Abstract
Introduction
Hepatic encephalopathy is defined as brain dysfunction caused by liver insufficiency and/or portosystemic shunting. Symptoms include nonspecific cognitive impairment, personality changes and changes in consciousness. Overt (symptomatic) hepatic encephalopathy is a common complication of cirrhosis that is associated with a poor prognosis. Patients with hepatic encephalopathy may present to healthcare providers who do not have primary responsibility for management of patients with cirrhosis. Therefore, we developed a series of ‘consensus points’ to provide some guidance on management.
Methods
Using a modified ‘Delphi’ process, consensus statements were developed that summarize our recommendations for the diagnosis and management of patients with hepatic encephalopathy. Points on which full consensus could not be reached are also discussed.
Results
Our recommendations emphasize the role of all healthcare providers in the identification of cognitive impairment in patients with cirrhosis and provide guidance on steps that might be considered to make a diagnosis of overt hepatic encephalopathy. In addition, treatment recommendations are summarized. Minimal hepatic encephalopathy can have a significant impact on patients; however, in most circumstances identification and management of minimal hepatic encephalopathy remains the responsibility of specialists in liver diseases.
Conclusion
Our opinion statements aim to define the roles and responsibilities of all healthcare providers who at times care for patients with cirrhosis and hepatic encephalopathy. We suggest that these recommendations be considered further by colleagues in other disciplines and hope that future guidelines consider the management of patients with cirrhosis and with a ‘suspicion’ of cognitive impairment through to a formal diagnosis of hepatic encephalopathy.
Keywords: hepatic encephalopathy, hypertension portal, lactulose, liver cirrhosis, rifaximin
Introduction
Hepatic encephalopathy (HE) is defined as ‘brain dysfunction caused by liver insufficiency and/or portosystemic shunting’ 1. Symptoms of overt HE include nonspecific cognitive impairment, personality changes and changes in consciousness – particularly disturbance of the sleep/wake cycle 1–3. In more severe HE, patients may become disorientated, acutely confused and suffer from agitation or coma 1,2,4. Minimal HE, the mildest form of HE, is clinically undetectable and requires the use of psychometric or neurophysiological testing to make a diagnosis 1,5.
Overt HE is a common complication of cirrhosis; at diagnosis approximately 10% of patients have HE 6,7 and 20–40% of patients with cirrhosis will develop HE during their disease course 8. In a multicentre study in Europe, of 2145 successive patients at different stages of liver disease 21% had HE 9. HE is also associated with a poor prognosis; 1-year mortality in patients with HE was 64% in one population-based series 7. Mortality may also be correlated with HE severity 9,10. In addition, HE affects the patient’s quality of life 11,12 and caregiver burden 13. There is also accumulating evidence that after apparent resolution of HE persistent cognitive dysfunction may occur 14,15.
New Insights into HE (http://www.hepaticencephalopathy.info and http://www.HEFastFacts.info) is an educational programme developed by a Steering Committee of hepatologists and gastroenterologists from across Europe. The aim of this programme is to improve the understanding and management of patients with HE. A series of Needs Assessment teleconferences were undertaken in November 2013 with 14 hepatologists and gastroenterologists managing patients with cirrhosis and HE. These open-question-led interviews were designed to identify educational needs around HE, which would inform the ‘New Insights into HE’ educational programme. As a result of these teleconferences, and subsequent discussions with the New Insights into HE Steering Committee and other faculty involved in developing the educational content, it was clear that a potential barrier to effective management of patients with HE is delay in identification and referral. The European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) guidelines present recommendations and options for the diagnosis and management of patients with HE (Fig. 1) 1. However, these guidelines reflect the uncertainties and variability in management and often a lack of high-quality evidence to support one approach over another. Therefore, the final recommendations present options that ‘…not all readers may necessarily agree with…’ 1. For the specialist in liver diseases this guideline therefore allows for management to reflect personal experience and preference while providing a framework for future developments. However, for those healthcare providers who are not hepatologists (or do not specialize in diseases of the liver), but have clinical responsibility for patients with cirrhosis, no guidance is provided to define their role in the identification and referral and/or management of patients with HE. To provide this guidance, eight members of the Steering Committee developed a series of Consensus Opinion Statements to better define the role of healthcare providers who do not specialize in liver diseases in the management of HE. The recommendations of this paper should therefore be reviewed alongside the EASL/AASLD treatment guidelines 1, and consideration should be given to an individual healthcare provider’s experience and expertise.
Fig. 1.
