Abstract
Many food-derived phytochemical compounds and their derivatives represent a cornucopia of new anticancer compounds. Despite extensive study of luteolin, the literature has no information on the exact mechanisms or molecular targets through which it deters cancer progression. This review discusses existing data on luteolin’s anticancer activities and then offers possible explanations for and molecular targets of its cancer-preventive action. Luteolin prevents tumor development largely by inactivating several signals and transcription pathways essential for cancer cells. This review also offers insights into the molecular mechanisms and targets through which luteolin either prevents cancer or mediates cancer cell death.
Keywords: cell cycle arrest, DNA methylation, histone modification, PI3K/Akt/mTOR pathway, Wnt/β-catenin signaling
Introduction
Compounds of natural origin could lead to new, innovative therapeutic agents for cancer. Several promising new anticancer agents have been developed and used in the clinic on the basis of their selective molecular targets (Rengarajan et al., 2014). Yet, the progress of modern technology enables us to design and synthesize drug molecules for specific molecular targets. Therefore, we can shift our attention from chemically synthetic drugs to purely natural ones (Ortholand and Ganesan, 2004; Montaser and Luesch, 2011). Luteolin (3,4,5,7-tetrahydroxy flavone) is a natural flavonoid present in several plants. Vegetables and fruits rich in luteolin include carrots, broccoli, onion leaves, parsley, celery, sweet bell peppers, and chrysanthemum flowers (Miean and Mohamed, 2001; Sun et al., 2007; Chen et al., 2012b; Lim et al., 2013). Like other flavonoids, luteolin is mainly glycosylated in plants. During digestion and intestinal absorption, luteolin’s glycosylated form is mainly hydrolyzed to free luteolin (Hempel et al., 1999). However, during passage through the intestinal stroma, some luteolin can reconvert into its glycosylated form (Shimoi et al., 1998). Luteolin is a heat-stable reagent that degrades relatively little during cooking (Le Marchand, 2002). Luteolin has potent activity against cancer, inflammation, and oxidation, and it can reverse multidrug resistance (MDR) in many types of cancer cells (Park et al., 2012; Ou et al., 2013; Chen et al., 2014; Jeon et al., 2014; Khan et al., 2014). Alone or with other chemotherapeutics, luteolin can sensitize MDR cancer cells (Dellafiora et al., 2014). It can also ameliorate the cytotoxicity that various chemotherapy drugs can cause. Despite luteolin’s well-documented anticancer properties, exactly how these work remains unclear. To the best of my knowledge, no seminal review has determined the potential mechanisms of luteolin’s anticancer activities, except that published by Lin et al. (2008).
Apoptosis pathways
Apoptosis occurs through two major pathways: intrinsic and extrinsic. The intrinsic apoptosis pathway operates by modulating mitochondrial membrane potential, which releases cytochrome c and inhibits the expression of antiapoptotic proteins Bcl-2 and Bcl-xL. The extrinsic apoptosis pathway operates through activation of caspase-3, -7, -8, and -9 and enhanced expression of death receptors and their downstream factors, such as DR4, DR5, tumor necrosis factor receptor apoptosis-inducing ligand (TRAIL), and Fas/FasL (Ham et al., 2014). When the signal of apoptosis is received, Fas-associated death domain binds and recruits the death-induced signaling complex, forming initiator caspases-8 and -10 (Park et al., 2013b). Any alteration or interruption in the mitochondrial membrane could activate both intrinsic and extrinsic apoptosis pathways by triggering caspase activities; promoting imbalance of the Bax/Bcl-xL ratio; and decreasing the expression of p21, survivin, Mcl-1, and mdm2 proteins (Chang et al., 2005; Lim do et al., 2007; Chen et al., 2012a). Researchers have implicated the endoplasmic reticulum as a third subcellular compartment involved in apoptosis (Nakagawa et al., 2000; Rao et al., 2004).
In many ways, luteolin can trigger both intrinsic and extrinsic apoptosis pathways in a variety of human cancer cells (Fig. 1). In part, luteolin can arrest the cell cycle and then induce apoptosis. For instance, in the SH-SY5Y neuroblastoma tumor cell line, luteolin arrests G0/G1 cell cycle growth, accompanied by loss of mitochondrial membrane potential and apoptosis (Wang et al., 2014). Furthermore, luteolin inhibits SMMC-7721 and BEL-7402 cell proliferation by arresting the cell cycle at the G1/S phase, enhancing the level of Bax and reducing the level of antiapoptotic protein Bcl-2, leading to apoptosis (Ding et al., 2014). Luteolin can also directly induce apoptosis by activating JNK, which inhibits the translocation of tumor necrosis factor α (TNF-α)-mediating nuclear factor-κB (NF-κB) p65 to the nucleus (Cai et al., 2011). Furthermore, in human non-small-cell lung cancer A549 cells, apoptosis occurs by phosphorylating JNK and inhibiting NF-κB translocation as a transcription factor from the nucleus (Hu et al., 2012). Surprisingly, although luteolin increased Bax and caspase-3 expression and upregulated Bcl-2 expression in liver carcinoma cells, it exerted almost no effect on normal liver HL-7702 cells (Ding et al., 2014).
Fig. 1.
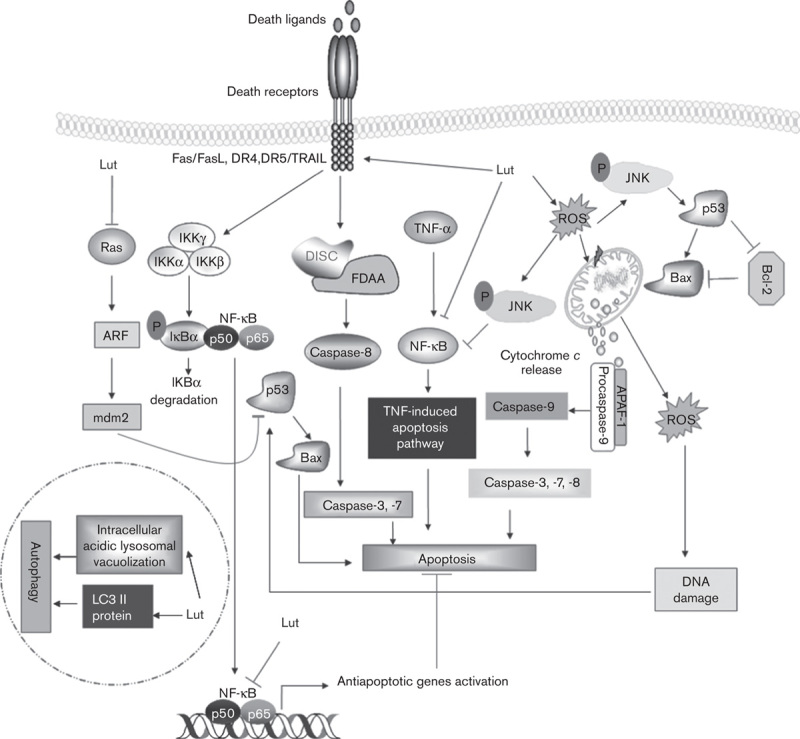
Mechanisms of luteolin (Lut)-induced apoptosis and autophagy in cancer cells. Luteolin mediates both the intrinsic and the extrinsic apoptosis pathways. Luteolin triggers the intrinsic apoptosis pathway by modulating mitochondrial membrane potential, releasing cytochrome c, and inhibiting the expression of Bcl-2 and Bcl-xL. Luteolin mediates extrinsic apoptosis by activating caspase activities; enhances expression of death receptors and their downstream factors such as Fas/FasL, DR4, DR5, and TRAIL; and suppresses other death receptor survival pathways. Luteolin also inhibits mdm2 activated by Ras; mdm2 expression triggers p53 degradation. p53, a tumor suppressor protein, mediates apoptosis by enhancing Bax levels and reducing levels of antiapoptotic protein Bcl-2. Luteolin can directly mediate apoptosis by mediating DNA damage induced by ROS. DNA damage signaling, in turn, enhances p53 production and activity. Luteolin activates JNK, which inhibits TNF-α-mediated NF-κB (p65) translocation, promoting TNF-α-induced apoptosis in cancer cells. However, luteolin can mediate autophagy as a cell death mechanism by triggering the intracellular acidic lysosomal vacuolization and accumulation of microtubule-associated LC3 II protein, which in turn enhances autophagy flux. IKK, I-κB kinase; LC3, light chain-3; NF-κB, nuclear factor-κB; ROS, reactive oxygen species; TNF-α, tumor necrosis factor α; TRAIL, tumor necrosis factor receptor apoptosis-inducing ligand.
Autophagy
Autophagy is a process of cellular self-eating activated by lysosomal activity caused by nutrient depletion. In addition to its role in maintaining cellular balance under normal physiological conditions, it is also implicated in the development of genetic diseases and drug resistance in cancer cells (Uekita et al., 2013; Gewirtz, 2014; Wang and Wu, 2014). Luteolin-induced autophagy functions as a cell death mechanism (Fig. 1) by accumulating microtubule-associated protein light chain-3 II protein, which in turn enhances autophagy flux (Park et al., 2013a). In metastatic MET4 cells, luteolin stimulated autophagy by triggering intracellular acidic lysosomal vacuolization (Verschooten et al., 2012).
Cell cycle regulation
The cell cycle, arranged in the following phases, leads to cell growth and division:
In the G1 phase, the cell grows and chromosomes prepare for replication.
In the S phase, DNA replicates and chromosomes duplicate.
