Abstract
Estrogen and progesterone are key factors in the development of breast cancer, but it remains unclear whether these hormones are associated with mammographic density phenotypes in premenopausal women. We measured percent mammographic density, nondense area, and absolute mammographic density using computer-assisted breast density readings (Madena) from digitized mammograms taken on a scheduled day of the menstrual cycle (day 7–12) among 202 healthy, premenopausal women (Energy Balance and Breast cancer Aspects Study-I). Daily salivary concentrations of 17β-estradiol and progesterone throughout an entire menstrual cycle and fasting morning serum concentrations of hormones on 3 specific days of the menstrual cycle were assessed. Salivary and serum 17β-estradiol and progesterone were positively associated with percent mammographic density, we observed by 1 SD increase in overall salivary estradiol (β-value equal to 2.07, P=0.044), luteal salivary progesterone (β-value equal to 2.40, P=0.020). Women with above-median percent mammographic density had a 20% higher mean salivary 17β-estradiol level throughout the menstrual cycle. The odds ratio for having above-median percent mammographic density (>28.5%) per 1 SD increase in overall salivary 17β-estradiol was 1.66 (95% confidence interval 1.13–2.45). Women in the top tertile of the overall average daily 17β-estradiol concentrations had an odds ratio of 2.54 (confidence interval 1.05–6.16) of above-median percent mammographic density compared with women in the bottom tertile. Our finding of a relationship between estrogen, progesterone, and percent mammographic density and not with other mammographic density phenotypes in premenopausal women is biologically plausible, but needs to be replicated in larger studies.
Keywords: 17β-estradiol, mammographic density, premenopausal women, progesterone
Introduction
Women with higher levels of mammographically measured breast density have a significantly increased risk of developing breast cancer (Pettersson et al., 2014). Absolute mammographic density reflects dense areas of the breast, hypothesized to be composed of epithelial and stromal tissues. Nondense area represents fat tissue and percent mammographic density reflects the relative amounts of fibroglandular and fat tissue (Stone et al., 2010; Boyd et al., 2011; Pettersson et al., 2011). Both percent mammographic density and absolute mammographic density are positively correlated with the number of epithelial cells at risk for malignant transformation. Although a high percent mammographic density is associated with a three-to-six-fold increase in the risk of breast cancer compared with a low percent mammographic density (Pettersson et al., 2014), the absolute dense area is considered to represent the actual target tissue for tumor development as ductal carcinoma in situ and invasive breast cancer more often occur in dense areas (Ursin et al., 2005; Gill et al., 2006; Pinto Pereira et al., 2011).
Recently, diverse processes including growth factors, hormones, and interactions among epithelial cells, and the breast microenvironment, including fibroblast and adipocytes, have been shown to influence breast phenotypes (Brower, 2010; Boyd et al., 2011; Pettersson et al., 2014). Various breast cancer risk factors, such as age, reproductive factors, and BMI, have been associated with different breast tissue compositions (Stone et al., 2010; Boyd et al., 2011; Pettersson et al., 2011). Moreover, estrogen and progesterone promote cellular and epithelial growth in the normal mammary gland (Henderson et al., 1982; Bernstein, 2002; Mctiernan et al., 2009), and are positively associated with an increased risk for breast cancer in both premenopausal and postmenopausal women (Key et al., 2003, 2013). Interestingly, randomized-controlled trials have shown that percent mammographic density increases significantly with administration of combined estrogen–progesterone menopausal hormone therapy (Greendale et al., 2003; Mctiernan et al., 2005), whereas the estrogen receptor antagonist Tamoxifen reduces percent mammographic density (Cuzick et al., 2004; Johansson et al., 2013). However, it is unclear whether estrogen and progesterone levels throughout the menstrual cycle in premenopausal women are associated with mammographic density or vary by mammographic density phenotypes (Boyd et al., 2002; Noh et al., 2006; Yong et al., 2009).
Previously, we observed in the Energy Balance and Breast cancer Aspects (EBBA) study-I that daily cyclic 17β-estradiol was associated with breast cancer risk factors including age at menarche (Emaus et al., 2008a) and body composition from birth to adult life (Furberg et al., 2005; Jasienska et al., 2006; Emaus et al., 2008b; Finstad et al., 2009; Barrett et al., 2013). Furthermore, in a subanalysis, we observed a positive association between daily progesterone concentrations and mammographic density in premenopausal women using a modified Wolfe’s classification (Furberg et al., 2005).
In the present study, we investigated the associations between salivary and serum sex hormones [estradiol, progesterone, testosterone, dehydroepiandrosterone-sulfate (DHEA-SO4), follicle-stimulating hormone (FSH), and luteinizing hormone (LH)] and mammographic density phenotypes (percent mammographic density, nondense area, and absolute mammographic density), assessed by the computer-assisted method (Madena; University of Southern California School of Medicine, Los Angeles, California, USA) (Ursin et al., 2003), among premenopausal women from the EBBA-I study.
Materials and methods
Study design, setting, and participants
The women participating in the Norwegian EBBA-I Study (2000–2002) were recruited through local media campaigns. A total of 204 women aged 25–35 years were included, and fulfilled the following criteria: regular menstrual cycles (cycle length: 22–38 days within the previous 3 months), no use of any regular (daily/weekly) medication, no pregnancy or lactation or use of steroid contraceptives over the previous 6 months, and no history of gynecological or chronic disorders (e.g. diabetes, hypo/hyperthyroidism, polycystic ovary syndrome) (Furberg et al., 2005; Iversen et al., 2011). Two women were excluded because of missing mammographic data, leaving data from 202 premenopausal women available for the present study.
We used a standardized questionnaire to collect information on reproductive history, previous hormone use, and lifestyle habits. The same trained nurse interviewed all participants.
Clinical examination
The participants underwent a clinical examination at three scheduled visits over the course of one menstrual cycle: first visit (days 1–5, early follicular phase), second visit (days 7–12, late follicular phase), and third visit (days 21–25, late luteal phase). They came in on the first possible day after the onset of menstrual bleeding for clinical examinations at the Clinical Research Center, University Hospital of North Norway (UNN), Tromsø. Fasting blood samples were collected and analyzed at the Department of Clinical Chemistry, UNN. Height was measured to the nearest 0.5 cm and weight to the nearest 0.1 kg on an electronic scale, and BMI was calculated in kg/m2. At the second visit, participants underwent a full-body scan to estimate total percent body fat using dual-energy X-ray absorptiometry (DPLX-L 2288; Lunar Radiation Corporation, Madison, Wisconsin, USA) (Furberg et al., 2005).