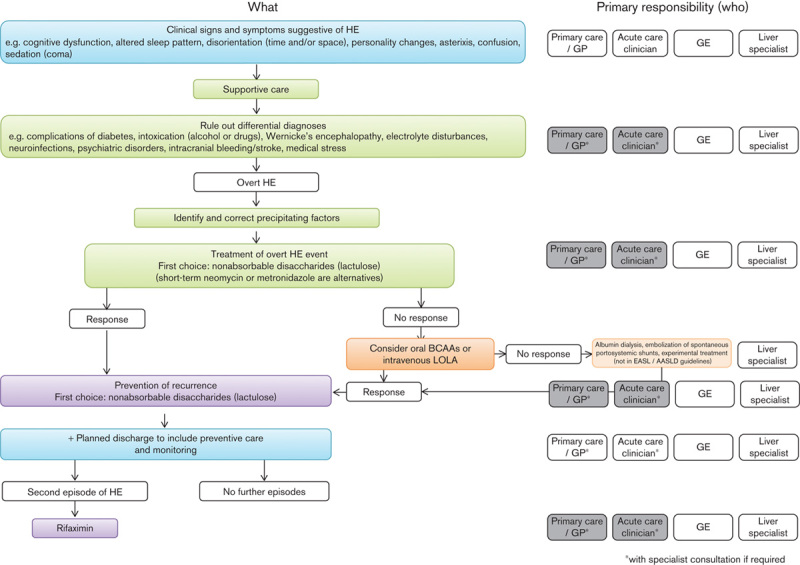
An overview of the latest European Association for the Study of the Liver/American Association for the Study of Liver Diseases (EASL/AASLD) treatment guidelines for overt hepatic encephalopathy 1, and the role of different medical specialists in the implementation of these guidelines according to our consensus recommendations (grey boxes indicate ‘optional’ involvement, if there is sufficient experience and confidence). BCAAs, branched chain amino acids; GE, gastroenterologist; GP, general practitioner; HE, hepatic encephalopathy; LOLA, l-ornithine l-aspartate.
Methods
On the basis of the results of the Needs Assessment survey and follow on discussions, a PubMed search was undertaken to identify additional management guidelines and recommendations for management of HE. The MESH term ‘hepatic encephalopathy’ combined with the subheadings ‘therapy’, ‘rehabilitation’, ‘diet therapy’, or ‘drug therapy’ was used as an initial search. This was then supplemented by a search combining the MESH term ‘hepatic encephalopathy’ and the article types ‘consensus development conference’, ‘consensus development conference, NIH’, or ‘guideline’. A total of 1146 possible publications were identified and a manual search of this list was then undertaken to identify publications that could inform the development of draft statements. Because of the paucity of suitable papers, a free text search (‘hepatic encephalopathy’ AND (guideline* OR algorithm)) and a MESH search (‘cognition disorders’ AND cirrhosis) were also undertaken. These did not retrieve any additional suitable citations.
A number of statements were then developed by a single, nonvoting author (I.E.J.M.) that attempted to better define the role of the general practitioner, acute care physician, general gastroenterologist and liver specialist/hepatologist in the management of HE. These statements were reviewed and scored by the eight expert authors (Panel) using a modified ‘Delphi’ process. The Delphi process has been explained in detail elsewhere but is briefly summarized 16,17. Each of the eight authors scored each statement on a scale of 1–5, where 1=strongly disagree, 2=disagree, 3=neutral, 4=agree and 5=strongly agree. Each member of the Panel was asked to provide comments, if any, or points of clarification. The scores were anonymized and collated and mean scores and ranges from the Panel’s first round were then recirculated to all members of the Panel with the collated comments. Each Panel member was invited to revise their scores in a second round. In addition, statements with a wide range of responses were revised, and in subsequent rounds the Panel was again asked to provide their scores. After the third round scores were collated to produce mean and mode scores, as well as the range of final scores. Using the definition of ‘Consensus’ as no more than one individual score being no greater than two scores from the mode, the majority of statements approached consensus after three rounds of voting and revision. These results were used to develop a ‘Discussion Document’ that used points of consensus and nonconsensus to develop the ‘Panel Recommendations’ defined below. As the final round of this process, the Discussion Document was reviewed and refined by teleconference, and agreement reached whether consensus was possible, or whether the final Panel Recommendations should reflect differences in opinion or clinical practice. These recommendations and points of consensus or nonconsensus are described below and summarized in Fig. 1.