The G2 phase represents the gap between DNA synthesis and mitosis.
In the M phase (mitosis), nuclear and cytoplasmic division occurs, yielding two daughter cells.
Luteolin can keep several human cancers from growing, but the precise molecular mechanisms are unclear. Figure 2 shows the molecular mechanisms underlying luteolin’s antiproliferative activities. Luteolin induces cell cycle arrest and apoptosis by decreasing the expression of AKT, PLK1, cyclin B1, cyclin A, CDC2, CDK2, Bcl-2, and Bcl-xL as well as increasing the expression of Bax, caspase-3, and p21 (Lee et al., 2012; Pandurangan et al., 2013). Luteolin also arrested colon cancer cell growth through Wnt/β-catenin/glycogen synthase kinase-3β (GSK-3β) signaling (Pandurangan et al., 2013). However, luteolin can obviously arrest the cell cycle by suppressing Akt phosphorylation, which dephosphorylates and activates GSK-3β. Activating GSK-3β enhances phosphorylation of cyclin D1 at Thr-286, followed by proteasomal degradation (Ong et al., 2010).
Fig. 2.
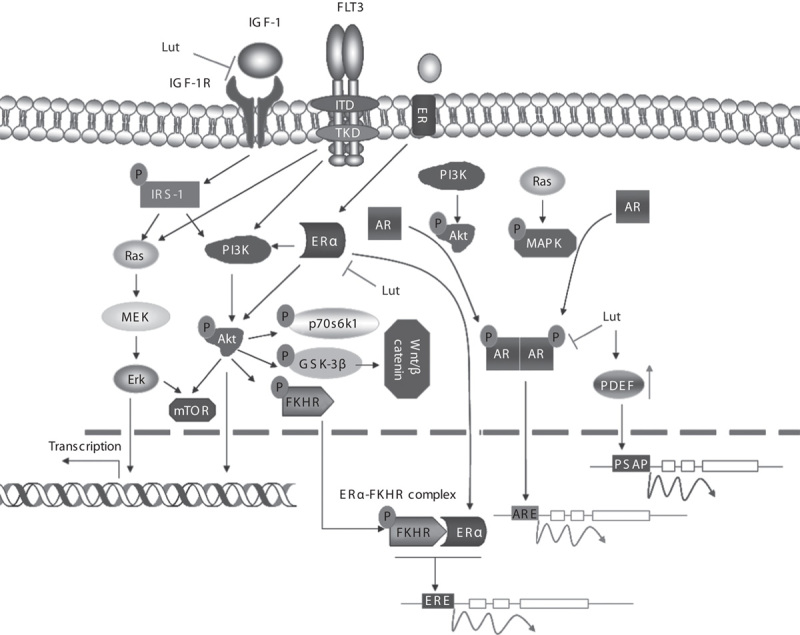
Luteolin (Lut) modulates cancer cell cycle progression. Luteolin’s antiproliferative activity is attributed to its ability to inhibit IGF-1 activation, thus preventing the phosphorylation of the intracellular IRS-1 and its downstream targets. Furthermore, luteolin inhibits IGF-1-mediated PI3K/Akt activation by reducing the expression of ERα. Estradiol receptor triggers the PI3K/AKT pathway, mediating FKHR phosphorylation, which functionally associates with ERα and forms a FKHR–ERα complex. Inhibiting AKT activity reduces phosphorylation of its downstream targets, including p70S6K1, GSK-3β, and FKHR. Luteolin suppresses prostate cancer cell proliferation by downregulating AR expression. Phosphorylation of the cytoplasmic AR by MAPK and AKT enables AR to form dimers and enhances ARE. By contrast, luteolin upregulates the expression of PDEF, which acts as an androgen-independent transcriptional activator of the prostate-specific antigen promoter. AR, androgen receptor; ARE, androgen response element; ER, estrogen receptor; Erk, extracellular signal-regulated kinase; FKHR, forkhead transcription factor; FLT3, Fms-like tyrosine kinase 3; GSK-3β, glycogen synthase kinase-3β; IGF-1, insulin-like growth factor 1; IRS-1, insulin receptor substrate 1; ITD, internal tandem duplications; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; PDEF, prostate-derived Ets factor; PSAP, prosaposin; TDK, tyrosine kinase domain.
Potential molecular targets of luteolin-mediated cell cycle arrest
Insulin-like growth factor 1 (IGF-1) is crucial in cellular growth, proliferation, and apoptosis (Katic and Kahn, 2005; Pollak, 2008). Altered IGF-1 function is implicated in tumorigenesis, metastasis, and resistance of human cancer cells (Lin et al., 2014). IGF-1 signaling begins when IGF-1 binds with its cell surface receptor, IGF-1R, forming a homodimer signaling complex, phosphorylating IGF-1R, which then phosphorylates intracellular insulin receptor substrate 1 (IRS-1) for its downstream targets (Chitnis et al., 2008; Aleksic et al., 2010). In HT-29 cells treated with luteolin, reduced IGF-1R signaling downregulated the PI3K/Akt and ERK1/2 pathways (Lim do et al., 2012). However, luteolin’s inhibitory action on IGF-1 extends beyond inhibiting IGF-1R; it can also inhibit Akt signaling (Fang et al., 2007). Inhibition of Akt signaling in turn dephosphorylates its downstream targets, including p70S6K1, GSK-3β, and FKHR/FKHRL1 (forkhead human transcription factor like 1). Moreover, in estrogen receptor (ER)-positive tumors and cell lines, IGF signaling can also cooperate with the ER to promote tumor growth and progression, while hindering the efforts of endocrine therapy (Zhang et al., 2011; Mancini et al., 2014). Targeting ERα is a possible mechanism of luteolin’s antiproliferative effect (Wang et al., 2012a). Using an ERα-specific small interfering RNA to knock down ERα in MCF-7 cells reduced luteolin’s ability to inhibit the growth of MCF-7 cells. This finding suggests that luteolin’s inhibitory effect on cancer cell growth may inhibit the IGF-1-mediated PI3K/Akt pathway depending on ERα expression. Thus, the downregulation of the PI3K/Akt and mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathways through luteolin’s reduction of IGF-1R/ERα signaling pathways may offer promising routes for cancer therapeutic agents.
Fms-like tyrosine kinase 3 (FLT3) is another potential means by which luteolin arrests the cell cycle. In one study, FLT3 was highly overexpressed in most patients with acute myeloid leukemia (Chin et al., 2013). Luteolin suppressed cell proliferation in MV4-11 cells with constitutively activated FLT3, suggesting that luteolin may be a potent FLT3 enzyme inhibitor.
Downregulated androgen receptor expression could be a main mechanism through which luteolin mediates its antiproliferative and anti-invasive effects in LNCaP human prostate cancer cells (Chiu and Lin, 2008). By contrast, luteolin upregulates the expression of prostate-derived Ets factor (PDEF) in LNCaP cells, which acts as an androgen-independent transcriptional activator of the prostate-specific antigen promoter (Tsui et al., 2012).
Molecular targets of luteolin-induced apoptosis
Nuclear factor-κB-induced and tumor necrosis factor α-induced apoptosis pathway
NF-κB is synthesized in the cytoplasm and complexed with its inhibitor I-κB; thus, NF-κB is released as an inactive form. To activate, I-κB must undergo phosphorylation, followed by proteasomal degradation of the NF-κB–p-κB complex. The free p-NF-κB then translocates to the nucleus to transcribe and activate genes to synthesize progrowth and antiapoptosis proteins (Lun et al., 2005). NF-κB is a heterodimer composed of two subunits: the DNA-binding subunit p50 and the transactivator p65. Phosphorylation of IκBα is mediated by the I-κB kinase (IKK) complex, which consists of NF-κB essential modulators IKKγ, IKKα, and IKKβ, degrading IκBα through a ubiquitin/proteasomal process (Thomas et al., 2009). Degrading IκBα allows insertion of NF-κB’s two subunits into the nucleus to transcribe and activate target genes.
The NF-κB transcription factor plays a major role in the development and progression of various cancers (Erez et al., 2013; Wu et al., 2013; Kagoya et al., 2014). In many cancers, TNF-α is one of the most important activators for NF-κB and plays a paramount role in activating pathways for both cancer cell death and survival. On the one hand, TNF-α’s activation of NF-κB abolishes TNF-induced cancer cell apoptosis, which plays a marginal role in the development of resistance in cancer cells. On the other, blocking NF-κB enhances TNF-α’s anticancer activity (Ju et al., 2007). Luteolin can suppress NF-κB, thus activating TNF-α-induced apoptosis (Fig. 3). A possible mechanism for this process is through its ability to mediate the release of reactive oxygen species, which suppresses NF-κB and activates JNK, stimulating cancer cells to undergo TNF-α-induced apoptosis (Ju et al., 2007). Hwang et al. (2011) suggested AMPK as a novel regulator of NF-κB in luteolin-induced cancer cell death (Hwang et al., 2011), as inhibiting AMPK activity restored luteolin-inhibited NF-κB DNA-binding activity.
Fig. 3.