Serum sex steroid hormone assessment procedures
Fasting serum samples were measured at three scheduled visits during the menstrual cycle. Concentrations of estradiol and progesterone were measured using a direct immunometric assay (Immuno-1; Bayer Diagnostics, at the Department of Clinical Chemistry, UNN, Tromsø, Norway) in fresh fasting serum samples at all three visits during the menstrual cycle. The sensitivity for estradiol was 0.01 nmol/l and the coefficient of variation (CV) was 3.9%. The sensitivity and CV for progesterone were 0.13 nmol/l and 5.7%, respectively. Sex hormone-binding globulin was measured using an immunometric method (both Diagnostic Products Corporation, Bierman GmbH, Bad Nauheim, Germany), with a CV of 5–10%. Serum testosterone was measured using an enhanced chemiluminescence immunoassay, using Elecsys 2010 from Roche Diagnostics (Mannheim, Germany), in 153 samples (74%), whereas for the remaining samples, Immuno-1 from Bayer Diagnostics (Tarrytown, New York, USA) was used. The CV was 0.97 in parallel runs of the testosterone assays and no correction formula was used. Serum DHEA-SO4 was measured using a competitive immunometric assay. LH and FSH were measured in serum samples using Techicon Immuno-1 immunometric assays (Bayer Diagnostics). Both assays were standardized against the WHO 2nd International Standard (for FSH: IRP 78/549 and for LH: IRP 68/40). The sensitivity of the FSH assay was 0.1 IU/l and the CV was less than 7%. For LH, the assay sensitivity was 0.3 IU/l and the CV was 5–10%.
Daily saliva sampling
The women collected daily morning saliva samples at home for one entire menstrual cycle, and sampling started on the first day of menstrual bleeding according to protocols established at the Reproductive Ecology Laboratory, Harvard University (USA) (Lipson and Ellison, 1989; Ellison and Lipson, 1999; Furberg et al., 2005). Levels of 17β-estradiol were measured in daily saliva samples from 20 days (reverse cycle day −5 to −24) and levels of progesterone from 14 days (reverse cycle days −1 to −14). 125I-labeled RIA kits (#39100; Diagnostic Systems Laboratories, Webster, Texas, USA) were used along with published modifications of the manufacturer’s protocols (Furberg et al., 2005). All samples were run in duplicate. All of a participant’s samples were run in the same batch, with women assigned to batches randomly. CVs were calculated on the basis of high and low value pools (appropriate to the range of each steroid) included in each assay (Furberg et al., 2005). In the present study, measurements of 17β-estradiol at the beginning and at the end of the cycles had higher CVs. The sensitivity of the 17β-estradiol assay (lowest value measurable by assay) was 4 pmol/l. The average intra-assay variability was 9% and the interassay variability ranged from 23% for low pools to 13% for high pools. For progesterone, the sensitivity of the assay was 13 pmol/l. The average intra-assay variability was 10%; the interassay variability ranged from 19% for low pools to 12% for high pools.
Alignment of the cycles was based on the identification of the mid-cycle decrease in estradiol (aligned cycle day 0), which provides a good estimate of the day of ovulation (Ellison and Lipson, 1999). Identification of the decrease in the salivary 17β-estradiol concentration was not satisfactory for 14 women; thus, their cycles were not aligned. The overall average salivary 17β-estradiol and progesterone were calculated for all women, and additional indices of average hormone concentrations were calculated for 188 women: follicular estradiol index (aligned cycle days −7 to −1), luteal estradiol index (aligned cycle days 0 to +6), mid-menstrual estradiol index (aligned cycle days −7 to +6), and early-mid luteal progesterone index (aligned cycle days 0 to +9).
Mammographic density phenotypes
Bilateral two-view mammograms were obtained from all women during the same menstrual cycle as the serum and salivary sampling were performed, late follicular phase, second visit (days 7–12) at the Center of Breast Imaging, UNN, using a standard protocol (Bjurstam et al., 2003; Furberg et al., 2005). Left craniocaudal mammograms were digitized and imported into a computerized mammographic density assessment program (Madena) (Ursin et al., 1998, 2003), and the breast areas were outlined by a trained research assistant using validated methods (Ursin et al., 1998). The total breast area was defined on the mammographic image using a special outlining tool. The region of interest (ROI) was then outlined. The mammogram reader used a tinting tool to apply yellow tint to areas considered to represent mammographically dense areas. The Madena software estimated the total number of pixels and the number of tinted pixels in the ROI.
Absolute mammographic density represents the number of the tinted pixels within the ROI. The nondense area reflects the total breast area, minus the dense area. Percent mammographic density is the ratio of absolute mammographic density to the total breast area (area of ROI) multiplied by 100. The mammograms were read in four batches, with an equal number of mammograms in each batch. A duplicate reading of 26 randomly selected mammograms from two of the batches showed a Pearson’s correlation coefficient of 0.97. The reader was blinded to all the characteristics of the study population.
Ethical considerations
All the participants signed an informed consent form and the Regional Committee for Medical Research Ethics and the Norwegian Data Protection Agency approved the study.
Statistical analysis
The associations between sex steroid hormones in both saliva and serum and mammographic density were analyzed using multivariable linear and logistic regression models. Mammographic density outcome variables were used as both continuous and dichotomized variables representing lower and higher density using median values as cut-off points: percent mammographic density (28.5%), nondense area (84.7 cm2), and absolute mammographic density (32.4 cm2). Both mammographic and hormone variables were approximately normally distributed, enabling data analyses by parametric tests.
On the basis of suggested biological mechanisms influencing levels of estradiol and progesterone or mammographic density, several models were used, including a variety of potentially confounding variables. Age, BMI, parity, previous oral contraceptives (OC) use, and current smoking were included as covariates in the final models. The adjusted β-values and odds ratio (ORs) of having above-median mammographic density were estimated according to a 1 SD higher level of ovarian hormones. The area under the curve for estradiol and progesterone was calculated for each participant with an aligned cycle using the trapezium rule (Matthews et al., 1990).
