooperation in simple liver-specific transgenic mouse models using noninvasive in vivo imaging. PLoS One 8:e59869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JH, Lee JO, Kim JH, Lee SK, You GY, Park SH, et al. (2010). Quercetin suppresses HeLa cell viability via AMPK-induced HSP70 and EGFR down-regulation. J Cell Physiol 223:408–414. [DOI] [PubMed] [Google Scholar]
- Khodadoust MS, Verhaegen M, Kappes F, Riveiro-Falkenbach E, Cigudosa JC, Kim DS, et al. (2009). Melanoma proliferation and chemoresistance controlled by the DEK oncogene. Cancer Res 69:6405–6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konson A, Pradeep S, Seger R. (2010). Phosphomimetic mutants of pigmented epithelium-derived factor with enhanced antiangiogenic activity as potent anticancer agents. Cancer Res 70:6247–6257. [DOI] [PubMed] [Google Scholar]
- Lan F, Cacicedo JM, Ruderman N, Ido Y. (2008). SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem 283:27628–27635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last AR, Wilson SA. (2006). Low-carbohydrate diets. Am Fam Physician 73:1942–1948. [PubMed] [Google Scholar]
- Lee CW, Wong LL, Tse EY, Liu HF, Leong VY, Lee JM, et al. (2012). AMPK promotes p53 acetylation via phosphorylation and inactivation of SIRT1 in liver cancer cells. Cancer Res 72:4394–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Vasioukhin V. (2008). Cell polarity and cancer – cell and tissue polarity as a non-canonical tumor suppressor. J Cell Sci 121 (Pt 8):1141–1150. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Puzio-Kuter AM. (2010). The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 330:1340–1344. [DOI] [PubMed] [Google Scholar]
- Levy C, Khaled M, Fisher DE. (2006). MITF; master regulator of melanocyte development and melanoma oncogene. Trends Mol Med 12:406–414. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu L, Tollefsbol TO. (2010). Glucose restriction can extend normal cell lifespan and impair precancerous cell growth through epigenetic control of hTERT and p16 expression. FASEB J 24:1442–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link A, Balaguer F, Goel A. (2010). Cancer chemoprevention by dietary polyphenols: promising role for epigenetics. Biochem Pharmacol 80:1771–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RH. (2004). Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr 134 (Suppl):3479S–3485S. [DOI] [PubMed] [Google Scholar]
- Liu T, Liu PY, Marshall GM. (2009). The critical role of the class III histone deacetylase SIRT1 in cancer. Cancer Res 69:1702–1705. [DOI] [PubMed] [Google Scholar]
- López-Lázaro M. (2008). The warburg effect: why and how do cancer cells activate glycolysis in the presence of oxygen? Anticancer Agents Med Chem 8:305–312. [DOI] [PubMed] [Google Scholar]
- Luo Z, Zang M, Guo W. (2010). AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol 6:457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masko EM, Thomas JA, Antonelli JA, Lloyd JC, Phillips TE, Poulton SH, et al. (2010). Low-carbohydrate diets and prostate cancer: how low is ‘low enough’? Cancer Prev Res 3:1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, et al. (2012). Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489:318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez JA, Oliveras-Ferraros C, Cufí S, Corominas-Faja B, Joven J, Martin-Castillo R, et al. (2012). Metformin is synthetically lethal with glucose withdrawal in cancer cells. Cell Cycle 11:2782–2792. [DOI] [PubMed] [Google Scholar]
- Nayagam VM, Wang X, Tan YC, Poulsen A, Goh KC, Ng T, et al. (2006). SIRT1 modulating compounds from high-throughput screening as anti-inflammatory and insulin-sensitizing agents. J Biomol Screen 11:959–967. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. (2004). Nutrition availability regulates SIRT1 through a forkhead-dependent pathway. Science 306:2105–2108. [DOI] [PubMed] [Google Scholar]
- Pan W, Yang H, Cao C, Song X, Wallin B, Kivlin R, et al. (2008). AMPK mediates curcumin-induced cell death in CaOV3 ovarian cancer cells. Oncol Rep 20:1553–1559. [PubMed] [Google Scholar]
- Park IJ, Lee YK, Hwang JT, Kwon DY, Ha J, Park OJ. (2009). Green tea catechin controls apoptosis in colon cancer cells by attenuation of H2O2-stimulated COX-2 expression via the AMPK signaling pathway at low-dose H2O2.. Ann N Y Acad Sci 1171:538–544. [DOI] [PubMed] [Google Scholar]
- Peters JM, Shah YM, Gonzalez FJ. (2012). The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat Rev Cancer 12:181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardiello L, Bazzoli F, Fogliano V. (2011). Phytochemicals and colorectal cancer prevention – myth or reality? Nat Rev Gastroenterol Hepatol 8:592–596. [DOI] [PubMed] [Google Scholar]
- Roth M, Chen WY. (2013). Sorting out functions of sirtuins in cancer. Oncogene 33:1609–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. (2010). AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab 298:E751–E760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EN, Gescher AJ, Steward WP, Brown K. (2009). Development of dietary phytochemical chemopreventive agents: biomarkers and choice of dose for early clinical trials. Cancer Prev Res (Phila) 2:525–530. [DOI] [PubMed] [Google Scholar]
- Sebastian S, Kenkare UW. (1997). Insulin-like growth factor I induces tumor hexokinase RNA expression in cancer cells. Biochem Biophys Res Commun 235:389–393. [DOI] [PubMed] [Google Scholar]
- Seyfried TN, Marsh J, Shelton LM, Huysentruyt LC, Mukherjee P. (2012). Is the restricted ketogenic diet a viable alternative to the standard of care for managing malignant brain cancer? Epilepsy Res 100:310–326. [DOI] [PubMed] [Google Scholar]
- Seymour EM, Bennink MR, Watts SW, Bolling SF. (2010). Whole grape intake impacts cardiac peroxisome proliferator-activated receptor and nuclear factor kappaB activity and cytokine expression in rats with diastolic dysfunction. Hypertension 55:1179–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford DB, Shaw RJ. (2009). The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer 9:563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Chen X, Ma J, Peng H, Wang F, Zha X, et al. (2011). Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci U S A 108:4129–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh YJ. (2003). Cancer chemoprevention with dietary phyrochemicals. Nat Rev 3:768–780. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Lu C, Mancuso A, Lemons JM, Ryczko M, Dennis JW, et al. (2010). The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev 24:2784–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Cao R, Xia L, Erdjument-Bromage H, Borchers C, Tempst P, Zhang Y. (2001). Purification and functional characterization of a histone H3-lysine 4-specific methyltransferase. Mol Cell 8:1207–1217. [DOI] [PubMed] [Google Scholar]
- Yang J, Splittgerber R, Yull FE, Kantrow S, Ayers GD, Karin M, Richmond A. (2010). Conditional ablation of Ikkb inhibits melanoma tumor development in mice. J Clin Invest 120:2563–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. (2004). Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23:2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]