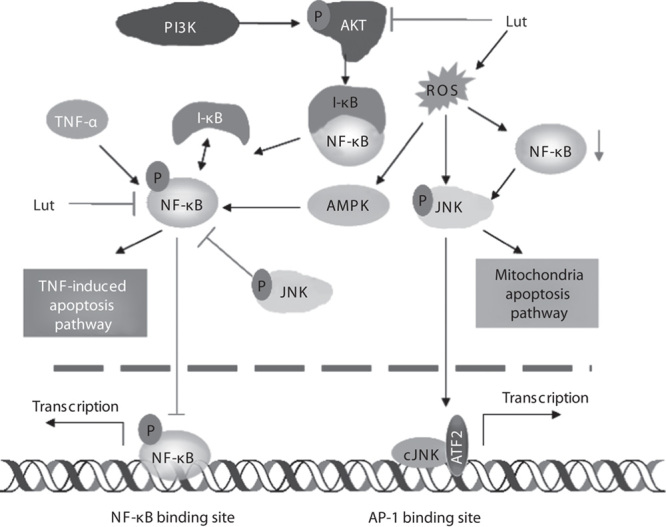
Mechanism of luteolin (Lut)-triggered TNF-α-induced cancer cell apoptosis. Free p-NF-κB translocates to the nucleus to mediate the transcriptional activation of genes. Luteolin can suppress the activity of NF-κB translocation, activating the TNF-α-induced apoptosis pathway. The generation of ROS caused by treatment with luteolin plays a marginal role in suppressing NF-κB, further enforcing JNK activation. ROS activate the AMPK signaling pathway, which interacts with the NF-κB pathway, thereby inhibiting NF-κB DNA-binding activity. Activating JNK activates the mitochondrial apoptosis pathway. Furthermore, luteolin’s inhibition of NF-κB activity augments and prolongs cJNK activation induced by TNF-α. AMPK, AMP-activated protein kinase; AP-1, activating protein 1; ATF2, activating transcription factor 2; JNK/cJNK, c-Jun N-terminal kinase; NF-κB, nuclear factor-κB; ROS, reactive oxygen species; TNF-α, tumor necrosis factor α.
Reactive oxygen species generation caused by luteolin treatment is the major mechanism through which luteolin activates AMPK (Hwang et al., 2011). However, luteolin can obviously induce apoptosis in human non-small-cell lung cancer A549 cells by phosphorylating JNK, activating the mitochondrial pathways of apoptosis while inhibiting NF-κB translocation (Hu et al., 2012). Furthermore, luteolin’s inhibition of NF-κB augmented and prolonged TNF-α-induced cJNK activation (Shi et al., 2004). Taken together, these findings indicate that luteolin’s sensitization of TNF-α-induced cancer cell death may encompass many cancer types. Interestingly, inhibiting NF-κB’s transcription activity also downregulated the expression of vascular endothelial growth factor (VEGF) mRNA, inhibiting VEGF secretion in pancreatic carcinoma cells (Cai et al., 2012). This finding suggested that luteolin had potent antiangiogenesis activity.
Tumor necrosis factor receptor apoptosis-inducing ligand
TRAIL is an endogenous protein belonging to the TNF family. TRAIL induces apoptosis in a wide variety of transformed and cancer cells, but has little or no effect on normal cells (Rushworth and Micheau, 2009). Luteolin can sensitize TRAIL-induced apoptosis in both TRAIL-sensitive cancer cells, including HeLa (Horinaka et al., 2005; Shi et al., 2005; Yan et al., 2012) and human 786-O renal cell carcinoma (Ou et al., 2013), and TRAIL-resistant cancer cells (CNE1, HT-29, and HepG2) (Shi et al., 2005). Luteolin is also a potential sensitizer of TRAIL in anticancer therapy against human renal cell carcinoma involving Akt and STAT3 inactivation (Ou et al., 2014). However, the Janus tyrosine kinases (Jak1) and tyrosine kinase 2 (Tyk2) mediate most, if not all, cellular responses to peptide hormones, cytokines, and interferons (IFNs) and are often hyperactivated in tumors (Muller et al., 2014). In fact, neither Jak1 nor Tyk2 has serine activities (Carbone and Fuchs, 2014); thus, they must undergo phosphorylation before they can act. Luteolin can sensitize the antiproliferative effect of IFN by enhancing phosphorylation of Jak1 and Tyk2, thus ensuring the activation of STAT1/2, which promotes STAT1 accumulation in the nucleus and endogenous IFN-α-regulated gene expression (Tai et al., 2014). Treatment with TRAIL and luteolin markedly reduced the growth of xenograft tumors in animals (Yan et al., 2012). Therefore, luteolin’s potent activity to sensitize both TRAIL-sensitive and TRAIL-resistant cancer cells may represent another dimension for the development of new techniques enabling us to conjugate luteolin or use it as a juvenile agent with other anticancer drugs.
Modulation of Wnt/β-catenin signaling
Wnt/β-catenin signaling regulates the proliferation and differentiation of many normal and malignant cells (Abdel-Magid, 2014; Draganova et al., 2015; Zhao and Carrasco, 2014). Luteolin’s antiproliferative effect on cancer may be attributed to its inhibitory effect on Wnt/β-catenin signaling. For instance, luteolin decreases the expression of Wnt/β-catenin/GSK-3β signaling, arresting the growth of colon cancer cells (Pandurangan et al., 2013). Wnt/β-catenin/GSK-3β signaling is also involved in luteolin-prevented azoxymethane-induced cellular proliferation (Pandurangan et al., 2014).
Topoisomerases
Topoisomerases, especially DNA topoisomerases, are among the most desired targets for chemotherapy drugs. Topoisomerase inhibition might correlate with the antioxidant capacity of the flavonoids (Topcu et al., 2008). Chowdhury et al. (2002) published the first report on luteolin functionally inhibiting the catalytic activity of topoisomerase. The second report was by Wu and Fang (2010), speculating that luteolin has chymotrypsin-like and trypsin-like catalytic activities in tumor cells. In a canine tumor cell line (DH82), luteolin was highly cytotoxic without causing considerable DNA damage (Silva et al., 2013). However, no studies have examined luteolin’s ability to modulate topoisomerases in human cancer cells. Further studies are needed.
Heat shock protein 90
Heat shock protein 90 (Hsp90) stabilizes newly synthesized proteins and helps maintain the functional competency of several signaling transducers involved in cell growth, survival, and oncogenesis. Therefore, interest grows in Hsp90 as an important target for molecular cancer therapy (Zhang et al., 2005; Beck et al., 2009). In the past few years, many specific inhibitors for Hsp90 have been developed, such as geldanamycin (GA) and its derivatives. However, GA is not used clinically because of serious toxic effects in the liver and kidney (Wang et al., 2006). Despite its effectiveness in clinical trials for cancer, 17-AAG (17-allylamino-17-demethoxygeldanamycin), a GA derivative, has several problems, including stability, solubility, and hepatotoxicity. Luteolin can block Hsp90 by inhibiting its association with STAT3 (Fu et al., 2012). This action degrades phosphor-STAT3 (Tyr-705) and phosphor-STAT3 (Ser-727)-phosphorylated STAT3 through a proteasome-dependent pathway. Hsp90 is one of the most important regulators of the Akt signaling pathway (Zhang et al., 2005; Beck et al., 2009). Surprisingly, a recent study presented protein phosphatase 2A (PP2A) as an alternative target for luteolin (Ou et al., 2013). This study suggests that PP2A activation may work with Hsp90 cleavage to inactivate Akt and lead to a vicious caspase-dependent apoptotic cycle.
Stabilization of tumor suppressor protein p53
The tumor suppressor protein p53, a transcription factor, controls the cell cycle (and arrests it in case of DNA damage). Inhibition of tumor growth through cell cycle arrest and induction of apoptosis are functionally related to p53 (Kobayashi et al., 2002; Didelot et al., 2003). Luteolin could mediate p53 stabilization and accumulation, which induces apoptosis and prevents cell proliferation in many cancer cell lines, including breast cancer (Momtazi-Borojeni et al., 2013), Eca109 (Wang et al., 2012b), gastric cancer AGS (Wu et al., 2008), HT-29 colon cancer (Lim do et al., 2007), and head and neck and lung cancer (Amin et al., 2010). In two human colorectal carcinoma-derived cell lines with microsatellite instability – CO115 with wild-type p53 and HCT15 harboring a p53 mutation – luteolin enhanced p53 expression (Xavier et al., 2011). In an in-vivo nude mouse xenograft model, luteolin enhanced cisplatin’s anticancer activity by promoting p53 stabilization and accumulation (Shi et al., 2007). Also, luteolin ameliorates cisplatin’s nephrotoxicity by downregulating the p53-dependent apoptotic pathway in the kidney (Kang et al., 2011).
Mammalian target of rapamycin signaling
Mammalian target of rapamycin (mTOR), a key regulator of various cellular activities, belongs to the family of PI3K-related kinases and is one of the most commonly activated signaling pathways in human cancer (Faivre et al., 2006). Chiang et al. (2007) showed that luteolin inhibited cell proliferation and mediated apoptosis in HER2-overexpressing cancer cells. Also, in nude mice with xenografted SKOV3.ip1-induced tumors, luteolin inhibited HER2 expression and tumor growth. In that study, but only at low doses, luteolin upregulated the expression of p21 and transiently inhibited mTOR signaling. That finding suggests luteolin’s inability to cause sustained Akt/mTOR inhibition, which may contribute to the p21 induction that may confer a survival advantage on HER2-overexpressing cancer cells (Fig. 4). Therefore, suppressing p21 expression along with mTOR inhibition may be a good way to improve anticancer drugs against HER2-overexpressing tumors.
Fig. 4.