To study in detail how measures of mammographic density vary among premenopausal women in groups of ‘low’, ‘medium’, and ‘high’ cyclic endogenous estrogen and progesterone levels, we used tertiles (T1–T3). Furthermore, we used adjusted linear mixed models for repeated measures to study variations in daily salivary 17β-estradiol and salivary progesterone across the menstrual cycle according to low and high (median split) levels of mammographic density phenotypes. The Toeplitz covariance structure yielded the best fit to the data and was used in all models.
All statistical tests were two-sided using a 5% significance level. Statistical analyses were carried out using SPSS version 21.0 (IBM Corporation, Armonk, New York, USA).
Results
Patient characteristics
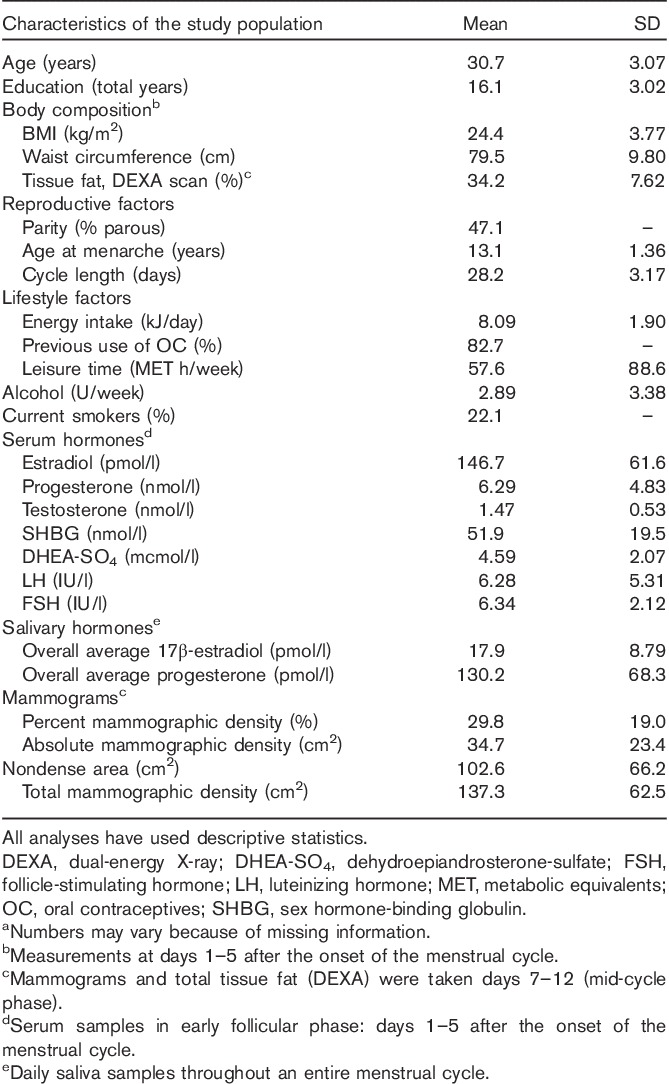
There were 204 healthy participants; their mean age was 30.7 years. The mean (median) percent mammographic density was 29.8% (28.5%), nondense area 102.6 cm2 (84.7 cm2), and the absolute mammographic density was 34.7 cm2 (32.4 cm2) (Table 1).
Table 1.
Descriptive characteristics of the study population: the Norwegian Energy Balance and Breast Cancer Aspects-I study (n=202)a
Association between hormones and mammographic density
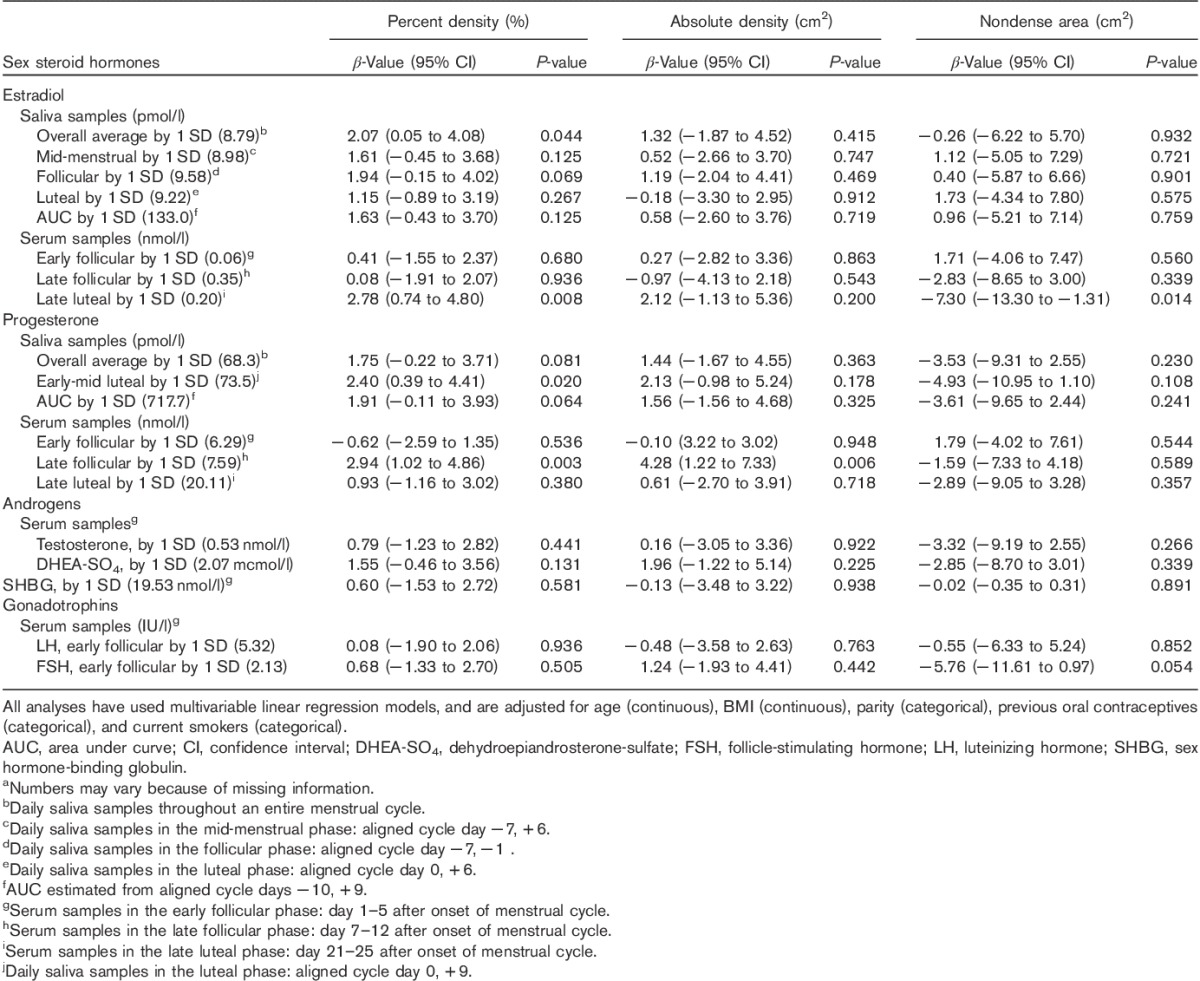
Percent mammographic density was positively associated with salivary estrogen and progesterone after adjustments. Both a 1 SD increase in the overall average salivary estradiol (β=2.07, P=0.044) and late luteal serum estradiol (β=2.78, P=0.008) were positively associated with percent mammographic density after