Abstract
Previous studies suggest that hormone therapy may play an important role in the development of meningioma. However, it is unclear whether medication with tamoxifen can prevent meningioma. Our study cohort included all women who were diagnosed with breast cancer between 1961 and 2010, and a total of 227 535 women were identified with breast cancer with a median age at diagnosis of 63 years. Women diagnosed with breast cancer after 1987 were defined as tamoxifen exposed; those diagnosed with breast cancer before or during 1987 were defined as not exposed to tamoxifen. Standardized incidence ratios (SIRs) were used to calculate the risk of subsequent meningioma. Of these women, 223 developed meningioma. For women without tamoxifen exposure, the risk of meningioma was significantly increased, with an SIR of 1.54 (95% confidence interval 1.30–1.81); the risk was not increased in those with tamoxifen exposure (SIR=1.06, 95% confidence interval 0.84–1.32). The increased risk of meningioma in women without tamoxifen exposure persisted during 10 years of follow-up. In this historical cohort study, we found that women diagnosed with breast cancer but not treated with tamoxifen had an increased incidence of meningioma, whereas the incidence was close to that of the general population in patients treated with tamoxifen. This suggests that tamoxifen may prevent the development of meningioma.
Keywords: breast cancer, meningioma, tamoxifen
Introduction
Meningioma is a common tumour in the central nervous system, and it accounts for 13–26% of all intracranial tumours (Barnholtz-Sloan and Kruchko, 2007; Wiemels et al., 2010). The only known risk factor for meningioma is ionizing radiation (Preston et al., 2002; El Ghissassi et al., 2009); other environmental and lifestyle risk factors show inconclusive results (Barnholtz-Sloan and Kruchko, 2007; Wiemels et al., 2010). Given the higher prevalence of meningioma in women than in men (ratio 2 : 1) (Hsu et al., 1997; Carroll et al., 2000; Claus et al., 2008), it was hypothesized that hormones may be a risk factor for meningioma. Its association with hormones was further supported by evidence from women receiving hormone replacement therapy, who were found to have an increased risk of meningioma (Claus et al., 2007; Benson et al., 2010; Korhonen et al., 2012). A possible association was also observed among women using oral contraceptives (Custer et al., 2006; Claus et al., 2007; Korhonen et al., 2010), and pregnancy and menopausal status were reported to influence the risk of meningioma (Lambe et al., 1997; Wigertz et al., 2008). Women diagnosed with breast cancer have an increased risk of meningioma and vice versa (Custer et al., 2002; Rao et al., 2009). Some studies confirmed the expression of oestrogen receptors in some meningiomas (Black et al., 1996; Carroll et al., 1999; Claus et al., 2008).
All the above observations suggest that oestrogen may play an important role in the development of meningioma. It is thus important to examine whether medication with tamoxifen, a selective oestrogen receptor modulator with an antioestrogenic effect (Al-Mubarak et al., 2014; Nazarali and Narod, 2014), can inhibit or prevent the development of meningioma. Tamoxifen has been used widely to treat patients with oestrogen receptor-positive breast cancer (Al-Mubarak et al., 2014; Nazarali and Narod, 2014), and was first used in Sweden in the late 1980s (Fornander et al., 1989; Chandanos et al., 2006). To study its influence on the development of meningioma, we tested in this study whether women diagnosed with breast cancer and treated with tamoxifen have a lower incidence of meningioma compared with women without tamoxifen treatment using a nationwide population-based cohort study.
Methods
This historical cohort study was approved by the Regional Ethical Review Board of Lund University, Sweden, in 2013 (Diarienummer 2012/795). Patients diagnosed with breast cancer during the period 1 January 1961 to 31 December 2010 were identified from the Swedish Cancer Registry. The Swedish Cancer Registry was founded in 1958, and has had nationwide coverage since 1961. The Swedish Cancer Registry is currently estimated to have close to 100% coverage at the national level (Hemminki et al., 2010). It is maintained by the National Board of Health and Welfare. In Sweden, all clinicians and pathologists/cytologists are obliged to report all newly diagnosed primary cancers to the Cancer Registry. A validation study showed that 99% of the recorded cancer cases are morphologically verified (Hemminki et al., 2010). Only primary tumours are recorded in this register; metastases are excluded. Women with breast cancer were identified from the Swedish Cancer Registry using the ICD-7 code 170. Only patients with a first primary breast cancer were included; patients with a previous cancer, including breast cancer, were excluded.