Results
Delphi process
Strong levels of consensus were reached regarding the role of gastroenterologists and liver specialists in the clinical diagnosis of HE, with lower levels of consensus regarding the role of the family physician. There was also consensus that all patients with cirrhosis should be assessed, not only those with a history of decompensation. As shown, full consensus was not reached regarding the specific diagnostic test or tests that should be used, nor the treatment of an overt HE episode. In contrast, there was general consensus that secondary prophylaxis was appropriate. During the final teleconference, agreement was reached to develop the ‘recommendations’ below with all members of the Panel providing agreement. The relevant consensus statements and level of consensus are also provided for each recommendation.
Panel recommendations
-
General practitioners, acute care physicians and others, who may encounter patients with cirrhosis, should be aware of the signs and symptoms of HE. If these healthcare providers identify cognitive impairment, or symptoms of HE, in patients with cirrhosis, additional clinical assessment/workup may be required. This may include specialist consultation if helpful.
All patients with cirrhosis should be assessed clinically for overt HE by their family physician: 38% strongly agree; 25% agree (mean score: 3.63/5).
Most patients with cirrhosis will be under appropriate specialist care. The primary care physician plays a vital role in the identification of patients with liver disease and should be aware of at-risk groups in addition to clinical signs and symptoms. Guidelines for the primary care provider on this are lacking. HE as a first presentation of cirrhosis would be unusual 7. However, HE should be considered in the differential diagnosis in appropriate clinical settings – for example, the patient with risk factors for liver disease, the patient with physical signs suggesting liver disease, or the patient with abnormal serum liver function tests. Unlike other decompensation events (e.g. variceal bleeding, ascites), patients with HE may not present to their usual specialist as a result of their symptoms 18, and patients with prior HE are more likely to present to their primary care provider or as an emergency admission 13. Therefore, it is necessary that patients with HE not presenting to their usual healthcare provider are identified and appropriate treatment initiated. Although assessment of cognition and psychiatric symptoms is relatively simple, making a definitive diagnosis of HE is more difficult and HE remains a diagnosis of exclusion 1. Symptoms of HE are relatively nonspecific (Table 1), which can lead to challenges in making a formal diagnosis of HE. Therefore, providers should be ready to consider HE as a cause of cognitive dysfunction and symptoms in patients with cirrhosis, and either work up the patient to make a formal diagnosis or, if necessary, be prepared to refer such patients to specialist care for further assessment. This prompt workup and/or referral should allow for early initiation of appropriate management, which might reduce the risk of hospitalization and thus reduce healthcare costs 19.
-
The lead clinician caring for patients with cirrhosis (gastroenterologist or liver specialist) should formally assess all patients for signs and symptoms of HE.
All patients with cirrhosis should be assessed clinically for overt HE by their gastroenterologist: 63% strongly agree; 25% agree (mean score: 4.5/5).
All patients with cirrhosis should be assessed clinically for overt HE by their liver specialist: 75% strongly agree; 25% agree (mean score: 4.75/5).
Although the general practitioner or acute care physician or other healthcare provider encountering patients with cirrhosis and cognitive impairment should have a low ‘threshold’ for suspecting HE as a possible cause, we believe that it is the responsibility of the lead clinician (gastroenterologist or liver specialist) to routinely assess patients with cirrhosis for symptoms of HE. According to current guidelines this may involve clinical assessment and/or psychometric testing 1. As stated below (point 3), the selection of additional psychometric testing remains a matter of personal experience and availability. Routine formal assessment will facilitate early identification of symptoms of HE, and therefore initiation of appropriate management.
-
Assessment for overt HE should principally be by clinical evaluation, with use of additional testing as required on an individual basis (and according to availability).
The assessment of overt HE outside a liver unit should be by clinical evaluation only: 25% strongly agree; 50% agree (mean score: 3.75/5).
Only near consensus was reached for this statement as one author strongly felt that even outside the liver unit additional testing beyond clinical assessment was necessary. However, on further discussion it was agreed that for many healthcare providers the facilities are not available to undertake additional tests. Therefore, the final statement above, which allows for additional testing ‘if available’, was agreed upon. The EASL/AASLD guidelines state that overt HE is primarily a clinical diagnosis (Table 1) 1. Psychometric and neurophysiological tests do not differentiate HE from other causes of cognitive dysfunction, and differential diagnoses include renal dysfunction, hyponatraemia, diabetes, sepsis, alcohol withdrawal and Wernicke’s encephalopathy 1. However, psychometric, psychophysical and neurophysiological tests can have some value in supporting the clinical diagnosis of lower grades of overt HE, in addition to their use to detect minimal HE. These include simple paper and pencil tests (including the Psychometric Hepatic Encephalopathy Score test) as well as more sophisticated psychometric tests including the Inhibitory Control Test and Repeated Battery for Assessment of Neurological State, neurophysiological testing by electroencephalogram, or psychophysical testing – for example, the Critical Flicker Frequency 1. Full consensus on the use of a specific test or tests could not be reached, reflecting differences in availability and clinical opinion. It should be noted that not all of the available tests are fully validated and many require administration by a trained examiner. The results of several of these including the Inhibitory Control Test and Psychometric Hepatic Encephalopathy Score test, can be affected by age, educational status and training effects, and for many tests validated ‘normals’ are not available for all countries/languages and populations. The availability of equipment for these tests varies, and in many centres pencil and paper tests are used and remain valuable. However, if available, the Critical Flicker Frequency test was felt by some authors to have several advantages, including lack of training effect, educational status, and age, and avoidance of interexaminer variability 20,21.