adjusting for age, BMI, parity, smoking, and previous OC use in linear regression analysis (Table 2). Furthermore, a one SD increase in luteal salivary progesterone (β=2.40, P=0.020), and late follicular serum progesterone (β=2.94, P=0.003) were positively associated with percent mammographic density (Table 2). No associations were observed between gonadotropins (LH, FSH) or androgens (testosterone, DHEA-SO4) and percent mammographic density, and no associations were observed between salivary 17β-estradiol, or progesterone with either absolute mammographic density or nondense area (Table 2). Late follicular serum progesterone was the only hormone positively associated with absolute mammographic density (β=4.28, P=0.006) (Table 2).
Table 2.
The association by 1 SD higher level of sex steroid hormones and mammographic density phenotypes in premenopausal women (n=202)a using multivariable linear regression models
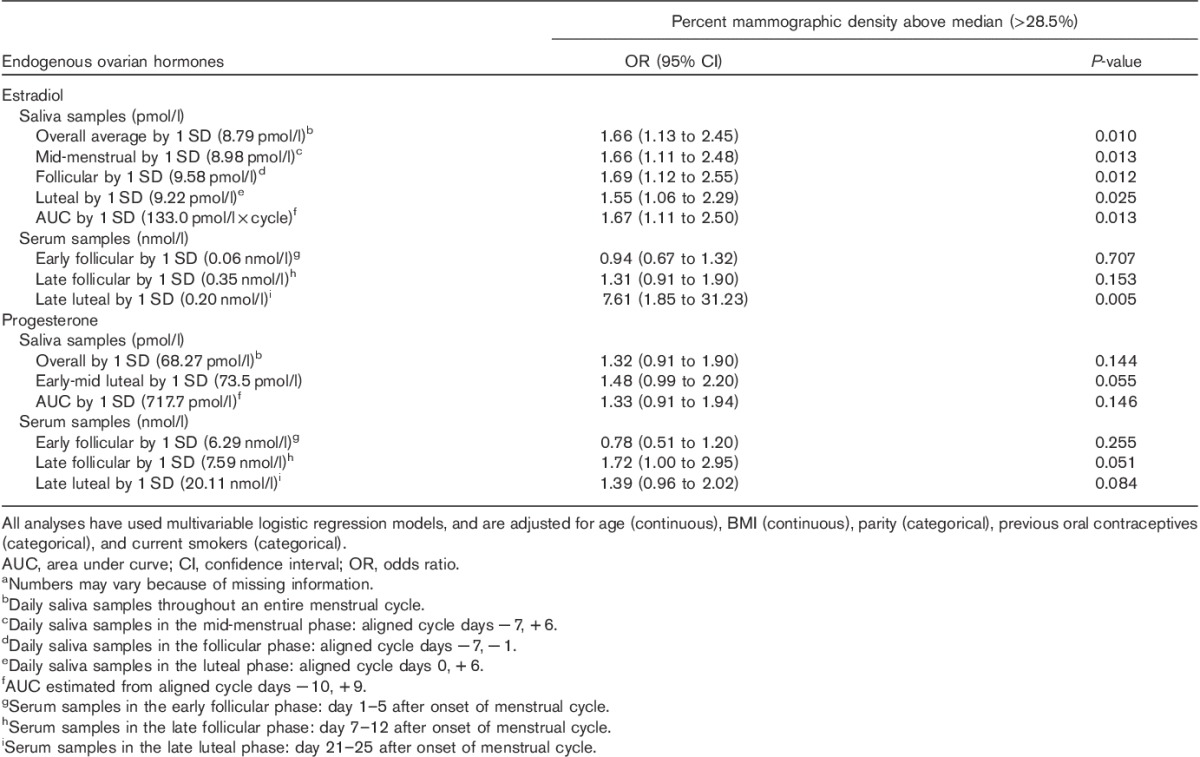
In adjusted logistic regression analysis, a 1 SD increase in salivary 17β-estradiol in all menstrual phases was associated with statistically significant 50–60% higher odds of above-median percent mammographic density (i.e. >28.5%) (Table 3). In addition, a 1 SD increase in late follicular serum progesterone was associated with an OR of 1.72 [95% confidence interval (CI) 1.00–2.95] for having above-median percent mammographic density. No associations were found between the dichotomized (above-median) nondense area or absolute mammographic density and levels of estradiol or progesterone (Supplementary Table 1).
Table 3.