All the patients in our cohort were followed until the diagnosis of meningioma, which was identified from the Swedish Cancer Registry using the ICD-7 code 1930. Histological confirmation was determined using the pathological anatomic diagnostic codes 461, 465 and 466. These codes were used during the entire study period. Additional linkages were made to the Swedish National Population and Housing Census (Population and Housing Census 1960–1990, 2011) to obtain information on individual-level characteristics, such as year of birth and sex; to the Cause of Death Register to identify date of death; and to the Emigration Registry to identify date of emigration. All linkages were performed using individual national identification numbers, which were replaced with serial numbers to preserve anonymity.
We calculated person-years at risk for our cohort from the date of diagnosis of first primary breast cancer to the earliest of the following: date of diagnosis of meningioma, death, emigration and end of the study period (31 December 2010). We estimated the risk of meningioma in our cohort using standardized incidence ratios (SIRs), which were calculated as the ratio of the observed to expected numbers of cases (Breslow and Day, 1987; Rothman and Greenland, 1998). The expected number of cases was calculated by multiplying the observed person-years at risk by the incidence rate of meningioma for all individuals without a diagnosis of primary breast cancer. SIRs were standardized by 5-year age group and 5-year time interval. For the SIRs, 95% confidence intervals (CIs) were calculated assuming a Poisson distribution and rounded to two decimal places (Esteve et al., 1994). To exclude surveillance bias, all meningiomas diagnosed within 1 year of follow-up after the primary breast cancer were excluded. As tamoxifen has been used widely in Sweden since the late 1980s, we defined patients with a diagnosis of breast cancer before the end of December 1987 (years 1961–1997) as not exposed to tamoxifen and those diagnosed with breast cancer in 1988–2010 as tamoxifen exposed. As this study is a nationwide study with a total of 227 535 patients with breast cancer, we have 100% power to detect an increasing prevalence of tamoxifen between the two cohorts on the basis of the assumption that the prevalence of tamoxifen was 5 and 50% before and after the year 1987, respectively.
In our exposure definition, all the patients diagnosed with breast cancer in 1988–2000 were defined as being tamoxifen exposed, which may lead to misclassification of exposure. In particular, only those breast cancer patients with ER+ could be treated with tamoxifen, and around 85% could be diagnosed with ER+ according to the published literature (Ferno et al., 2000). We thus calculated the adjusted risk ratio on the basis of the following formula:
 |
We assumed that all the patients with ER− will not be treated with tamoxifen, and they will have the same incidence as those breast cancer patients diagnosed before 1988 (SIR=1.56). All analyses were carried out using SAS, version 9.1 (SAS Institute, Cary, North Carolina, USA).
Results
In Table 1, we present the basic characteristics of Swedish women diagnosed with breast cancer from 1961 to 2000. A total of 227 535 women were diagnosed with breast cancer, with a median age at diagnosis of 63 years. Among these women, 223 developed meningioma during 1 715 497 person-years of follow-up. The number of women without tamoxifen exposure (diagnosed between 1961 and 1987) was 98 681; 128 854 women (diagnosed between 1988 and 2010) had tamoxifen exposure. A total of 145 meningiomas were found in women without tamoxifen exposure and 78 meningiomas were found in women with tamoxifen exposure.
Table 1.