-
For all patients with HE, consideration should usually be given for referral to a liver unit or physician who specializes in liver disease.
All patients with overt HE should be referred to a liver unit: 50% strongly agree; 38% agree (mean score: 4.38/5).
HE is a decompensation event and all healthcare providers managing patients with a diagnosis of HE should consider whether the patient should be referred to a liver specialist. Although policies vary with respect to wait-listing patients with HE and a number of allocation scoring systems have been proposed 22, further assessment of patients with HE is warranted as they may be eligible for assessment for liver transplantation 1.
-
All patients with overt HE should be treated (including checking for precipitants).
All patients with HE should be treated: 75% strongly agree; 25% agree (mean score: 4.75/5).
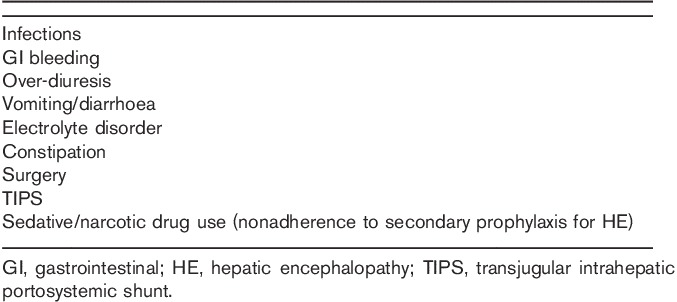
The most important step in the management of HE is evaluation for precipitants (Table 2) 1,23 and appropriate intervention. In addition, all patients with a diagnosis of overt HE should be treated 1. The most commonly used treatments are nonabsorbable disaccharides 1. A meta-analysis indicated that lactulose or lactitol therapy resulted in clinical improvement, although there was no effect on mortality 24. Although the same meta-analysis reported that nonabsorbable disaccharides were inferior to short-course antibiotic therapy 24, lactulose is the usual ‘first choice’ treatment for overt HE 1. Antibiotics are, however, commonly used, whereas other therapies available in some countries include oral branched chain amino acids 25 and intravenous l-ornithine l-aspartate (LOLA) 26. Other treatment options specialists may consider include albumin dialysis 27 and embolization of large spontaneous portal–systemic shunts 28, although these are not part of the EASL/AASLD recommendations 1. If helpful, advice on treatment should be sought from a specialist in liver diseases, particularly for more severe cases, and/or those for whom a nonstandard therapy is being considered.
-
After recovery/improvement of HE symptoms, secondary prophylaxis should be considered in all patients.
After recovery/improvement of overt HE, patients should receive secondary prophylaxis: 83% strongly agree; 16% agree (mean score: 4.83/5).
Prior HE is associated with an increased risk for subsequent episodes 8,9. In one study ∼50% of patients with at least one prior HE event (median 1) suffered recurrence within 1 year 29, and in another, 46% of those with at least two prior events suffered recurrence within 6 months 30. Therefore, it is appropriate to offer secondary prophylaxis to reduce recurrence in most, if not all, patients recovering from an episode of HE 1. Secondary prophylaxis should be initiated by the clinician managing an episode of HE, with advice from a gastroenterologist or specialist in liver disease if helpful.
In patients with a median of one prior episode, lactulose was associated with a significant reduction in the risk for recurrence over a median 14 months’ follow-up 27. Patients receiving lactulose should have the dose adjusted so that there are two or three bowel movements daily but without diarrhoea 1. In patients with at least two prior episodes of HE in the previous 6 months (the majority of whom were receiving lactulose), rifaximin-α (rifaximin) 550 mg twice daily was associated with a significant reduction in the risk for recurrent HE over 6 months and a reduction in the risk for HE-related hospitalization 28. The EASL/AASLD guidelines suggest rifaximin 550 mg twice daily add-on to lactulose after the second episode of HE 1. It may also be appropriate to consider rifaximin monotherapy in patients intolerant to lactulose despite patient education, and dose adjustment to achieve two or three bowel movements each day.