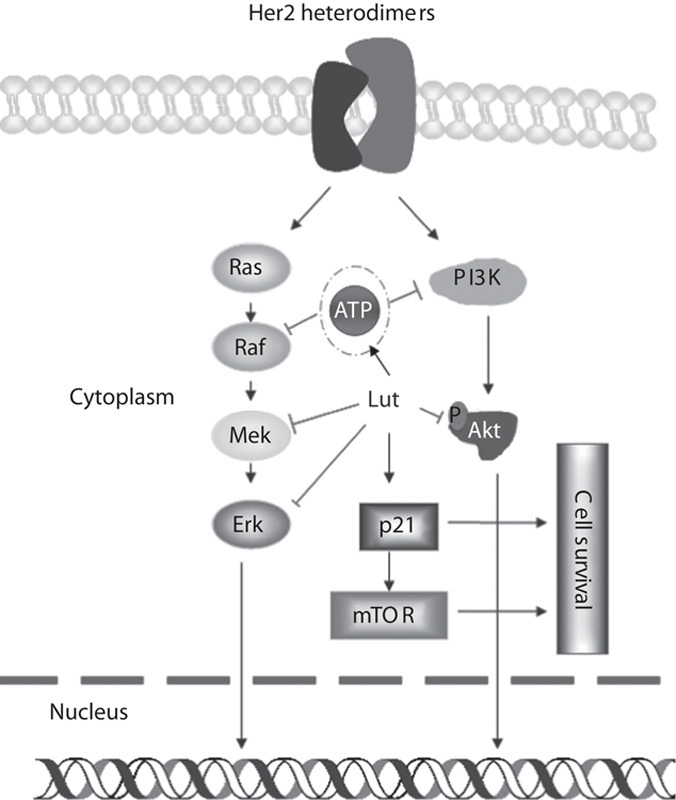
Luteolin (Lut) modulates the Raf/PI3K signaling pathway in KRAS and BRAF mutated cancer cells and HER2-overexpressing cancer cells. Luteolin inhibits Ras’s downstream client proteins and PI3K signaling pathways. Luteolin noncompetitively binds with ATP to abolish Raf activity and competitively binds with ATP to inhibit PI3K activity. However, p21 induction by luteolin could confer on cancer cells a survival advantage by activating mTOR signaling. mTOR, mammalian target of rapamycin.
Raf and PI3K
KRAS and BRAF mutations are common in colorectal carcinoma and can activate proliferation and survival through MAPK/ERK and/or PI3K signaling pathways. In KRAS-mutated HCT15 cells, luteolin decreased ERK phosphorylation, whereas it had no effect on phospho-ERK in BRAF-mutated CO115 cells. This finding suggests that luteolin’s antiproliferative and apoptotic effects can be attributed to its activity on KRAS and PI3K, but not on BRAF (Xavier et al., 2009). In another study, luteolin inhibited Raf and PI3K activities and attenuated phosphorylation of MEK and Akt (Kim et al., 2013). The potential mechanism for this event is that luteolin noncompetitively binds with ATP to suppress Raf activity and competitively binds with ATP to inhibit PI3K activity (Fig. 4).
Preventing tumor invasion and metastasis
Metastasis is the major cause of death from cancer (Weng and Yen, 2012; Lin et al., 2013). In metastasis, cancer cells migrate from the primary tumor to other sites, forming secondary tumors. Several reports showed that flavonoids naturally inhibit cancer invasion and metastasis. As discussed above, studies have confirmed luteolin’s antiproliferative activities in many cancer cell lines, but how it affects invasion by cancer cells remains unclear. Figure 5 shows the possible molecular targets whereby luteolin inhibits the invasion of cancer cells.
Fig. 5.
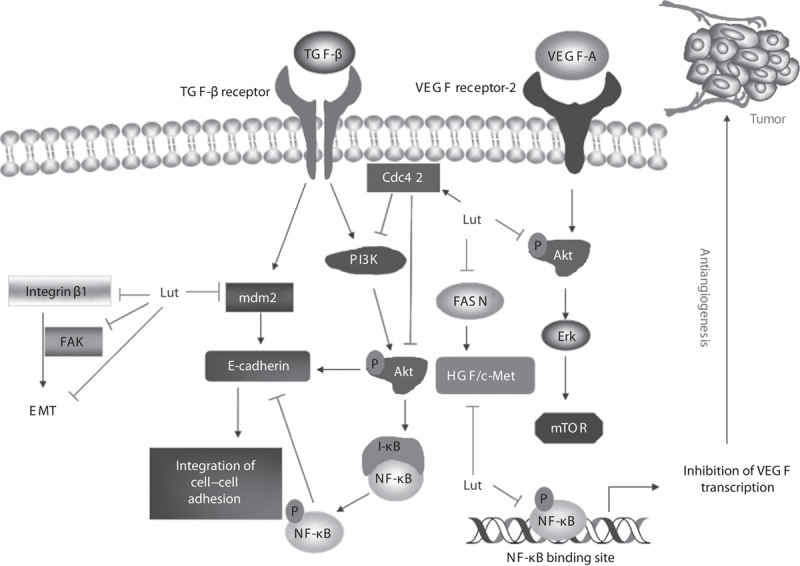
Possible molecular targets whereby luteolin (Lut) inhibits the invasion of cancer cells. Luteolin inhibits hypoxia-induced EMT, at least in part, by inhibiting the expression of integrin β1 and FAK. Luteolin prevents cancer cell migration by activating the modulator protein of cell division, Cdc42, which modulates PI3K/AKT activity by facilitating its degradation through the proteasomal pathway. Luteolin acts as a novel HGF/c-Met inhibitor by suppressing phosphorylation of c-Met tyrosine kinase, induced by HGF, thereby inhibiting cancer cell invasion. Because it can reduce AKT phosphorylation, luteolin mediates inhibition of mdm2, upregulating E-cadherin. Of note, downregulation of E-cadherin results in the loss of cell–cell adhesion. Luteolin could interfere in the PI3K–Akt–NF-κB–Snail pathway, thus attenuating TGF-β1-induced EMT of cancer cells. Luteolin reduces the expression of VEGF mRNA by inhibiting NF-κB transcription activity, thus inhibiting VEGF secretion. Furthermore, luteolin suppresses VEGF-A-induced phosphorylation of VEGF receptor 2 and their downstream protein kinases AKT, ERK, and mTOR, thus reducing cell viability and possibly leading to apoptosis. Cdc42, cycle 42; EMT, epithelial–mesenchymal transition; ERK, extracellular signal-regulated kinase; FAK, focal adhesion kinase; FASN, fatty acid synthesis; HGF, hepatocyte growth factor; ; mdm2, mouse double minute 2 homolog; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; TGF-1, transforming growth factor-1; VEGF, vascular endothelial growth factor.
Integrin β1 and focal adhesion kinase
Hypoxia-induced epithelial–mesenchymal transition (EMT) is an essential step in cancer metastasis. Luteolin inhibits the expression of integrin β1 and focal adhesion kinase (FAK), which are closely related to EMT formation. This relationship suggests that luteolin inhibits hypoxia-induced EMT, at least in part, by inhibiting the expression of integrin β1 and FAK (Ruan et al., 2012a). Luteolin also inhibits EMT in malignant melanoma cells both in vitro and in vivo by regulating β3 integrin (Ruan et al., 2012b). Taken together, these findings show luteolin’s potential as an anticancer chemopreventive and chemotherapeutic agent to prevent EMT.
Cycle 42
A recent study showed that luteolin prevents the migration of glioblastoma cells by affecting PI3K/AKT activation, modulating the expression of cell division protein cycle 42 (Cdc42), and facilitating its degradation by the proteasome pathway (Cheng et al., 2013). This finding suggests that pharmacological inhibition of migration by luteolin is likely to preferentially facilitate the degradation of Cdc42. Understanding Cdc42’s function and degradation by specific inhibitors adds another dimension for the development of potent therapeutic modalities in the context of invasion and metastasis and may be useful for cancer patients.
Fatty acid synthesis
Fatty acid synthesis is now associated with clinically aggressive tumor behavior and tumor cell growth and has become a novel target pathway for chemotherapy development (Cheng et al., 2014; Hamada et al., 2014). Coleman et al. (2009) reported a novel connection between fatty acid synthesis activity and c-Met protein expression, suggesting that luteolin could act as a novel hepatocyte growth factor (HGF)/c-Met inhibitor by reducing the expression of this receptor. However, adding palmitate prevented luteolin from suppressing c-Met protein expression.
c-Met tyrosine kinase
c-Met tyrosine kinase plays paramount roles in cancer invasion and metastasis in many types of cancer cells. c-Met tyrosine kinase acts as a membrane receptor for HGF. Aberrant activation of the HGF/MET signaling is strongly implicated in the malignant transformation and progression of many tumors which are characterized by an aggressive metastatic phenotype and a poor prognosis (Hack et al., 2014; Lee et al., 2014; Vigna and Comoglio, 2014). Luteolin acts as a novel HGF/c-Met inhibitor by suppressing phosphorylation of c-Met tyrosine kinase. Luteolin thus inhibits HGF-induced cell invasion in human DU145 prostate and hepatoma HepG2 cancer cells (Lee et al., 2006; Coleman et al., 2009). Luteolin’s inhibition of HGF/MET signaling represents a validated and effective therapeutic tool in the battle against cancer.
E-cadherin
E-cadherin, a marker of epithelial cells, maintains cell–cell adhesion. Decreased expression of E-cadherin thus leads to a prominent increase of cell invasion (Borchers et al., 1997; Soncin et al., 2009; Chen et al., 2010; Lin et al., 2011).