Odds ratio with 95% confidence interval for above-median percent mammographic density (>28.5%) by 1 SD higher level of ovarian hormones among premenopausal women (n=202)a
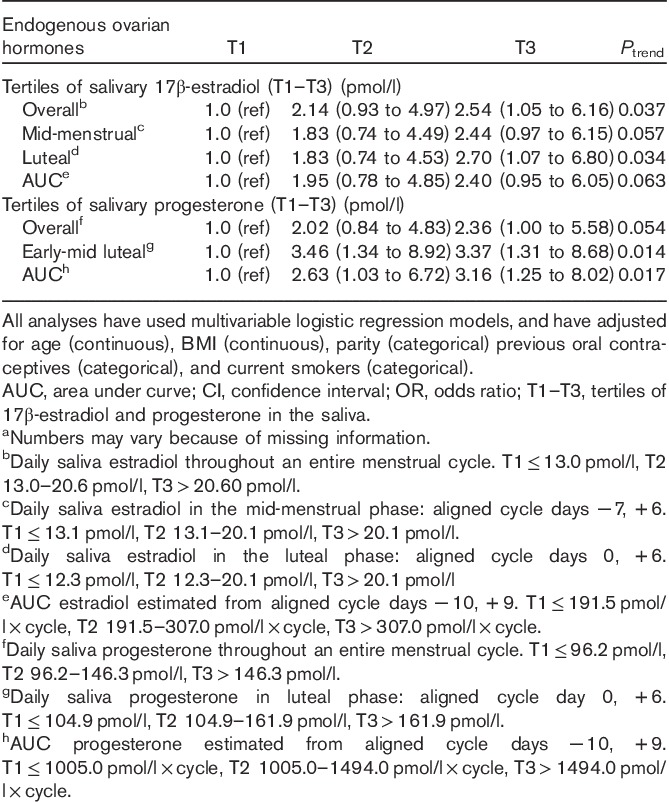
Women in the top tertile of the overall average salivary 17β-estradiol concentration had a 2.5 times higher odds of above-median percent mammographic density (≥28.5%) compared with women in the bottom tertile [T3 vs. T1: OR 2.54 (95% CI 1.05–6.16) Ptrend=0.037] (Table 4). Similarly, associations were observed for mid-menstrual, luteal, and area under the curve measures of salivary 17β-estradiol. Women in the two top tertiles of salivary luteal progesterone had a 3.4 times higher odds of above-median percent mammographic density compared with women in the bottom tertile [T2 vs. T1: OR 3.46 (95% CI 1.34–8.92), T3 vs. T1: OR 3.37 (95% CI 1.31–8.68), Ptrend=0.014] (Table 4).
Table 4.
Odds ratios for higher percent mammographic density (>28.5%) associated with endogenous ovarian hormones by tertiles of salivary 17β-estradiol and progesterone (n=202)a
Associations between repeated measures of hormones and mammographic density
In the mixed linear regression models, we found that women with above-median percent mammographic density had 20% higher mean salivary 17β-estradiol levels throughout the menstrual cycle compared with women with below-median percent mammographic density (P=0.011) (Fig. 1a). No associations were observed between salivary estradiol and progesterone with either absolute mammographic density or nondense area (Fig. 1b–f).
Fig. 1.
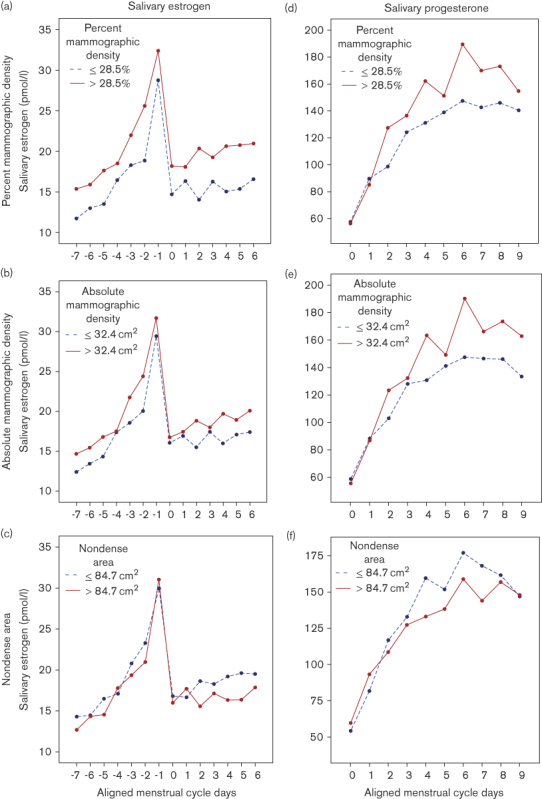
Adjusted mean salivary ovarian hormones by high (red line) and low (blue line) percent mammographic density (a, c), absolute mammographic density (b, e), and nondense area (c, f) among 202 premenopausal women. Note that all analyses have used linear mixed models for repeated measures, adjusted for age (continuous), BMI (kg/m2) (continuous), parity (categorical), previous oral contraceptives (categorical), and current smokers (categorical). Mean salivary estradiol throughout the menstrual cycle. (a) Percent mammographic density: ≤28.5%, 16.3 pmol/l; >28.5%, 20.4 pmol/l (P=0.011). (b) Absolute mammographic density: ≤32.4 cm2, 17.4 pmol/l; >32.4 cm2, 19.4 pmol/l (P=0.148). (c) Nondense area: ≤84.7 cm2, 18.9 pmol/l; >84.7 cm2, 17.8 pmol/l (P=0.501). Mean salivary progesterone level throughout the menstrual cycle: (d) percent mammographic density: ≤28.5%, 121.8 pmol/l; >28.5%, 140.7 pmol/l (P=0.118). (e) Absolute mammographic density: ≤32.4 cm2, 122 pmol/l; >32.4 cm2, 140 pmol/l (P=0.092). (f) Nondense area: ≤84.7 cm2, 134.9 pmol/l; >84.7 cm2, 126.3 pmol/l (P=0.490).
Discussion
In this study of premenopausal women, positive associations were observed between salivary and serum estradiol and progesterone, and percent mammographic density, but no clear associations were observed between these hormones and other mammographic density phenotypes. Women in the top tertiles of overall average daily 17β-estradiol and luteal progesterone concentrations had about two to three times higher odds of having above-median percent mammographic density compared with women in the bottom tertile.