A number of other therapies used for secondary prophylaxis are not included in the EASL/AASLD guidelines 1 but were felt by some authors to have a role. Full consensus could not be reached on these treatments, partly because of differences in availability and clinical experience. These included laxatives 31, branched chain amino acids 25,32,33 and LOLA 26,34,35. For patients with hyperammonaemia and HE, LOLA was felt to be particularly helpful by one author, although only intravenous LOLA was reported by the EASL/AASLD guidelines as being effective for the treatment of HE episodes but not for secondary prophylaxis 1. There was also experience of using oral branched chain amino acids in patients with chronic HE who were protein intolerant, as recommended by the International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) guidelines on nutrition 36. Patients receiving secondary prophylaxis should be encouraged to remain on therapy to maximize the benefits of treatment. It is the responsibility of all healthcare professionals who encounter patients with cirrhosis and HE to encourage compliance with therapy.
-
Liver specialists may assess patients with cirrhosis for minimal HE; other healthcare professionals caring for patients with cirrhosis should not be expected to routinely assess patients for minimal HE.
Patients with cirrhosis should be assessed for minimal HE by their gastroenterologist: 38% strongly agree; 38% agree (mean score: 4.13/5).
Patients with cirrhosis should be assessed for minimal HE by their liver specialist: 88% strongly agree; 13% agree (mean score: 4.88/5).
Substantial expertise is required to make a diagnosis of minimal HE and the diagnosis is defined by the test used. In addition, there are no therapies with a specific approval for its treatment. Therefore, it may be reasonable to expect that diagnosis and management of minimal HE should remain the responsibility of liver specialists. Although treatment for minimal HE has been shown to improve cognitive test score and quality of life 37–39, it is not yet possible to confirm benefits in terms of reduced episodes of overt HE or other clinical outcomes. As a result, there is no agreement over potential treatment strategies, including the optimal duration of therapy 1.
It is appropriate for those healthcare providers caring for patients with cirrhosis, who have the expertise and experience, to utilize psychometric or neurophysiological tests on a case-by-case basis if this will help clinical decision making. This reflects the EASL/AASLD guidelines, which suggest that consideration be given to testing patients for minimal HE and providing counselling in some circumstances – for example, potential consequences on driving, flying, train drivers, or operating machinery 1.
-
If diagnosed, consideration should be given to treatment of patients with minimal HE.
All patients with a diagnosis of minimal HE should be treated: 38% strongly agree; 38% agree (mean score: 4.13/5).
If a diagnosis of minimal HE has been made it is reasonable to consider treatment, accepting the caveats above regarding uncertainties surrounding the endpoints of treatment and lack of licensed therapies. Such a decision should be taken in consultation with a specialist in liver diseases.
Table 1.
Signs and symptoms of hepatic encephalopathy (modified from EASL/AASLD, Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. Journal of Hepatology 61, 642–659, Elsevier, © 2014 1)
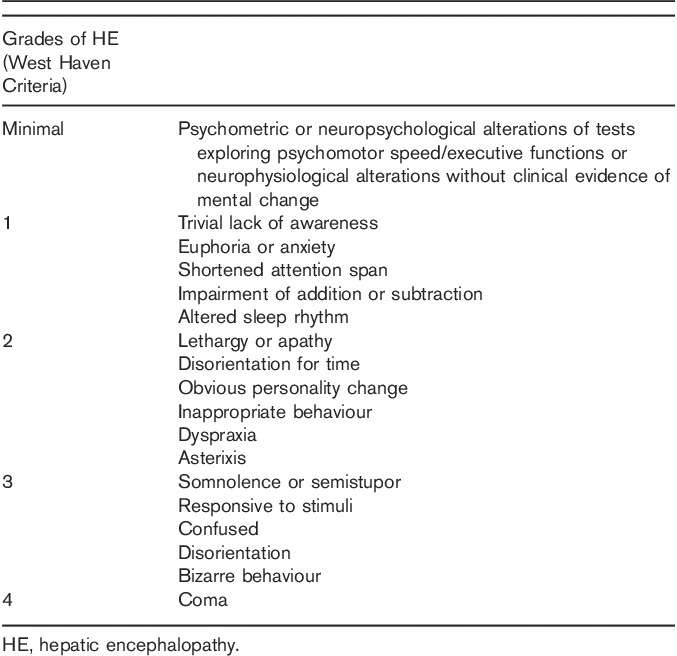
Table 2.