Luteolin prevents the invasion of prostate cancer PC3 cells by inhibiting mdm2 expression and inducing E-cadherin expression (Zhou et al., 2009). Moreover, pretreatment of A549 lung cancer cells with luteolin prevented TGF-β1 from downregulating E-cadherin, maintained normal morphological appearance, and prevented EMT of lung cancer cells (Chen et al., 2013). Furthermore, TGF-β1’s activation of the PI3K–Akt–IκBα–NF-κB–Snail pathway reduced the activity of E-cadherin, which pretreatment with luteolin prevented. This finding suggests that luteolin could be involved as a juvenile agent with chemotherapeutics to prevent EMT of a wide spectrum of cancer cells.
Angiogenesis
Angiogenesis, the formation of new blood vessels from existing vascular beds, plays a marginal role in tumor growth, invasion, and metastasis. Luteolin exerted strong antiangiogenesis activity in chick chorioallantoic membrane and anti-invasive activity on breast cancer cells. It also downregulates the expression of astrocyte elevated gene 1 (AEG-1), a novel oncoprotein, and matrix metalloproteinase-2 (MMP-2) (Jiang et al., 2013). Luteolin can inhibit the in-vivo growth of gastric tumors; this mechanism may correlate with downregulated expression of VEGF-A and MMP-9 (Lu et al., 2013). In prostate cancer cells, luteolin suppressed VEGF-A-induced phosphorylation of VEGF receptor 2 and their downstream protein kinases AKT, ERK, and mTOR, reducing cell viability, followed by induction of apoptosis (Pratheeshkumar et al., 2012). Alternatively, luteolin can reduce the expression of VEGF mRNA by inhibiting NF-κB transcription activity, inhibiting VEGF secretion in pancreatic carcinoma cells (Cai et al., 2012).
Luteolin with other anticancer drugs
MDR is an obstacle in cancer treatment, often because less drug accumulates in tumor cells owing to enhanced drug efflux (Limtrakul et al., 2004). In oxaliplatin-resistant cell lines, luteolin inhibited the Nrf2 pathway and reversed MDR (Chian et al., 2014a). Furthermore, in non-small-cell lung cancer, luteolin inhibits the Nrf2 pathway in vivo and can serve as an adjuvant in chemotherapy (Chian et al., 2014b). Pretreatment of BxPC-3 human pancreatic cancer with luteolin, followed by gemcitabine inhibited protein expression of nuclear GSK-3β and NF-κB p65, was accompanied by increased proapoptotic cytosolic cytochrome c (Johnson and Gonzalez de Mejia, 2013). Coadministration of luteolin and paclitaxel activated caspase-8 and -3 and increased expression of Fas by blocking STAT3 (Yang et al., 2014). In an in-vivo nude mouse xenograft model, luteolin enhanced p53 accumulation, reinforcing cisplatin’s therapeutic activity (Shi et al., 2007). Surprisingly, luteolin prevented cisplatin from causing nephrotoxicity by downregulating the p53-dependent apoptotic pathway in the kidney (Kang et al., 2011). Finally, luteolin may act against metastasis because it can suppress the production of MMP-9 and MMP-2 and upregulate TIMP2 gene expression (Pandurangan et al., 2014). Taken together, these findings show that luteolin can serve as an adjuvant – not only to enhance the potency of chemotherapeutics but also to reduce their cytotoxicity.
Epigenetic regulation
In recent years, researchers have extensively documented that epigenetic mechanisms such as DNA methylation and histone modification regulate activities of many cancer cells (Mirza et al., 2013; Yu et al., 2013; Farkas et al., 2014). Therefore, epigenetic regulation is an attractive target for cancer therapeutics (Ptak and Petronis, 2008). In fact, the human genome has four DNA methyltransferase genes (DNMT), encoding proteins with distinct functions (Mirza et al., 2013). However, histone tails (and their modifications) regulate diverse biological processes such as transcription, DNA repair, cell division, and differentiation (Van Attikum and Gasser, 2005; Duncan et al., 2008). Unfortunately, the literature offers no precise information on the epigenetic regulation of luteolin in cancer cells. In a study on the HeLa cell line, luteolin-induced E3 ubiquitin-protein ligase UHRF1 and DNMT1 downregulation was accompanied by global DNA hypomethylation (Krifa et al., 2013). Attoub et al. (2011) first presented luteolin as a potent histone deacetylase (HDAC) inhibitor that enhances cisplatin cytotoxicity in LNM35 cells and reduces the growth of LNM35 tumor xenografts in athymic mice (Attoub et al., 2011). However, an urgent need remains to study epigenetic regulation of luteolin in different cancer cell lines. By taking advantage of epigenetic modifications, we can use HDAC and DNMT inhibitors to control various cancer cell activities. Moreover, luteolin may be a promising HDAC inhibitor for cancer treatment. The US Food and Drug Administration has already approved some HDAC and DNMT inhibitors, such as azanucleoside drugs, to treat myelodysplastic syndromes and acute myeloid leukemia (Garcia-Manero and Fenaux, 2011; Yu et al., 2013).
Conclusion
Luteolin is a potent anticancer agent that could halt a wide spectrum of tumors and cancer cells, including MDR cells. Preclinical and clinical trials using luteolin as an adjuvant supplement for cancer therapy should place this fascinating agent at the forefront of new therapeutic approaches and then translate this study’s concepts into clinical applications.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- Abdel-Magid AF. (2014). Wnt/beta-catenin signaling pathway inhibitors: a promising cancer therapy. ACS Med Chem Lett 5:956–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksic T, Chitnis MM, Perestenko OV, Gao S, Thomas PH, Turner GD, et al. (2010). Type 1 insulin-like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Res 70:6412–6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin AR, Wang D, Zhang H, Peng S, Shin HJ, Brandes JC, et al. (2010). Enhanced anti-tumor activity by the combination of the natural compounds (−)-epigallocatechin-3-gallate and luteolin: potential role of p53. J Biol Chem 285:34557–34565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attoub S, Hassan AH, Vanhoecke B, Iratni R, Takahashi T, Gaben AM, et al. (2011). Inhibition of cell survival, invasion, tumor growth and histone deacetylase activity by the dietary flavonoid luteolin in human epithelioid cancer cells. Eur J Pharmacol 651:18–25. [DOI] [PubMed] [Google Scholar]
- Beck R, Verrax J, Gonze T, Zappone M, Pedrosa RC, Taper H, et al. (2009). Hsp90 cleavage by an oxidative stress leads to its client proteins degradation and cancer cell death. Biochem Pharmacol 77:375–383. [DOI] [PubMed] [Google Scholar]
- Borchers AH, Sanders LA, Bowden GT. (1997). Regulation of matrilysin expression in cells of squamous cell carcinoma by E-cadherin-mediated cell–cell contact. J Cancer Res Clin Oncol 123:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Ye T, Liu C, Lu W, Lu M, Zhang J, et al. (2011). Luteolin induced G2 phase cell cycle arrest and apoptosis on non-small cell lung cancer cells. Toxicol In Vitro 25:1385–1391. [DOI] [PubMed] [Google Scholar]
- Cai X, Lu W, Ye T, Lu M, Wang J, Huo J, et al. (2012). The molecular mechanism of luteolin-induced apoptosis is potentially related to inhibition of angiogenesis in human pancreatic carcinoma cells. Oncol Rep 28:1353–1361. [DOI] [PubMed] [Google Scholar]
- Carbone CJ, Fuchs SY. (2014). Eliminative signaling by Janus kinases: role in the downregulation of associated receptors. J Cell Biochem 115:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Hsu Y, Kuo P, Kuo Y, Chiang L, Lin C. (2005). Increase of Bax/Bcl-XL ratio and arrest of cell cycle by luteolin in immortalized human hepatoma cell line. Life Sci 76:1883–1893. [DOI] [PubMed] [Google Scholar]
- Cheng WY, Chiao MT, Liang YJ, Yang YC, Shen CC, Yang CY. (2013). Luteolin inhibits migration of human glioblastoma U-87 MG and T98G cells through downregulation of Cdc42 expression and PI3K/AKT activity. Mol Biol Rep 40:5315–5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CS, Wang Z, Chen J. (2014). Targeting FASN in breast cancer and the discovery of promising inhibitors from natural products derived from traditional chinese medicine. Evid Based Complement Alternat Med 2014:232946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Yuan D, Wei B, Jiang J, Kang J, Ling K, et al. (2010). E-cadherin-mediated cell–cell contact is critical for induced pluripotent stem cell generation. Stem Cells 28:1315–1325. [DOI] [PubMed] [Google Scholar]
- Chen Q, Liu S, Chen J, Zhang Q, Lin S, Chen Z, Jiang J. (2012a). Luteolin induces mitochondria-dependent apoptosis in human lung adenocarcinoma cell. Nat Prod Commun 7:29–32. [PubMed] [Google Scholar]