Basic characteristics of Swedish women diagnosed with breast cancer in 1961–2010
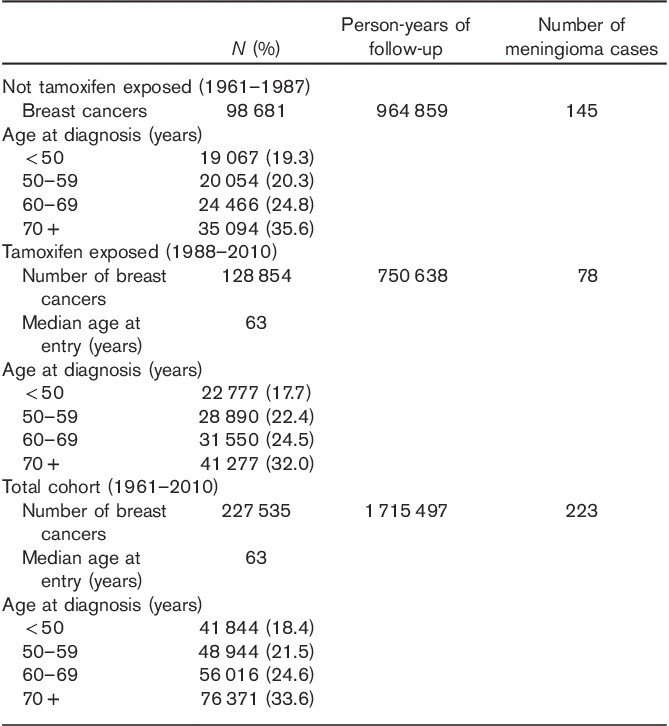
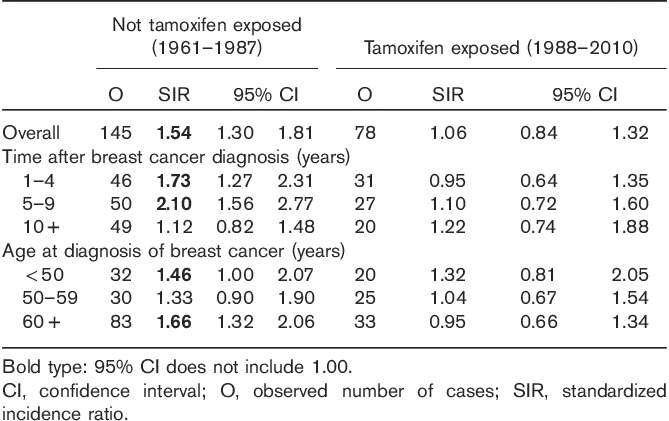
The risk of meningioma in our cohort is presented in Table 2 according to tamoxifen exposure status. For women without tamoxifen exposure, the risk of meningioma was significantly increased, with an SIR of 1.54 (95% CI 1.30–1.81). By contrast, the risk of meningioma was not increased in women with tamoxifen treatment (SIR=1.06, 95% CI 0.84–1.32). The risk of meningioma was further analysed by follow-up interval and age at diagnosis of breast cancer. For women without tamoxifen exposure, the increased risk of meningioma can persist for 10 years after the first breast cancer, and the risk was increased for women diagnosed with breast cancer before 50 years and after 59 years. In addition, we calculated the risk of meningioma among breast cancer patients diagnosed between 1988 and 2000 and those between 2001 and 2010, separately. The SIR was 1.08 (N=55, 95% CI 0.81–1.41) for those diagnosed between 1988 and 2000 and 1.01 (N=23, 95% CI 0.64–1.51) for those diagnosed between 2001 and 2010.
Table 2.
Risk of meningioma in Swedish women with a breast cancer diagnosis in 1961–2010
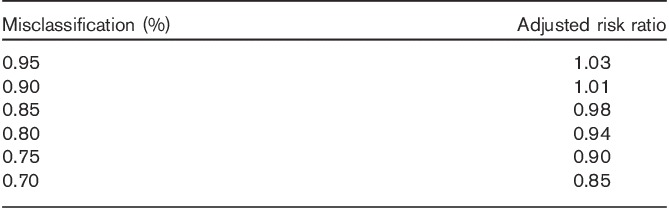
In Table 3, we present the adjusted risk ratio on the basis of the percentage of misclassification of exposure. The adjusted risk ratio decreased with an increased percentage of misclassification of exposure. The risk ratio was 1.01 when misclassification of exposure was 10%.
Table 3.