Discussion
The recently published guidelines from the EASL/AASLD provide a framework for the diagnosis and management of patients with HE 1. Our opinion statements aim to define the role and responsibilities of all those healthcare providers who at times care for patients with cirrhosis and HE (Fig. 1). Although developed before the EASL/AASLD guidelines were published, the expert consensus opinion statements discussed here do not differ significantly from these guidelines.
In developing these statements we have acknowledged that making a definitive diagnosis of HE can be difficult. However, it is possible for all those healthcare providers who encounter patients with cirrhosis to identify cognitive impairment and be aware of HE in terms of the impact on patients. For those healthcare providers who do not have primary responsibility for management of patients with cirrhosis we believe that it is reasonable that patients with suspected HE are referred to their gastroenterologist or liver specialist. It is then the responsibility of the primary provider (gastroenterologist or liver specialist) to ensure that appropriate clinical assessments are undertaken to make a diagnosis of overt HE. The use of psychometric and neurophysiological tests is essential to identify minimal HE, but the authors also felt they can be useful in the assessment of lower grades of overt HE. Gastroenterologists who have appropriate training and facilities/equipment should be prepared to supplement clinical assessment with such tests. If appropriate facilities or trained staff are not available, discussion with a liver specialist or liver unit should be considered. As HE is a decompensation event, such a discussion can be helpful to allow prompt consideration of whether the patient should be evaluated for liver transplantation.
Specific therapy for overt HE episodes includes supportive care, treatment of underlying precipitants, and initiation of specific therapy; first-line choices are nonabsorbable disaccharides or short-course antibiotics 1. All patients recovering from an acute episode of overt HE should also receive secondary prophylaxis to reduce the risk of recurrence; the evidence-based choices include nonabsorbable disaccharides and/or rifaximin 1. Appropriate therapy can reduce the duration of admission and reduce the risk of subsequent readmission. However, to maximize the benefits from therapy, early diagnosis is essential to allow prompt initiation of recommended treatment.
We recommend that our conclusions and recommendations be considered further by our colleagues in gastroenterology and hepatology and those in other disciplines and hope that these recommendations assist in the development of regional/national cross-speciality guidelines to improve the identification and triage of patients who may have HE and facilitate appropriate management. We also hope that future guidelines consider the management of patients with HE from initial ‘suspicion’ of cognitive impairment, which may be by a clinician or healthcare provider not routinely responsible for management of an individual’s liver disease.
Acknowledgements
D.L.S., A.A.D., R.J., G.K., R.J.de.K., W.L., J.K.R. and H.W. contributed to the Delphi process (voting and commentary), developed the consensus statements and revised the manuscript. I.E.J.M. coordinated the Delphi process and developed the first draft of the manuscript
The Delphi process was supported by an independent grant from Norgine. Norgine had no input into the development of these consensus statements, nor in this publication.
Conflicts of interest
Debbie L. Shawcross has served as a speaker, consultant and an advisory board member for Norgine and has received research funding from Higher Education Funding Council for England (HEFCE), The Wellcome Trust, The European Foundation for Alcohol Research (ERAB), The Royal Society, The Foundation for Liver Research, and Norgine. Arthur A. Dunk has received an honorarium from Norgine. Rajiv Jalan has received an honorarium for speaking from 4C Consultants/Norgine, has served as a consultant for Grifols Inc., Ocera Therapeutics and Conatus, and has received research funding from Grifols Inc., Ocera Therapeutics Inc., Sequana Medical AG and Norgine BV. Gerald Kircheis serves on the Speaker’s Bureau for Merz Pharmaceuticals and Norgine and is a joint patent holder for a bedside Critical Flicker Frequency Analyser. Robert J. de Knegt has received an honorarium for speaking or consulting from AbbVie, BMS, Gilead, Janssen-Cilag, Medtronic, Merck/Schering-Plough, Norgine and Roche, and has received research grants from BMS, Janssen-Cilag, Medtronic and Roche. Wim Laleman has served as a consultant to Norgine, Gilead, MSD, Roche, Gore and BMS. John K. Ramage has received an honorarium from Norgine. Heiner Wedemeyer has received honoraria for speaking or consulting from Abbott, Achillon, Abbvie, BMS, Gilead, Eiger, Janssen, Merck, Novartis, Roche and Transgene, has received honoraria from Falk and 4C Consultants/Norgine, and received research support from Roche, BMS, Novartis, Roche Diagnostics and Abbott. Ian E.J. Morgan is a director of 4C Consultants International, which has received funding from Norgine.