- Chen Z, Kong S, Song F, Li L, Jiang H. (2012b). Pharmacokinetic study of luteolin, apigenin, chrysoeriol and diosmetin after oral administration of Flos Chrysanthemi extract in rats. Fitoterapia 83:1616–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KC, Chen CY, Lin CJ, Yang TY, Chen TH, Wu LC, Wu CC. (2013). Luteolin attenuates TGF-beta1-induced epithelial–mesenchymal transition of lung cancer cells by interfering in the PI3K/Akt–NF-kappaB–Snail pathway. Life Sci 93:924–933. [DOI] [PubMed] [Google Scholar]
- Chen R, Hollborn M, Grosche A, Reichenbach A, Wiedemann P, Bringmann A, Kohen L. (2014). Effects of the vegetable polyphenols epigallocatechin-3-gallate, luteolin, apigenin, myricetin, quercetin, and cyanidin in primary cultures of human retinal pigment epithelial cells. Mol Vis 20: , 242–258. [PMC free article] [PubMed] [Google Scholar]
- Chian S, Li YY, Wang XJ, Tang XW. (2014a). Luteolin sensitizes two oxaliplatin-resistant colorectal cancer cell lines to chemotherapeutic drugs via inhibition of the Nrf2 pathway. Asian Pac J Cancer Prev 15:2911–2916. [DOI] [PubMed] [Google Scholar]
- Chian S, Thapa R, Chi Z, Wang XJ, Tang X. (2014b). Luteolin inhibits the Nrf2 signaling pathway and tumor growth in vivo. Biochem Biophys Res Commun 447:602–608. [DOI] [PubMed] [Google Scholar]
- Chiang CT, Way TD, Lin JK. (2007). Sensitizing HER2-overexpressing cancer cells to luteolin-induced apoptosis through suppressing p21(WAF1/CIP1) expression with rapamycin. Mol Cancer Ther 6:2127–2138. [DOI] [PubMed] [Google Scholar]
- Chin YW, Kong JY, Han SY. (2013). Flavonoids as receptor tyrosine kinase FLT3 inhibitors. Bioorg Med Chem Lett 23:1768–1770. [DOI] [PubMed] [Google Scholar]
- Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM. (2008). The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res 14:6364–6370. [DOI] [PubMed] [Google Scholar]
- Chiu FL, Lin JK. (2008). Downregulation of androgen receptor expression by luteolin causes inhibition of cell proliferation and induction of apoptosis in human prostate cancer cells and xenografts. Prostate 68:61–71. [DOI] [PubMed] [Google Scholar]
- Chowdhury AR, Sharma S, Mandal S, Goswami A, Mukhopadhyay S, Majumder HK. (2002). Luteolin, an emerging anti-cancer flavonoid, poisons eukaryotic DNA topoisomerase I. Biochem J 366:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman DT, Bigelow R, Cardelli JA. (2009). Inhibition of fatty acid synthase by luteolin post-transcriptionally down-regulates c-Met expression independent of proteosomal/lysosomal degradation. Mol Cancer Ther 8:214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellafiora L, Mena P, Del Rio D, Cozzini P. (2014). Modelling the effect of phase II conjugations on topoisomerase I poisoning: pilot study with luteolin and quercetin. J Agric Food Chem 62:5881–5886. [DOI] [PubMed] [Google Scholar]
- Didelot C, Mirjolet JF, Barberi-Heyob M, Ramacci C, Teiten MH, Merlin JL. (2003). Oncoprotein expression of E6 and E7 does not prevent 5-fluorouracil (5FU) mediated G1/S arrest and apoptosis in 5FU resistant carcinoma cell lines. Int J Oncol 23:81–87. [DOI] [PubMed] [Google Scholar]
- Ding S, Hu A, Hu Y, Ma J, Weng P, Dai J. (2014). Anti-hepatoma cells function of luteolin through inducing apoptosis and cell cycle arrest. Tumour Biol 35:3053–3060. [DOI] [PubMed] [Google Scholar]
- Draganova K, Zemke M, Zurkirchen L, Valenta T, Cantu C, Okoniewski M, et al. (2015). Wnt/beta-catenin signaling regulates sequential fate decisions of murine cortical precursor cells. Stem Cells 33:170–182. [DOI] [PubMed] [Google Scholar]
- Duncan EM, Muratore-Schroeder TL, Cook RG, Garcia BA, Shabanowitz J, Hunt DF, Allis CD. (2008). Cathepsin L proteolytically processes histone H3 during mouse embryonic stem cell differentiation. Cell 135:284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez N, Glanz S, Raz Y, Avivi C, Barshack I. (2013). Cancer associated fibroblasts express pro-inflammatory factors in human breast and ovarian tumors. Biochem Biophys Res Commun 437:397–402. [DOI] [PubMed] [Google Scholar]
- Faivre S, Kroemer G, Raymond E. (2006). Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov 5:671–688. [DOI] [PubMed] [Google Scholar]
- Fang J, Zhou Q, Shi XL, Jiang BH. (2007). Luteolin inhibits insulin-like growth factor 1 receptor signaling in prostate cancer cells. Carcinogenesis 28:713–723. [DOI] [PubMed] [Google Scholar]
- Farkas SA, Vymetalkova V, Vodickova L, Vodicka P, Nilsson TK. (2014). DNA methylation changes in genes frequently mutated in sporadic colorectal cancer and in the DNA repair and Wnt/beta-catenin signaling pathway genes. Epigenomics 6:179–191. [DOI] [PubMed] [Google Scholar]
- Fu J, Chen D, Zhao B, Zhao Z, Zhou J, Xu Y, et al. (2012). Luteolin induces carcinoma cell apoptosis through binding Hsp90 to suppress constitutive activation of STAT3. PLoS One 7:e49194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Manero G, Fenaux P. (2011). Hypomethylating agents and other novel strategies in myelodysplastic syndromes. J Clin Oncol 29:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz DA. (2014). An autophagic switch in the response of tumor cells to radiation and chemotherapy. Biochem Pharmacol 90:208–211. [DOI] [PubMed] [Google Scholar]
- Hack SP, Bruey JM, Koeppen H. (2014). HGF/MET-directed therapeutics in gastroesophageal cancer: a review of clinical and biomarker development. Oncotarget 5:2866–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham S, Kim KH, Kwon TH, Bak Y, Lee DH, Song YS, et al. (2014). Luteolin induces intrinsic apoptosis via inhibition of E6/E7 oncogenes and activation of extrinsic and intrinsic signaling pathways in HPV-18-associated cells. Oncol Rep 31:2683–2691. [DOI] [PubMed] [Google Scholar]
- Hamada S, Horiguchi A, Asano T, Kuroda K, Asakuma J, Ito K, et al. (2014). Prognostic impact of fatty acid synthase expression in upper urinary tract urothelial carcinoma. Jpn J Clin Oncol 44:486–492. [DOI] [PubMed] [Google Scholar]
- Hempel J, Pforte H, Raab B, Engst W, Böhm H, Jacobasch G. (1999). Flavonols and flavones of parsley cell suspension culture change the antioxidative capacity of plasma in rats. Nahrung 43:201–204. [DOI] [PubMed] [Google Scholar]
- Horinaka M, Yoshida T, Shiraishi T, Nakata S, Wakada M, Nakanishi R, et al. (2005). The combination of TRAIL and luteolin enhances apoptosis in human cervical cancer HeLa cells. Biochem Biophys Res Commun 333:833–838. [DOI] [PubMed] [Google Scholar]
- Hu C, Cai X, Hu T, Lu W, Cao P. (2012). Mechanism of growth inhibition effect of 3′,4′,5,7-tetrahydroxyflavone on A549 cells. Zhongguo Zhong Yao Za Zhi 37:1259–1264. [PubMed] [Google Scholar]
- Hwang JT, Park OJ, Lee YK, Sung MJ, Hur HJ, Kim MS, et al. (2011). Anti-tumor effect of luteolin is accompanied by AMP-activated protein kinase and nuclear factor-kappaB modulation in HepG2 hepatocarcinoma cells. Int J Mol Med 28:25–31. [DOI] [PubMed] [Google Scholar]
- Jeon IH, Kim HS, Kang HJ, Lee HS, Jeong SI, Kim SJ, Jang SI. (2014). Anti-inflammatory and antipruritic effects of luteolin from Perilla (P. frutescens L.) leaves. Molecules 19:6941–6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Xie KP, Huo HN, Wang LM, Zou W, Xie MJ. (2013). Inhibitory effect of luteolin on the angiogenesis of chick chorioallantoic membrane and invasion of breast cancer cells via downregulation of AEG-1 and MMP-2. Sheng Li Xue Bao 65:513–518. [PubMed] [Google Scholar]
- Johnson JL, Gonzalez de Mejia E. (2013). Interactions between dietary flavonoids apigenin or luteolin and chemotherapeutic drugs to potentiate anti-proliferative effect on human pancreatic cancer cells, in vitro. Food Chem Toxicol 60: , 83–91. [DOI] [PubMed] [Google Scholar]
- Ju W, Wang X, Shi H, Chen W, Belinsky SA, Lin Y. (2007). A critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor-kappaB pathway and sensitization of apoptosis in lung cancer cells. Mol Pharmacol 71:1381–1388. [DOI] [PubMed] [Google Scholar]
- Kagoya Y, Yoshimi A, Kataoka K, Nakagawa M, Kumano K, Arai S, et al. (2014). Positive feedback between NF-kappaB and TNF-alpha promotes leukemia-initiating cell capacity. J Clin Invest 124:528–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KP, Park SK, Kim DH, Sung MJ, Jung YJ, Lee AS, et al. (2011). Luteolin ameliorates cisplatin-induced acute kidney injury in mice by regulation of p53-dependent renal tubular apoptosis. Nephrol Dial Transplant 26:814–822. [DOI] [PubMed] [Google Scholar]