These results are unique, but partly supported (Noh et al., 2006; Yong et al., 2009). A positive association was observed between serum estradiol concentrations (n=192) (Yong et al., 2009) and serum concentration of progesterone (n=204) (Noh et al., 2006), and percent mammographic density among older premenopausal women. In addition, urinary estrogen metabolites were associated with both percent and absolute mammographic density among premenopausal women (Walker et al., 2009). However, the associations observed between endogenous hormones and mammographic density phenotypes were weaker or inconclusive when BMI was included as a covariate (Boyd et al., 2002; Tamimi et al., 2005; Johansson et al., 2008; Walker et al., 2009). Thus, the associations observed between endogenous hormones and different mammographic density phenotypes have been divergent (Boyd et al., 2002; Walker et al., 2009).
The present study provides novel information by including both fasting serum levels of 17β-estradiol and progesterone from 3 scheduled days, and daily salivary levels of both hormones over an entire menstrual cycle. Previous studies have measured only blood hormones at discrete periods (Boyd et al., 2002; Noh et al., 2006; Walker et al., 2009; Yong et al., 2009; Maskarinec et al., 2012). Our study provides a more accurate estimate of likely exposure to estradiol and progesterone across the entire menstrual cycle (Bellem et al., 2011). However, we observed no correlations between serum and salivary hormones among all the participants, which is consistent with the results of others (Lu et al., 1999) as a correlation between salivary and serum concentrations within the individual was observed, but not in total. This may be explained by the fact that serum hormones are dependent on the protein-binding capacity, which differs markedly among individuals. Moreover, the serum levels in one individual cannot be predicted from salivary concentrations in others (Ellison and Lipson, 1999; Lu et al., 1999). Interestingly, almost all of our observed associations were in relation to estrogen and progesterone and percent mammographic density, and not with other mammographic phenotypes. Recently, a meta-analysis of 13 case–control studies including both premenopausal and postmenopausal women found that percent mammographic density was a stronger risk factor for breast cancer than absolute mammographic density, potentially suggesting that the ratio of fibroglandular to fat tissue may be important in relation to the development of breast cancer (Pettersson et al., 2014). Recently, the importance of both percent and absolute mammographic density has been considered, in addition to established risk factors, in a predictive model for breast cancer (Rauh et al., 2012). However, a recent study found absolute breast density to be a better breast cancer risk marker in women with an unfavorable metabolic profile as percent density was correlated negatively with nondense area and adiposity (Schetter et al., 2014). However, the women in our study had a mean BMI of 24.4 kg/m2.
Mammographic density reflects proliferation of epithelial and stromal cells, as well as the cumulative exposure of the breast to different mitogens including sex hormones (Boyd et al., 2011), and in the ‘Pike model’, breast tissue aging is hypothesized to reflect reproductive factors and the cumulative hormone exposure. The rate of breast tissue aging is most rapid at the time of menarche, slows with each pregnancy, and slows further in the menopausal phase (Pike et al., 1983); the same pattern has been observed in mammographic density (Boyd et al., 2007; Boyd, 2013). Interestingly, local estrogen production in the breast, rather than circulating estrogen levels, has been suggested to be more relevant to breast density in postmenopausal women (Pettersson et al., 2014), whereas one may hypothesize that percent mammographic density may be a better marker in premenopausal women.
Whether percent mammographic density or a specific threshold of percent mammographic density in early adulthood is predictive of breast cancer risk later in life remains unclear. However, previous studies in premenopausal (Van Gils et al., 2000) and postmenopausal (Yaghjyan et al., 2013) women have found a two-fold to three-fold increase in breast cancer risk for women with percent mammographic density above 25%. These observations support comparison of groups of women with above-versus below-median percent mammographic density, as we did in our study.
There are several strengths, but also limitations to our study. Fasting serum samples were collected at three scheduled visits during the menstrual cycle, and salivary measurements of unbound estradiol and progesterone concentrations were collected daily over an entire menstrual cycle, following strict procedures (Lipson and Ellison, 1989; Ellison and Lipson, 1999; Gann et al., 2001; Furberg et al., 2005; Jasienska and Jasienski, 2008). Using noninvasive daily salivary samples, we could measure the free biologically active forms of estrogen and progesterone, which are considered ideal measures among premenopausal women (Ellison and Lipson, 1999; Bellem et al., 2011). In addition, we measured the total sex steroid hormone, and could capture the total sex steroid hormone exposure throughout the entire menstrual cycle. All mammograms were assessed within a narrow time frame (between days 7 and 12), thereby avoiding the bias of variation in mammographic density during the menstrual cycle (Morrow et al., 2010). We used a validated computer-assisted method to quantify mammographic density (Ursin et al., 1998), and all mammograms were read by one experienced blinded reader. The study population was homogeneous and included healthy women aged 25–35 years from the same cultural background.
However, our sample size was small, the study design was cross-sectional, and because of ethical concerns, we could only obtain one measure of mammographic density, and therefore could not measure changes in density patterns across the menstrual cycle. Finally, all serum samples from each woman were not measured in the same batches, which could have potentially introduced some error. However, serum concentrations of estradiol and progesterone in premenopausal women are high, with lower CVs, and therefore there is generally less likelihood for measurement error compared with values in postmenopausal women. Each woman’s salivary estradiol and progesterone, however, were assayed in the same batch. These similar results observed in salivary and serum concentrations suggested that the associations using serum values are valid. The salivary samples were only measured during one menstrual cycle, and we could not capture the intercycle variations (Chatterton et al., 2005). However, among stable-weight women, marked changes in hormonal levels from cycle to cycle are not expected, thus lowering the required number of measured cycles. Even a single cycle per woman would be sufficient to provide adequate statistical power (Jasienska and Jasienski, 2008).
Conclusion
Our study provides novel data linking endogenous sex hormones throughout the menstrual cycle to percent mammographic density in particular. The present observations are biologically plausible, and may be of potential clinical interest. However, larger studies including estrogen and progesterone across the menstrual cycle in various populations are needed to define the clinical implications of these findings.