Adjusted risk ratio according to the percentage of misclassification of the exposure
Discussion
In this historical population-based cohort study, we identified a total of 227 535 women diagnosed with breast cancer during 1961–2010. The main finding is that the incidence of meningioma was significantly increased in women diagnosed with breast cancer between 1961 and 1987, when tamoxifen was not used for the treatment of breast cancer in Sweden. In addition, we found that the incidence of meningioma in women diagnosed with breast cancer during 1988–2010, when tamoxifen was used widely in Sweden, was close that in the general population, suggesting that tamoxifen may prevent the development of meningioma.
The increased risk of meningioma in women diagnosed with breast cancer before 1987 is in agreement with a few previous reports (Markopoulos et al., 1998; Malmer et al., 2000; Custer et al., 2002; Rao et al., 2009). One Swedish study found the relative risk of meningioma to be around 1.6 after the diagnosis of breast cancer (Malmer et al., 2000). Another study in the USA using the Washington State Cancer Registry found that the risk of meningioma in women with breast cancer and the risk of breast cancer in women with meningioma were increased (Custer et al., 2002). One subsequent study confirmed the observed association between meningioma and breast cancer in women, but not in men (Rao et al., 2009). The strong association between meningioma and breast cancer suggests that the two tumour types share aetiological factors. One explanation for the increased incidence of meningioma after breast cancer could be related to the radiation and chemotherapy used to treat primary cancers. However, the observed increase in the risk of meningioma in our study persisted for 10 years of follow-up. This suggests that the treatment effect may only play a small role because therapeutic effects are usually observed in the late follow-up period instead of the initial follow-up period (Ji and Hemminki, 2006). Another explanation is that the two primary cancers share genetic or environmental risk factors. However, a previous study suggests that the contribution of BCRA1 and BCRA2 mutations to the development of meningioma is minimal (Kirsch et al., 1997). On the basis of our discussion above, the strong association between breast cancer and meningiomas may be related to oestrogen, which could contribute to the development of these two types of tumour.
Women with breast cancer have been treated with tamoxifen since the late 1980s in Sweden (Rutqvist, 2004), and the prognosis has been found to be markedly improved among patients with oestrogen receptor-positive tumours. In addition, many studies have examined the effectiveness of tamoxifen in preventing breast cancer (Freedman et al., 2003; Kramer and Brown, 2004). It was found that administration of tamoxifen to women daily for 5 years can reduce their risk of developing breast cancer by about one-half (Freedman et al., 2003; Kramer and Brown, 2004). However, it is not known whether tamoxifen can prevent the development of meningioma, although oestrogen may play an important role in the development of meningioma.
In this study, we found that the incidence of meningioma in breast cancer patients diagnosed after 1987 and treated with tamoxifen was close to that in the general population. To our knowledge, no previous study has examined whether tamoxifen can inhibit the development of meningioma. Our data suggest that tamoxifen may inhibit the development of meningioma, which may be helpful for individuals at high risk of meningioma, such as those with neurofibromatosis type 2 gene (NF2) mutations: mutations in the NF2 gene may account for half of all meningioma cases (Simon et al., 2007). Two small studies explored the effect of tamoxifen treatment on meningioma, but the results were inconclusive (Markwalder et al., 1985; Goodwin et al., 1993). A study by Markwalder et al. (1985) in which six meningioma patients were treated with tamoxifen found no significant improvement in tumour growth. Another study by Goodwin et al. (1993), based on 21 patients with nonresectable refractory meningiomas, did not recommend the use of tamoxifen in refractory meningioma.
The present study has some important strengths. First, all primary breast cancers and subsequent meningiomas were identified from the Swedish Cancer Registry, which has high quality and nationwide coverage. Diagnostic bias is unlikely because the Swedish Cancer Registry is based on the compulsory clinical reports provided by physicians, pathologists, and cytologists, ensuring diagnostic accuracy at the national level. In addition, this study is a nationwide study and enough patients were included to ensure reliable risk estimates. The prospective study design and the completeness of the follow-up of patients are other major advantages of the present study.