- Katic M, Kahn CR. (2005). The role of insulin and IGF-1 signaling in longevity. Cell Mol Life Sci 62:320–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan HY, Zubair H, Faisal M, Ullah MF, Farhan M, Sarkar FH, et al. (2014). Plant polyphenol induced cell death in human cancer cells involves mobilization of intracellular copper ions and reactive oxygen species generation: a mechanism for cancer chemopreventive action. Mol Nutr Food Res 58:437–446. [DOI] [PubMed] [Google Scholar]
- Kim HY, Jung SK, Byun S, Son JE, Oh MH, Lee J, et al. (2013). Raf and PI3K are the molecular targets for the anti-metastatic effect of luteolin. Phytother Res 27:1481–1488. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Nakata T, Kuzumaki T. (2002). Effect of flavonoids on cell cycle progression in prostate cancer cells. Cancer Lett 176:17–23. [DOI] [PubMed] [Google Scholar]
- Krifa M, Alhosin M, Muller CD, Gies JP, Chekir-Ghedira L, Ghedira K, et al. (2013). Limoniastrum guyonianum aqueous gall extract induces apoptosis in human cervical cancer cells involving p16 INK4A re-expression related to UHRF1 and DNMT1 down-regulation. J Exp Clin Cancer Res 32: , 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Marchand L. (2002). Cancer preventive effects of flavonoids – a review. Biomed Pharmacother 56:296–301. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Wu LF, Chen WK, Wang CJ, Tseng TH. (2006). Inhibitory effect of luteolin on hepatocyte growth factor/scatter factor-induced HepG2 cell invasion involving both MAPK/ERKs and PI3K–Akt pathways. Chem Biol Interact 160:123–133. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Oh SY, Sung MK. (2012). Luteolin exerts anti-tumor activity through the suppression of epidermal growth factor receptor-mediated pathway in MDA-MB-231 ER-negative breast cancer cells. Food Chem Toxicol 50:4136–4143. [DOI] [PubMed] [Google Scholar]
- Lee YH, Morrison BL, Bottaro DP. (2014). Synergistic signaling of tumor cell invasiveness by hepatocyte growth factor and hypoxia. J Biol Chem 289:20448–20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SH, Jung SK, Byun S, Lee EJ, Hwang JA, Seo SG, et al. (2013). Luteolin suppresses UVB-induced photoageing by targeting JNK1 and p90 RSK2. J Cell Mol Med 17:672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim do Y, Jeong Y, Tyner AL, Park JH. (2007). Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am J Physiol Gastrointest Liver Physiol 292:G66–G75. [DOI] [PubMed] [Google Scholar]
- Lim do Y, Cho HJ, Kim J, Nho CW, Lee KW, Park JH. (2012). Luteolin decreases IGF-II production and downregulates insulin-like growth factor-I receptor signaling in HT-29 human colon cancer cells. BMC Gastroenterol 12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limtrakul P, Anuchapreeda S, Buddhasukh D. (2004). Modulation of human multidrug-resistance MDR-1 gene by natural curcuminoids. BMC Cancer 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Shi R, Wang X, Shen HM. (2008). Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets 8:634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YS, Tsai PH, Kandaswami CC, Cheng CH, Ke FC, Lee PP, et al. (2011). Effects of dietary flavonoids, luteolin, and quercetin on the reversal of epithelial–mesenchymal transition in A431 epidermal cancer cells. Cancer Sci 102:1829–1839. [DOI] [PubMed] [Google Scholar]
- Lin YC, Tsai PH, Lin CY, Cheng CH, Lin TH, Lee KP, et al. (2013). Impact of flavonoids on matrix metalloproteinase secretion and invadopodia formation in highly invasive A431-III cancer cells. PLoS One 8:e71903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Lin JC, Hung CM, Chen Y, Liu LC, Chang TC, et al. (2014). Osthole inhibits insulin-like growth factor-1-induced epithelial to mesenchymal transition via the inhibition of PI3K/Akt signaling pathway in human brain cancer cells. J Agric Food Chem 62:5061–5071. [DOI] [PubMed] [Google Scholar]
- Lu XY, Li YH, Xiao XW, Li XB. (2013). Inhibitory effects of luteolin on human gastric carcinoma xenografts in nude mice and its mechanism. Zhonghua Yi Xue Za Zhi 93:142–146. [PubMed] [Google Scholar]
- Lun M, Zhang PL, Pellitteri PK, Law A, Kennedy TL, Brown RE. (2005). Nuclear factor-kappaB pathway as a therapeutic target in head and neck squamous cell carcinoma: pharmaceutical and molecular validation in human cell lines using Velcade and siRNA/NF-kappaB. Ann Clin Lab Sci 35:251–258. [PubMed] [Google Scholar]
- Mancini M, Gariboldi MB, Taiana E, Bonzi MC, Craparotta I, Pagin M, Monti E. (2014). Co-targeting the IGF system and HIF-1 inhibits migration and invasion by (triple-negative) breast cancer cells. Br J Cancer 110:2865–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miean KH, Mohamed S. (2001). Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem 49:3106–3112. [DOI] [PubMed] [Google Scholar]
- Mirza S, Sharma G, Parshad R, Gupta SD, Pandya P, Ralhan R. (2013). Expression of DNA methyltransferases in breast cancer patients and to analyze the effect of natural compounds on DNA methyltransferases and associated proteins. J Breast Cancer 16:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momtazi-Borojeni AA, Behbahani M, Sadeghi-Aliabadi H. (2013). Antiproliferative activity and apoptosis induction of crude extract and fractions of Avicennia marina. Iran J Basic Med Sci 16:1203–1208. [PMC free article] [PubMed] [Google Scholar]
- Montaser R, Luesch H. (2011). Marine natural products: a new wave of drugs? Future Med Chem 3:1475–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S, Chen Y, Ginter T, Schäfer C, Buchwald M, Schmitz LM, et al. (2014). SIAH2 antagonizes TYK2-STAT3 signaling in lung carcinoma cells. Oncotarget 5:3184–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. (2000). Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98–103. [DOI] [PubMed] [Google Scholar]
- Ong CS, Zhou J, Ong CN, Shen HM. (2010). Luteolin induces G1 arrest in human nasopharyngeal carcinoma cells via the Akt–GSK-3beta–cyclin D1 pathway. Cancer Lett 298:167–175. [DOI] [PubMed] [Google Scholar]
- Ortholand JY, Ganesan A. (2004). Natural products and combinatorial chemistry: back to the future. Curr Opin Chem Biol 8:271–280. [DOI] [PubMed] [Google Scholar]
- Ou YC, Kuan YH, Li JR, Raung SL, Wang CC, Hung YY, Chen CJ. (2013). Induction of apoptosis by luteolin involving akt inactivation in human 786-o renal cell carcinoma cells. Evid Based Complement Alternat Med 2013:109105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou YC, Li JR, Kuan YH, Raung SL, Wang CC, Hung YY, et al. (2014). Luteolin sensitizes human 786-O renal cell carcinoma cells to TRAIL-induced apoptosis. Life Sci 100:110–117. [DOI] [PubMed] [Google Scholar]
- Pandurangan AK, Dharmalingam P, Sadagopan SK, Ramar M, Munusamy A, Ganapasam S. (2013). Luteolin induces growth arrest in colon cancer cells through involvement of Wnt/beta-catenin/GSK-3beta signaling. J Environ Pathol Toxicol Oncol 32:131–139. [DOI] [PubMed] [Google Scholar]
- Pandurangan AK, Dharmalingam P, Sadagopan SK, Ganapasam S. (2014). Luteolin inhibits matrix metalloproteinase 9 and 2 in azoxymethane-induced colon carcinogenesis. Hum Exp Toxicol 33:1176–1185. [DOI] [PubMed] [Google Scholar]
- Park SW, Cho CS, Jun HO, Ryu NH, Kim JH, Yu YS, et al. (2012). Anti-angiogenic effect of luteolin on retinal neovascularization via blockade of reactive oxygen species production. Invest Ophthalmol Vis Sci 53:7718–7726. [DOI] [PubMed] [Google Scholar]
- Park SH, Park HS, Lee JH, Chi GY, Kim GY, Moon SK, et al. (2013a). Induction of endoplasmic reticulum stress-mediated apoptosis and non-canonical autophagy by luteolin in NCI-H460 lung carcinoma cells. Food Chem Toxicol 56:100–109. [DOI] [PubMed] [Google Scholar]
- Park W, Amin AR, Chen ZG, Shin DM. (2013b). New perspectives of curcumin in cancer prevention. Cancer Prev Res (Phila) 6:387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M. (2008). Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 8:915–928. [DOI] [PubMed] [Google Scholar]
- Pratheeshkumar P, Son YO, Budhraja A, Wang X, Ding S, Wang L, et al. (2012). Luteolin inhibits human prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. PLoS One 7:e52279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak C, Petronis A. (2008). Epigenetics and complex disease: from etiology to new therapeutics. Annu Rev Pharmacol Toxicol 48: , 257–276. [DOI] [PubMed] [Google Scholar]
- Rao RV, Ellerby HM, Bredesen DE. (2004). Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ 11:372–380. [DOI] [PubMed] [Google Scholar]
- Rengarajan T, Nandakumar N, Rajendran P, Haribabu L, Nishigaki I, Balasubramanian MP. (2014). d-Pinitol promotes apoptosis in MCF-7 cells via induction of p53 and Bax and inhibition of Bcl-2 and NF-kappaB. Asian Pac J Cancer Prev 15:1757–1762. [DOI] [PubMed] [Google Scholar]