Acknowledgements
The authors thank the participants in the EBBA-I study. They are grateful to Gunn Kristin Knudsen, Heidi Jakobsen, Anna-Kirsti Kvitnes, Sissel Andersen, and Nils Bjurstam, and the Clinical Research Department and Center of Breast Imaging, University Hospital of North Norway, for professional assistance in the data collection. This work was supported by the Foundation for the Norwegian Health and Rehabilitation Organization (59010-2000, 59010-2001, 59010-2002); the Norwegian Cancer Society (05087, TP 49 258); the Aakre Foundation (5695-2000, 5754-2002); the Northern Norway Regional Health Authority (SFP-563-06); the Norwegian research Council (213997); and a PhD grant from University of Tromsø.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Anita Iversen and Hanne Frydenberg contributed equally to the writing of this article.
All supplementary digital content is available directly from the corresponding author.
References
- Barrett ES, Thune I, Lipson SF, Furberg AS, Ellison PT. (2013). A factor analysis approach to examining relationships among ovarian steroid concentrations, gonadotrophin concentrations and menstrual cycle length characteristics in healthy, cycling women. Hum Reprod 28:801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellem A, Meiyappan S, Romans S, Einstein G. (2011). Measuring estrogens and progestagens in humans: an overview of methods. Gend Med 8:283–299. [DOI] [PubMed] [Google Scholar]
- Bernstein L. (2002). Epidemiology of endocrine-related risk factors for breast cancer. J Mammary Gland Biol Neoplasia 7:3–15. [DOI] [PubMed] [Google Scholar]
- Bjurstam N, Björneld L, Warwick J, Sala E, Duffy SW, Nyström L, et al. (2003). The Gothenburg breast screening trial. Cancer 97:2387–2396. [DOI] [PubMed] [Google Scholar]
- Boyd NF. (2013). Mammographic density and risk of breast cancer. Am Soc Clin Oncol Educ Book . doi: 10.1200/EdBook_AM.2013.33.e57. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Stone J, Martin LJ, Jong R, Fishell E, Yaffe M, et al. (2002). The association of breast mitogens with mammographic densities. Br J Cancer 87:876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. (2007). Mammographic density and the risk and detection of breast cancer. N Engl J Med 356:227–236. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Martin LJ, Yaffe MJ, Minkin S. (2011). Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res 13:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower V. (2010). Homing in on mechanisms linking breast density to breast cancer risk. J Natl Cancer Inst 102:843–845. [DOI] [PubMed] [Google Scholar]
- Chatterton RT, Jr, Mateo ET, Hou N, Rademaker AW, Acharya S, Jordan VC, Morrow M. (2005). Characteristics of salivary profiles of oestradiol and progesterone in premenopausal women. J Endocrinol 186:77–84. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Warwick J, Pinney E, Warren RM, Duffy SW. (2004). Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst 96:621–628. [DOI] [PubMed] [Google Scholar]
- Ellison PT, Lipson SF. (1999). Salivary estradiol – a viable alternative? Fertil Steril 72:951–952. [DOI] [PubMed] [Google Scholar]
- Emaus A, Espetvedt S, Veierød MB, Ballard-Barbash R, Furberg AS, Ellison PT, et al. (2008a). 17-beta-estradiol in relation to age at menarche and adult obesity in premenopausal women. Hum Reprod 23:919–927. [DOI] [PubMed] [Google Scholar]
- Emaus A, Veierød MB, Furberg AS, Espetvedt S, Friedenreich C, Ellison PT, et al. (2008b). Physical activity, heart rate, metabolic profile, and estradiol in premenopausal women. Med Sci Sports Exerc 40:1022–1030. [DOI] [PubMed] [Google Scholar]
- Finstad SE, Emaus A, Potischman N, Barrett E, Furberg AS, Ellison PT, et al. (2009). Influence of birth weight and adult body composition on 17beta-estradiol levels in young women. Cancer Causes Control 20:233–242. [DOI] [PubMed] [Google Scholar]
- Furberg AS, Jasienska G, Bjurstam N, Torjesen PA, Emaus A, Lipson SF, et al. (2005). Metabolic and hormonal profiles: HDL cholesterol as a plausible biomarker of breast cancer risk. The Norwegian EBBA Study. Cancer Epidemiol Biomarkers Prev 14:33–40. [PubMed] [Google Scholar]
- Gann PH, Giovanazzi S, Van Horn L, Branning A, Chatterton RT., Jr (2001). Saliva as a medium for investigating intra- and interindividual differences in sex hormone levels in premenopausal women. Cancer Epidemiol Biomarkers Prev 10:59–64. [PubMed] [Google Scholar]
- Gill JK, Maskarinec G, Pagano I, Kolonel LN. (2006). The association of mammographic density with ductal carcinoma in situ of the breast: the Multiethnic Cohort. Breast Cancer Res 8:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greendale GA, Reboussin BA, Slone S, Wasilauskas C, Pike MC, Ursin G. (2003). Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst 95:30–37. [DOI] [PubMed] [Google Scholar]
- Henderson BE, Ross RK, Pike MC, Casagrande JT. (1982). Endogenous hormones as a major factor in human cancer. Cancer Res 42:3232–3239. [PubMed] [Google Scholar]
- Iversen A, Thune I, McTiernan A, Emaus A, Finstad SE, Flote V, et al. (2011). Ovarian hormones and reproductive risk factors for breast cancer in premenopausal women: the Norwegian EBBA-I study. Hum Reprod 26:1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasienska G, Jasienski M. (2008). Interpopulation, interindividual, intercycle, and intracycle natural variation in progesterone levels: a quantitative assessment and implications for population studies. Am J Hum Biol 20:35–42. [DOI] [PubMed] [Google Scholar]
- Jasienska G, Ziomkiewicz A, Lipson SF, Thune I, Ellison PT. (2006). High ponderal index at birth predicts high estradiol levels in adult women. Am J Hum Biol 18:133–140. [DOI] [PubMed] [Google Scholar]
- Johansson H, Gandini S, Bonanni B, Mariette F, Guerrieri-Gonzaga A, Serrano D, et al. (2008). Relationships between circulating hormone levels, mammographic percent density and breast cancer risk factors in postmenopausal women. Breast Cancer Res Treat 108:57–67. [DOI] [PubMed] [Google Scholar]