One major limitation of this study is that data on medication with tamoxifen in our cohort at the individual level were lacking, and we defined tamoxifen exposure by year of diagnosis of breast cancer on the basis of evidence that tamoxifen has been used widely in Sweden since the late 1980s. However, such a definition may have led to nondifferential misclassification of tamoxifen exposure, thereby diluting the true observations and biasing our results towards the null. The adjusted risk ratio decreased with an increased percentage of misclassification (Table 3), providing further evidence that nondifferential misclassification will bias our results towards the null. The use of tamoxifen was infrequent in Sweden before 1990, but increased continuously during the 1990s (Kemetli et al., 2009). In 2005, the proportion of operable breast cancer patients treated with adjuvant endocrine therapy was around 60–80%, although the prevalence varied somewhat depending on the age at diagnosis (Kemetli et al., 2009). In addition, treatment with tamoxifen at the beginning lasted only for 2 years, and then increased to 5 years (Ferno et al., 2000). The increased prevalence and time for the treatment of breast cancer with tamoxifen suggest that the risk of meningioma could be varied depending on the time period. Indeed, our study found that the risk of meningioma was somewhat higher among those patients diagnosed between 1988 and 2000 compared with those between 2001 and 2010, further supporting the evidence that treatment with tamoxifen may decrease the risk of meningioma. It is known that the therapy and diagnosis of meningioma have changed during the study period, but the SIR in this study was adjusted for the time period, which can minimize the confounding effect. In addition, adjuvant chemotherapy for breast cancer has also increased during the study period (Kemetli et al., 2009), but no evidence suggests that adjuvant chemotherapy could protect against the development of meningioma. The mortality of breast cancer has decreased continuously during the past decades (Schopper and De Wolf, 2009), but the risk of meningioma could be higher among women with low mortality because of the long duration of follow-up compared with those with high mortality. Data on individual-level risk factors such as alcohol consumption, smoking and dietary factors were lacking, which may have partly confounded our conclusion. However, there is no conclusive evidence that these factors are associated with meningioma, which suggests that their effects are minimal.
Conclusion
Women diagnosed with breast cancer who were not treated with tamoxifen had an increased risk of meningioma, suggesting that the two types of tumour may share aetiological factors such as oestrogen. By contrast, the incidence of meningioma in women with breast cancer who were treated with tamoxifen was close to that in the general population, which suggests that tamoxifen may prevent the development of meningioma.
Acknowledgements
The authors wish to thank the CPF’s Science Editor, Stephen Gilliver, for his valuable comments on the text. This work was supported by grants awarded to Dr Kristina Sundquist by the Swedish Research Council (K2009-70X-15428-05-3; K2012-70X-15428-08-3) and to Dr Jan Sundquist by the Swedish Council for Working Life and Social Research (2007-1754), as well as by ALF funding from Region Skåne awarded to Jan Sundquist, Kristina Sundquist and Dr Jianguang Ji.
J.J., K.S. and J.S. were responsible for the study concept and design. J.S., K.S. and J.J. obtained funding. K.S. and J.S. acquired the data. J.J. analysed and interpreted the data. J.J. drafted the manuscript. J.J., K.S. and J.S. revised the manuscript for important intellectual content.
Conflicts of interest
There are no conflicts of interest.