- Ruan J, Zhang L, Yan L, Liu Y, Yue Z, Chen L, et al. (2012a). Inhibition of hypoxia-induced epithelial mesenchymal transition by luteolin in non-small cell lung cancer cells. Mol Med Rep 6:232–238. [DOI] [PubMed] [Google Scholar]
- Ruan JS, Liu YP, Zhang L, Yan LG, Fan FT, Shen CS, et al. (2012b). Luteolin reduces the invasive potential of malignant melanoma cells by targeting beta3 integrin and the epithelial–mesenchymal transition. Acta Pharmacol Sin 33:1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth SA, Micheau O. (2009). Molecular crosstalk between TRAIL and natural antioxidants in the treatment of cancer. Br J Pharmacol 157:1186–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi RX, Ong CN, Shen HM. (2004). Luteolin sensitizes tumor necrosis factor-alpha-induced apoptosis in human tumor cells. Oncogene 23:7712–7721. [DOI] [PubMed] [Google Scholar]
- Shi RX, Ong CN, Shen HM. (2005). Protein kinase C inhibition and X-linked inhibitor of apoptosis protein degradation contribute to the sensitization effect of luteolin on tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in cancer cells. Cancer Res 65:7815–7823. [DOI] [PubMed] [Google Scholar]
- Shi R, Huang Q, Zhu X, Ong YB, Zhao B, Lu J, et al. (2007). Luteolin sensitizes the anticancer effect of cisplatin via c-Jun NH2-terminal kinase-mediated p53 phosphorylation and stabilization. Mol Cancer Ther 6:1338–1347. [DOI] [PubMed] [Google Scholar]
- Shimoi K, Okada H, Furugori M, Goda T, Takase S, Suzuki M, et al. (1998). Intestinal absorption of luteolin and luteolin 7-O-beta-glucoside in rats and humans. FEBS Lett 438:220–224. [DOI] [PubMed] [Google Scholar]
- Silva G, Fachin AL, Beleboni RO, França SC, Marins M. (2013). In vitro action of flavonoids in the canine malignant histiocytic cell line DH82. Molecules 18:15448–15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soncin F, Mohamet L, Eckardt D, Ritson S, Eastham AM, Bobola N, et al. (2009). Abrogation of E-cadherin-mediated cell-cell contact in mouse embryonic stem cells results in reversible LIF-independent self-renewal. Stem Cells 27:2069–2080. [DOI] [PubMed] [Google Scholar]
- Sun T, Xu Z, Wu CT, Janes M, Prinyawiwatkul W, No HK. (2007). Antioxidant activities of different colored sweet bell peppers (Capsicum annuum L.). J Food Sci 72:S98–S102. [DOI] [PubMed] [Google Scholar]
- Tai Z, Lin Y, He Y, Huang J, Guo J, Yang L, et al. (2014). Luteolin sensitizes the antiproliferative effect of interferon α/β by activation of Janus kinase/signal transducer and activator of transcription pathway signaling through protein kinase A-mediated inhibition of protein tyrosine phosphatase SHP-2 in cancer cells. Cell Signal 26:619–628. [DOI] [PubMed] [Google Scholar]
- Thomas GS, Zhang L, Blackwell K, Habelhah H. (2009). Phosphorylation of TRAF2 within its RING domain inhibits stress-induced cell death by promoting IKK and suppressing JNK activation. Cancer Res 69:3665–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topcu Z, Ozturk B, Kucukoglu O, Kilinc E. (2008). Flavonoids in Helichrysum pamphylicum inhibit mammalian type I DNA topoisomerase. Z Naturforsch C 63:69–74. [DOI] [PubMed] [Google Scholar]
- Tsui KH, Chung LC, Feng TH, Chang PL, Juang HH. (2012). Upregulation of prostate-derived Ets factor by luteolin causes inhibition of cell proliferation and cell invasion in prostate carcinoma cells. Int J Cancer 130:2812–2823. [DOI] [PubMed] [Google Scholar]
- Uekita T, Fujii S, Miyazawa Y, Hashiguchi A, Abe H, Sakamoto M, Sakai R. (2013). Suppression of autophagy by CUB domain-containing protein 1 signaling is essential for anchorage-independent survival of lung cancer cells. Cancer Sci 104:865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Attikum H, Gasser SM. (2005). The histone code at DNA breaks: a guide to repair? Nat Rev Mol Cell Biol 6:757–765. [DOI] [PubMed] [Google Scholar]
- Verschooten L, Barrette K, Van Kelst S, Rubio Romero N, Proby C, De Vos R, et al. (2012). Autophagy inhibitor chloroquine enhanced the cell death inducing effect of the flavonoid luteolin in metastatic squamous cell carcinoma cells. PLoS One 7:e48264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigna E, Comoglio PM. (2014). Targeting the oncogenic Met receptor by antibodies and gene therapy. Available at: http://www.nature.com/onc/journal/vaop/ncurrent/full/onc2014142a.html. [Accessed May 2014] [DOI] [PubMed] [Google Scholar]
- Wang F, Gao F, Pan S, Zhao S, Xue Y. (2014). Luteolin induces apoptosis, G0/G1 cell cycle growth arrest and mitochondrial membrane potential loss in neuroblastoma brain tumor cells. Available at: http://www.ncbi.nlm.nih.gov/pubmed/?term=Luteolin+induces+apoptosis%2C+G0%2FG1+Cell+cycle+growth+arrest+and+mitochondrial+membrane+potential+loss+in+neu-+roblastoma+brain+tumor+cells. [Accessed May 2014] [DOI] [PubMed] [Google Scholar]
- Wang J, Wu GS. (2014). Role of autophagy in cisplatin resistance in ovarian cancer cells. J Biol Chem 289:17163–17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LM, Xie KP, Huo HN, Shang F, Zou W, Xie MJ. (2012a). Luteolin inhibits proliferation induced by IGF-1 pathway dependent ERalpha in human breast cancer MCF-7 cells. Asian Pac J Cancer Prev 13:1431–1437. [DOI] [PubMed] [Google Scholar]
- Wang TT, Wang SK, Huang GL, Sun GJ. (2012b). Luteolin induced-growth inhibition and apoptosis of human esophageal squamous carcinoma cell line Eca109 cells in vitro. Asian Pac J Cancer Prev 13:5455–5461. [DOI] [PubMed] [Google Scholar]
- Wang X, Ju W, Renouard J, Aden J, Belinsky SA, Lin Y. (2006). 17-Allylamino-17-demethoxygeldanamycin synergistically potentiates tumor necrosis factor-induced lung cancer cell death by blocking the nuclear factor-kappaB pathway. Cancer Res 66:1089–1095. [DOI] [PubMed] [Google Scholar]
- Weng CJ, Yen GC. (2012). Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev 31:323–351. [DOI] [PubMed] [Google Scholar]
- Wu B, Zhang Q, Shen W, Zhu J. (2008). Anti-proliferative and chemosensitizing effects of luteolin on human gastric cancer AGS cell line. Mol Cell Biochem 313:125–132. [DOI] [PubMed] [Google Scholar]
- Wu YX, Fang X. (2010). Apigenin, chrysin, and luteolin selectively inhibit chymotrypsin-like and trypsin-like proteasome catalytic activities in tumor cells. Planta Med 76:128–132. [DOI] [PubMed] [Google Scholar]
- Wu Z, Peng X, Li J, Zhang Y, Hu L. (2013). Constitutive activation of nuclear factor kappaB contributes to cystic fibrosis transmembrane conductance regulator expression and promotes human cervical cancer progression and poor prognosis. Int J Gynecol Cancer 23:906–915. [DOI] [PubMed] [Google Scholar]
- Xavier CP, Lima CF, Preto A, Seruca R, Fernandes-Ferreira M, Pereira-Wilson C. (2009). Luteolin, quercetin and ursolic acid are potent inhibitors of proliferation and inducers of apoptosis in both KRAS and BRAF mutated human colorectal cancer cells. Cancer Lett 281:162–170. [DOI] [PubMed] [Google Scholar]
- Xavier CP, Lima CF, Rohde M, Pereira-Wilson C. (2011). Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother Pharmacol 68:1449–1457. [DOI] [PubMed] [Google Scholar]
- Yan J, Wang Q, Zheng X, Sun H, Zhou Y, Li D, et al. (2012). Luteolin enhances TNF-related apoptosis-inducing ligand’s anticancer activity in a lung cancer xenograft mouse model. Biochem Biophys Res Commun 417:842–846. [DOI] [PubMed] [Google Scholar]
- Yang MY, Wang CJ, Chen NF, Ho WH, Lu FJ, Tseng TH. (2014). Luteolin enhances paclitaxel-induced apoptosis in human breast cancer MDA-MB-231 cells by blocking STAT3. Chem Biol Interact 213:60–68. [DOI] [PubMed] [Google Scholar]
- Yu J, Peng Y, Wu LC, Xie Z, Deng Y, Hughes T, et al. (2013). Curcumin down-regulates DNA methyltransferase 1 and plays an anti-leukemic role in acute myeloid leukemia. PLoS One 8:e55934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Luo D, Miao R, Bai L, Ge Q, Sessa WC, Min W. (2005). Hsp90-Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene 24:3954–3963. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Moerkens M, Ramaiahgari S, de Bont H, Price L, Meerman J, van de Water B. (2011). Elevated insulin-like growth factor 1 receptor signaling induces antiestrogen resistance through the MAPK/ERK and PI3K/Akt signaling routes. Breast Cancer Res 13:R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JJ, Carrasco RD. (2014). Crosstalk between microRNA30a/b/c/d/e-5p and the canonical Wnt pathway: implications for multiple myeloma therapy. Cancer Res 74:5351–5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Yan B, Hu X, Li XB, Zhang J, Fang J. (2009). Luteolin inhibits invasion of prostate cancer PC3 cells through E-cadherin. Mol Cancer Ther 8:1684–1691. [DOI] [PubMed] [Google Scholar]