- Johansson H, Bonanni B, Gandini S, Guerrieri-Gonzaga A, Cazzaniga M, Serrano D, et al. (2013). Circulating hormones and breast cancer risk in premenopausal women: a randomized trial of low-dose tamoxifen and fenretinide. Breast Cancer Res Treat 142:569–578. [DOI] [PubMed] [Google Scholar]
- Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, et al. (2003). Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst 95:1218–1226. [DOI] [PubMed] [Google Scholar]
- Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, Barricarte A, et al. (2013). Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol 14:1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson SF, Ellison PT. (1989). Development of protocols for the application of salivary steroid analysis to field conditions. Am J Hum Biol 1:249–255. [DOI] [PubMed] [Google Scholar]
- Lu Y, Bentley GR, Gann PH, Hodges KR, Chatterton RT. (1999). Salivary estradiol and progesterone levels in conception and nonconception cycles in women: evaluation of a new assay for salivary estradiol. Fertil Steril 71:863–868. [DOI] [PubMed] [Google Scholar]
- Maskarinec G, Heak S, Morimoto Y, Custer L, Franke AA. (2012). The relation of urinary estrogen metabolites with mammographic densities in premenopausal women. Cancer Epidemiol 36:e310–e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JN, Altman DG, Campbell MJ, Royston P. (1990). Analysis of serial measurements in medical research. BMJ 300:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTiernan A, Martin CF, Peck JD, Aragaki AK, Chlebowski RT, Pisano ED, et al. (2005). Estrogen-plus-progestin use and mammographic density in postmenopausal women: Women’s Health Initiative randomized trial. J Natl Cancer Inst 97:1366–1376. [DOI] [PubMed] [Google Scholar]
- McTiernan A, Chlebowski RT, Martin C, Peck JD, Aragaki A, Pisano ED, et al. (2009). Conjugated equine estrogen influence on mammographic density in postmenopausal women in a substudy of the women's health initiative randomized trial. J Clin Oncol 27:6135–6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow M, Chatterton RT, Jr, Rademaker AW, Hou N, Jordan VC, Hendrick RE, Khan SA. (2010). A prospective study of variability in mammographic density during the menstrual cycle. Breast Cancer Res Treat 121:565–574. [DOI] [PubMed] [Google Scholar]
- Noh JJ, Maskarinec G, Pagano I, Cheung LW, Stanczyk FZ. (2006). Mammographic densities and circulating hormones: a cross-sectional study in premenopausal women. Breast 15:20–28. [DOI] [PubMed] [Google Scholar]
- Pettersson A, Hankinson SE, Willett WC, Lagiou P, Trichopoulos D, Tamimi RM. (2011). Nondense mammographic area and risk of breast cancer. Breast Cancer Res 13:R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson A, Graff RE, Ursin G, Santos Silva ID, McCormack V, Baglietto L, et al. (2014). Mammographic density phenotypes and risk of breast cancer: a meta-analysis. J Natl Cancer Inst 106doi: 10.1093/jnci/dju078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike MC, Krailo MD, Henderson BE, Casagrande JT, Hoel DG. (1983). ‘Hormonal’ risk factors, ‘breast tissue age’ and the age-incidence of breast cancer. Nature 303:767–770. [DOI] [PubMed] [Google Scholar]
- Pinto Pereira SM, McCormack VA, Hipwell JH, Record C, Wilkinson LS, Moss SM, et al. (2011). Localized fibroglandular tissue as a predictor of future tumor location within the breast. Cancer Epidemiol Biomarkers Prev 20:1718–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh C, Hack CC, Häberle L, Hein A, Engel A, Schrauder MG, et al. (2012). Percent mammographic density and dense area as risk factors for breast cancer. Geburtshilfe Frauenheilkd 72:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetter SE, Hartman TJ, Liao J, Richie JP, Prokopczyk B, DuBrock C, et al. (2014). Differential impact of body mass index on absolute and percent breast density: implications regarding their use as breast cancer risk biomarkers. Breast Cancer Res Treat 146:355–363. [DOI] [PubMed] [Google Scholar]
- Stone J, Ding J, Warren RM, Duffy SW, Hopper JL. (2010). Using mammographic density to predict breast cancer risk: dense area or percentage dense area. Breast Cancer Res 12:R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamimi RM, Hankinson SE, Colditz GA, Byrne C. (2005). Endogenous sex hormone levels and mammographic density among postmenopausal women. Cancer Epidemiol Biomarkers Prev 14 (Pt 1):2641–2647. [DOI] [PubMed] [Google Scholar]
- Ursin G, Astrahan MA, Salane M, Parisky YR, Pearce JG, Daniels JR, et al. (1998). The detection of changes in mammographic densities. Cancer Epidemiol Biomarkers Prev 7:43–47. [PubMed] [Google Scholar]
- Ursin G, Ma H, Wu AH, Bernstein L, Salane M, Parisky YR, et al. (2003). Mammographic density and breast cancer in three ethnic groups. Cancer Epidemiol Biomarkers Prev 12:332–338. [PubMed] [Google Scholar]
- Ursin G, Hovanessian-Larsen L, Parisky YR, Pike MC, Wu AH. (2005). Greatly increased occurrence of breast cancers in areas of mammographically dense tissue. Breast Cancer Res 7:R605–R608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gils CH, Hendriks JH, Otten JD, Holland R, Verbeek AL. (2000). Parity and mammographic breast density in relation to breast cancer risk: indication of interaction. Eur J Cancer Prev 9:105–111. [DOI] [PubMed] [Google Scholar]
- Walker K, Fletcher O, Johnson N, Coupland B, McCormack VA, Folkerd E, et al. (2009). Premenopausal mammographic density in relation to cyclic variations in endogenous sex hormone levels, prolactin, and insulin-like growth factors. Cancer Res 69:6490–6499. [DOI] [PubMed] [Google Scholar]
- Yaghjyan L, Colditz GA, Rosner B, Tamimi RM. (2013). Mammographic breast density and subsequent risk of breast cancer in postmenopausal women according to the time since the mammogram. Cancer Epidemiol Biomarkers Prev 22:1110–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong M, Atkinson C, Newton KM, Aiello Bowles EJ, Stanczyk FZ, Westerlind KC, et al. (2009). Associations between endogenous sex hormone levels and mammographic and bone densities in premenopausal women. Cancer Causes Control 20:1039–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]