References
- Al-Mubarak M, Tibau A, Templeton AJ, Cescon DW, Ocana A, Seruga B, Amir E. (2014). Extended adjuvant tamoxifen for early breast cancer: a meta-analysis. PLoS One 9:e88238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnholtz-Sloan JS, Kruchko C. (2007). Meningiomas: causes and risk factors. Neurosurgical Focus 23:E2. [DOI] [PubMed] [Google Scholar]
- Benson VS, Pirie K, Green J, Bull D, Casabonne D, Reeves GK, Beral V, Million Women Study Collaborators (2010). Hormone replacement therapy and incidence of central nervous system tumours in the Million Women Study. Int J Cancer 127:1692–1698. [DOI] [PubMed] [Google Scholar]
- Black P, Carroll R, Zhang J. (1996). The molecular biology of hormone and growth factor receptors in meningiomas. Acta Neurochir Suppl 65:50–53. [DOI] [PubMed] [Google Scholar]
- Breslow NE, Day NE. (1987). Statistical methods in cancer research. Volume 2 – The design and analysis of cohort studies. Lyon, France: International Agency for Research on Cancer. [PubMed] [Google Scholar]
- Carroll RS, Zhang J, Black PM. (1999). Expression of estrogen receptors alpha and beta in human meningiomas. J Neurooncol 42:109–116. [DOI] [PubMed] [Google Scholar]
- Carroll RS, Brown M, Zhang J, DiRenzo J, Font De Mora J, Black PM. (2000). Expression of a subset of steroid receptor cofactors is associated with progesterone receptor expression in meningiomas. Clin Cancer Res 6:3570–3575. [PubMed] [Google Scholar]
- Chandanos E, Lindblad M, Jia C, Rubio CA, Ye W, Lagergren J. (2006). Tamoxifen exposure and risk of oesophageal and gastric adenocarcinoma: a population-based cohort study of breast cancer patients in Sweden. Br J Cancer 95:118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus EB, Black PM, Bondy ML, Calvocoressi L, Schildkraut JM, Wiemels JL, Wrensch M. (2007). Exogenous hormone use and meningioma risk: what do we tell our patients? Cancer 110:471–476. [DOI] [PubMed] [Google Scholar]
- Claus EB, Park PJ, Carroll R, Chan J, Black PM. (2008). Specific genes expressed in association with progesterone receptors in meningioma. Cancer Res 68:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer BS, Koepsell TD, Mueller BA. (2002). The association between breast carcinoma and meningioma in women. Cancer 94:1626–1635. [DOI] [PubMed] [Google Scholar]
- Custer B, Longstreth WT, Jr, Phillips LE, Koepsell TD, van Belle G. (2006). Hormonal exposures and the risk of intracranial meningioma in women: a population-based case–control study. BMC Cancer 6:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ghissassi F, Baan R, Straif K, Grosse Y, Secretan B, Bouvard V, et al. (2009). A review of human carcinogens – part D: radiation. Lancet Oncol 10:751–752. [DOI] [PubMed] [Google Scholar]
- Esteve J, Benhamou E, Raymond L. (1994). Statistical methods in cancer research. Lyon, France: IARC. [PubMed] [Google Scholar]
- Ferno M, Stal O, Baldetorp B, Hatschek T, Kallstrom AC, Malmstrom P, et al. (2000). Results of two or five years of adjuvant tamoxifen correlated to steroid receptor and S-phase levels. South Sweden Breast Cancer Group, and South-East Sweden Breast Cancer Group. Breast Cancer Res Treat 59:69–76. [DOI] [PubMed] [Google Scholar]
- Fornander T, Rutqvist LE, Cedermark B, Glas U, Mattsson A, Silfverswärd C, et al. (1989). Adjuvant tamoxifen in early breast cancer: occurrence of new primary cancers. Lancet 1:117–120. [DOI] [PubMed] [Google Scholar]
- Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. (2003). Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst 95:526–532. [DOI] [PubMed] [Google Scholar]
- Goodwin JW, Crowley J, Eyre HJ, Stafford B, Jaeckle KA, Townsend JJ. (1993). A phase II evaluation of tamoxifen in unresectable or refractory meningiomas: a Southwest Oncology Group study. J Neurooncol 15:75–77. [DOI] [PubMed] [Google Scholar]