References
- 1.EASL/AASLD. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol 2014; 61:642–659. [DOI] [PubMed] [Google Scholar]
- 2.Conn HO, Leevy CM, Vlahcevic ZR, Rodgers JB, Maddrey WC, Seeff L, et al. Comparison of lactulose and neomycin in the treatment of chronic portal–systemic encephalopathy. Gastroenterology 1977; 72:573–583. [PubMed] [Google Scholar]
- 3.Montagnese S, De Pitta C, De Rui M, Corrias M, Turco M, Merkel C, et al. Sleep–wake abnormalities in patients with cirrhosis. Hepatology 2014; 59:705–712. [DOI] [PubMed] [Google Scholar]
- 4.Weissenborn K. Diagnosis of encephalopathy. Digestion 1998; 59:22–24. [DOI] [PubMed] [Google Scholar]
- 5.Weissenborn K, Ennen JC, Schomerus J, Ruckert N, Hecker H. Neurophysiological characterization of hepatic encephalopathy. J Hepatol 2001; 34:768–773. [DOI] [PubMed] [Google Scholar]
- 6.Saunders JB, Walters JRF, Davies P, Paton A. A 20-year prospective study of cirrhosis. BMJ 1981; 282:263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jepsen P, Ott P, Andersen PK, Sorensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology 2010; 51:1675–1682. [DOI] [PubMed] [Google Scholar]
- 8.Amodio P, Del Piccolo F, Petteno E, Mapelli D, Angeli P, Iemmolo R, et al. Prevalence and prognostic value of quantified electroencephalogram (EEG) alterations in cirrhotic patients. J Hepatol 2001; 35:37–45. [DOI] [PubMed] [Google Scholar]
- 9.Cordoba J, Ventura-Cots M, Simon-Talero M, Amorós À, Pavesi M, Vilstrup H, et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF). J Hepatol 2014; 60:275–281. [DOI] [PubMed] [Google Scholar]
- 10.Stewart CA, Malinchoc M, Kim WR, Kamath PS. Hepatic encephalopathy as a predictor of survival in patients with end-stage liver disease. Liver Transpl 2007; 13:1366–1371. [DOI] [PubMed] [Google Scholar]
- 11.Arguedas MR, DeLawrence TG, McGuire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Dig Dis Sci 2003; 48:1622–1626. [DOI] [PubMed] [Google Scholar]
- 12.Les I, Doval E, Flavia M, Jacas C, Cárdenas G, Esteban R, et al. Quality of life in cirrhosis is related to potentially treatable factors. Eur J Gastroenterol Hepatol 2010; 22:221–227. [DOI] [PubMed] [Google Scholar]
- 13.Orr JG, Morgan CL, Jenkins-Jones S, Hudson M, Conway P, Radwan A, et al. Resource use associated with hepatic encephalopathy in patients with liver disease [abstract P478]. Presented at the European Association for the Study of the Liver Annual Meeting; 9-13 April 2014; London.
- 14.Sotil EU, Gottstein J, Ayala E, Randolph C, Blei AT. Impact of preoperative overt hepatic encephalopathy on neurocognitive function after liver transplantation. Liver Transpl 2009; 15:184–192. [DOI] [PubMed] [Google Scholar]
- 15.Bajaj JS, Schubert CM, Heuman DM, Wade JB, Gibson DP, Topaz A, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology 2010; 138:2332–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu C-C, Sandford BA. The Delphi technique: making sense of consensus. Pract Assess Res Eval 2007; 12:1–8. [Google Scholar]
- 17.Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One 2011; 6:e20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grattagliano I, Ubaldi E, Bonfrate L, Portincasa P. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol 2011; 17:2273–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poole CD, Conway P, Nanuwa K, Joseph B, Bannister C, Currie CJ. Cost effectiveness of rifaximin-α in the reduction of recurrence of overt hepatic encephalopathy [abstract P451]. Presented at the European Association for the Study of the Liver Annual Meeting; 9-13 April 2014; London.
- 20.Kircheis G, Wettstein M, Timmermann L, Schnitzler A, Häussinger D. Critical flicker frequency for quantification of low-grade hepatic encephalopathy. Hepatology 2002; 35:357–366. [DOI] [PubMed] [Google Scholar]
- 21.Sharma P, Kumar A, Singh S, Tyagi P, Kumar A. Inhibitory control test, critical flicker frequency, and psychometric tests in the diagnosis of minimal hepatic encephalopathy in cirrhosis. Saudi J Gastroenterol 2013; 19:40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cholongitas E, Germani G, Burroughs AK. Prioritisation for liver transplantation. Nat Rev Gastroenterol Hepatol 2010; 7:659–668. [DOI] [PubMed] [Google Scholar]
- 23.Bajaj J. Review article: the modern management of hepatic encephalopathy. Aliment Pharmacol Ther 2010; 31:537–547. [DOI] [PubMed] [Google Scholar]
- 24.Als-Nielsen B, Gluud LL, Gluud C. Non-absorbable disaccharides for hepatic encephalopathy: systematic review of randomised trials. BMJ 2004; 328:1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gluud LL, Dam G, Borre M, Les I, Cordoba J, Marchesini G, et al. Oral branched-chain amino acids have a beneficial effect on manifestations of hepatic encephalopathy in a systematic review with meta-analyses of randomized controlled trials. J Nutr 2013; 143:1263–1268. [DOI] [PubMed] [Google Scholar]
- 26.Kircheis G, Wettstein M, vom Dahl S, Häussinger D. Clinical efficacy of l-ornithine–l-aspartate in the management of hepatic encephalopathy. Metab Brain Dis 2002; 17:453–463. [DOI] [PubMed] [Google Scholar]
- 27.Hassanein TI, Tofteng F, Brown RS, Jr, McGuire B, Lynch P, Mehta R, et al. Randomized controlled study of extracorporeal albumin dialysis for hepatic encephalopathy in advanced cirrhosis. Hepatology 2007; 46:1853–1862. [DOI] [PubMed] [Google Scholar]
- 28.Laleman W, Simon-Talero M, Maleux G, Perez M, Ameloot K, Soriano G, et al. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology 2013; 57:2448–2457. [DOI] [PubMed] [Google Scholar]
- 29.Sharma BC, Sharma P, Agrawal A, Sarin SK. Secondary prophylaxis of hepatic encephalopathy: an open label randomised controlled trial of lactulose verses placebo. Gastroenterology 2009; 137:885–891. [DOI] [PubMed] [Google Scholar]
- 30.Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010; 362:1071–1081. [DOI] [PubMed] [Google Scholar]
- 31.Uribe M, Campollo O, Campollo O, Vargas F, Ravelli GP, Mundo F, et al. Acidifying enemas (lactitol and lactose) versus nonacidifying enemas (tap water) to treat acute portal-systemic encephalopathy: a double-blind randomized clinical trial. Hepatology 1987; 7:639–643. [DOI] [PubMed] [Google Scholar]
- 32.Naylor CD, O’Rourke K, Detsky AS, Baker JP. Parenteral nutrition with branched-chain amino acids in hepatic encephalopathy. A meta-analysis. Gastroenterology 1989; 97:1033–1042. [DOI] [PubMed] [Google Scholar]
- 33.Marchesini G, Dioguardi FS, Bianchi GP, Zoli M, Bellati G, Roffi L, et al. Long-term oral branched-chain amino acid treatment in chronic hepatic encephalopathy. A randomized double-blind casein-controlled trial. J Hepatol 1990; 11:1–10. [DOI] [PubMed] [Google Scholar]
- 34.Stauch S, Kircheis G, Adler G, Beckh K, Ditschuneit H, Gortelmeyer R, et al. Oral l-ornithine–l-aspartate therapy of chronic hepatic encephalopathy: results of a placebo-controlled double-blind study. J Hepatol 1998; 28:856–864. [DOI] [PubMed] [Google Scholar]
- 35.Alvares-da-Silva MR, de Araujo A, Vicenzi JR, da Silva GV, Oliveira FB, Schacher F, et al. Oral l-ornithine–l-aspartate in minimal hepatic encephalopathy: a randomized, double-blind, placebo-controlled trial. Hepatol Res 2014; 44:956–963. [DOI] [PubMed] [Google Scholar]
- 36.Amodio P, Bemeur C, Butterworth R, Cordoba J, Kato A, Montagnese S, et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology 2013; 58:325–336. [DOI] [PubMed] [Google Scholar]
- 37.Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improved cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 2007; 45:549–559. [DOI] [PubMed] [Google Scholar]
- 38.Bajaj JS, Heuman DM, Wade JB, Gibson DP, Saeian K, Wegelin JA, et al. Rifaximin improves driving simulator performance in a randomized trial of patients with minimal hepatic encephalopathy. Gastroenterology 2011; 140:478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sidhu SS, Goyal O, Mishra BP, Sood A, Chhina RS, Soni RK. Rifaximin improves psychometric performance and health-related quality of life in patients with minimal hepatic encephalopathy (The RIME Trial). Am J Gastroenterol 2011; 106:307–316. [DOI] [PubMed] [Google Scholar]