- Hemminki K, Ji J, Brandt A, Mousavi SM, Sundquist J. (2010). The Swedish Family-Cancer Database 2009: prospects for histology-specific and immigrant studies. Int J Cancer 126:2259–2267. [DOI] [PubMed] [Google Scholar]
- Hsu DW, Efird JT, Hedley-Whyte ET. (1997). Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg 86:113–120. [DOI] [PubMed] [Google Scholar]
- Ji J, Hemminki K. (2006). Second primary malignancies among patients with soft tissue tumors in Sweden. Int J Cancer 119:909–914. [DOI] [PubMed] [Google Scholar]
- Kemetli L, Rutqvist LE, Jonsson H, Nystrom L, Lenner P, Tornberg S. (2009). Temporal trends in the use of adjuvant systemic therapy in breast cancer: a population based study in Sweden 1976–2005. Acta Oncol 48:59–66. [DOI] [PubMed] [Google Scholar]
- Kirsch M, Zhu JJ, Black PM. (1997). Analysis of the BRCA1 and BRCA2 genes in sporadic meningiomas. Genes Chromosomes Cancer 20:53–59. [PubMed] [Google Scholar]
- Korhonen K, Raitanen J, Isola J, Haapasalo H, Salminen T, Auvinen A. (2010). Exogenous sex hormone use and risk of meningioma: a population-based case–control study in Finland. Cancer Causes Control 21:2149–2156. [DOI] [PubMed] [Google Scholar]
- Korhonen K, Auvinen A, Lyytinen H, Ylikorkala O, Pukkala E. (2012). A nationwide cohort study on the incidence of meningioma in women using postmenopausal hormone therapy in Finland. Am J Epidemiol 175:309–314. [DOI] [PubMed] [Google Scholar]
- Kramer R, Brown P. (2004). Should tamoxifen be used in breast cancer prevention? Drug Saf 27:979–989. [DOI] [PubMed] [Google Scholar]
- Lambe M, Coogan P, Baron J. (1997). Reproductive factors and the risk of brain tumors: a population-based study in Sweden. Int J Cancer 72:389–393. [DOI] [PubMed] [Google Scholar]
- Malmer B, Tavelin B, Henriksson R, Gronberg H. (2000). Primary brain tumours as second primary: a novel association between meningioma and colorectal cancer. Int J Cancer 85:78–81. [DOI] [PubMed] [Google Scholar]
- Markopoulos C, Sampalis F, Givalos N, Gogas H. (1998). Association of breast cancer with meningioma. Eur J Surg Oncol 24:332–334. [DOI] [PubMed] [Google Scholar]
- Markwalder TM, Seiler RW, Zava DT. (1985). Antiestrogenic therapy of meningiomas – a pilot study. Surg Neuro 24:245–249. [DOI] [PubMed] [Google Scholar]
- Nazarali SA, Narod SA. (2014). Tamoxifen for women at high risk of breast cancer. Breast Cancer 6:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Population and Housing Census 1960-1990 (2011). Statistics Sweden (SCB). Available at: http://www.scb.se/Pages/Product____7158.aspx. [Accessed 22 March 2011].
- Preston DL, Ron E, Yonehara S, Kobuke T, Fujii H, Kishikawa M, et al. (2002). Tumors of the nervous system and pituitary gland associated with atomic bomb radiation exposure. J Natl Cancer Inst 94:1555–1563. [DOI] [PubMed] [Google Scholar]
- Rao G, Giordano SH, Liu J, McCutcheon IE. (2009). The association of breast cancer and meningioma in men and women. Neurosurgery 65:483–489, discussion 489. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S. (1998). Modern epidemiology, 2nd ed Philadelphia, PA: Lippincott-Raven Publishers. [Google Scholar]
- Rutqvist LE. (2004). Adjuvant endocrine therapy. Best Pract Res Clin Endocrinol Metab 18:81–95. [DOI] [PubMed] [Google Scholar]
- Schopper D, de Wolf C. (2009). How effective are breast cancer screening programmes by mammography? Review of the current evidence. Eur J Cancer 45:1916–1923. [DOI] [PubMed] [Google Scholar]
- Simon M, Bostrom JP, Hartmann C. (2007). Molecular genetics of meningiomas: from basic research to potential clinical applications. Neurosurgery 60:787–798. Discussion 787–798. [DOI] [PubMed] [Google Scholar]
- Wiemels J, Wrensch M, Claus EB. (2010). Epidemiology and etiology of meningioma. J Neurooncol 99:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigertz A, Lonn S, Hall P, Auvinen A, Christensen HC, Johansen C, et al. (2008). Reproductive factors and risk of meningioma and glioma. Cancer Epidemiol Biomarkers Prev 17:2663–2670. [DOI] [PubMed] [Google Scholar]


