Supplemental Digital Content is available in the text.
Keywords: diffuse, fluorine-18 fluorodeoxyglucose, large B-cell, lymphoma, positron-emission tomography, prognosis
Abstract
Objectives
The aim of this study is to determine the correlation of pretreatment fluorine-18 fluorodeoxyglucose uptake with clinicopathological factors and its prognostic value in patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL).
Patients and methods
A cohort of 162 patients with newly diagnosed DLBCL who had undergone pretreatment PET/computed tomography was retrospectively reviewed. The relationship of pretreatment maximum standard uptake value (SUVmax) with clinical factors, molecular markers, and efficacy was evaluated. The value of SUVmax in predicting progression-free survival (PFS) and overall survival was analyzed.
Results
In all, 72.9% of the patients received R-CHOP treatment; the rest received CHOP chemotherapy. The median follow-up duration was 30 months (range, 4–124 months). The median SUVmax was 12.2 (range, 1.7–42.7). SUVmax between groups differed significantly with respect to each of International Prognostic Index (IPI) factors, except for age and performance status. High SUVmax was associated with high Ki-67 and Glut-3 protein expression, but not with Glut-1. Complete remission rate differed significantly between the low (SUVmax≤9.0) and the high SUVmax (SUVmax>9.0) groups (91.7 vs. 61.1%, P=0.000). Patients with low SUVmax showed favorable survival (3-year PFS: 92.2 vs. 63.6%, P=0.000; 3-year overall survival: 95.5 vs. 78.3%, P=0.003). On multivariate analyses, SUVmax predicted PFS independent of revised-IPI (SUVmax: P=0.011, hazard ratio 4.784; revised-IPI: P=0.004, hazard ratio 2.551).
Conclusion
Pretreatment SUVmax was associated with clinicopathological factors, efficacy, and survival outcome. A novel prognostic model on the basis of IPI score/pretreatment SUVmax might be useful for risk stratification of patients with newly diagnosed DLBCL Video abstract: http://links.lww.com/NMC/A55.
Introduction
Non-Hodgkin lymphoma (NHL) is a common malignant proliferative disease of the lymphatic system. Its prevalence is increasing gradually in both developed and developing countries. Approximately 360 000 patients are newly diagnosed with NHL every year worldwide and NHL has an annual mortality of 1.9–3.6/100 000 1. Diffuse large B-cell lymphoma (DLBCL) is the most common type of NHL, accounting for ∼30–50% of all NHL cases 2.
Fluorine-18 fluorodeoxyglucose PET/computed tomography (18F-FDG PET/CT, referred to hereafter as PET/CT) is an emerging functional and metabolic technique for the molecular imaging of tumors. The role of PET/CT in the assessment of treatment response in DLBCL patients has been confirmed widely. In 2007, the International Workshop Criteria (IWG) included PET/CT findings as a criterion for the assessment of the response of malignant lymphoma to treatment 3. Recent clinical studies have carried out PET/CT using the Deauville criteria or the reduction in the maximum standard uptake value (SUVmax) and verified the prognostic value of interim PET/CT in DLBCL 4,5. In contrast, pretreatment PET/CT is currently applied only to identify the area of involvement and to stage the lymphoma 6; its clinical value in other aspects of clinical management remains unclear.
SUV is the most commonly used semiquantitative parameter in PET/CT and can directly reveal the 18F-FDG uptake by tumor tissues, which in turn reflects the status of glucose metabolism in these tissues. 18F-FDG uptake is affected by a variety of clinicopathological factors, such as the biological characteristics of the tumor, including proliferation activity, aggressiveness, and glucose transporter (Glut) expression 7.
The rates of cell proliferation and glucose metabolism in malignant tumors often reflect tumor aggressiveness and affect the response to therapy. The Ki-67 protein is a biological marker of cell proliferation and is expressed in all phases of the cell cycle, except for the G0 phase. Gluts are the major carriers mediating the uptake of glucose, and are widespread in various human tissues and cells. Glut-1 and Glut-3 show a higher affinity for glucose and are associated closely with tumor metabolism. Recent studies have shown that Ki-67, Glut-1, and Glut-3 are important factors affecting the mechanism of 18F-FDG uptake in some solid tumors 8–10. However, the precise roles of Ki-67 and Glut expression in 18F-FDG uptake in patients with DLBCL remain unknown, and only a few small studies with contradictory conclusions have been reported.
Several studies have shown that pretreatment SUVmax is associated with survival in patients with non-small-cell lung cancer, esophageal cancer, colorectal cancer, or other solid tumors 11–13; however, other studies have reported different conclusions. The studies carried out by Vu et al. 14 and Brown et al. 15 do not support the use of SUVmax on pretreatment PET scans as a prognostic tool for patients with non-small-cell lung cancer and esophageal cancer. In the case of DLBCL, only a few studies have investigated the prognostic value of pretreatment PET/CT and obtained varying results; thus, there is a lack of clear and consistent conclusions 16–18.
Therefore, in this study, we retrospectively analyzed 162 cases of newly diagnosed DLBCL to determine whether pretreatment 18F-FDG uptake was correlated with clinicopathologic factors, treatment response, and prognosis.
Materials and methods
Patients and treatment protocols
This single-center retrospective study included patients with newly diagnosed DLBCL who were admitted to our hospital and underwent pretreatment PET/CT between August 2004 and May 2014. In all patients, DLBCL was diagnosed on the basis of a pathological examination of biopsy or surgically resected specimens. Two hematopathologists reviewed the diagnoses to confirm that they fulfilled the 2008 WHO criteria 19. The following clinical and laboratory data were available before the initiation of treatment: physical exam, Eastern Cooperative Oncology Group performance status (PS) evaluation, laboratory studies [including blood counts, biochemical tests, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and β2-microglobulin], bone marrow examination, and PET/CT scan, as well as additional gastrointestinal tract or central nervous system examination if necessary. Furthermore, staging was performed according to the Ann Arbor classification and risk groups were determined using the International Prognostic Index (IPI) and the Revised International Prognostic Index (R-IPI). The local ethics committee approved the study.
Patients received treatment with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOP regimens as the first-line therapy. In the present study, the result of PET/CT before treatment did not affect treatment decisions. Response to treatment was evaluated after three to four treatment cycles using the IWG criteria 3. Patients who were responsive to the treatment were administered additional treatment consisting of three to four courses of the same regimen, whereas patients in whom the treatment was ineffective or in whom progression was detected were shifted to other second-line chemotherapy regimens. The response was assessed again after six or eight treatment cycles, on the basis of which follow-up or continued treatment with adjusted regimens was performed. Follow-up was performed by the referring physician once every 3–6 months for the first 5 years and yearly thereafter. The examinations would be performed when changes occurred in the clinical condition to detect disease progression or recurrence. Methods of assessment and follow-up were as follows: physical exam, relevant laboratory tests (as those done for staging), imaging examinations, and bone marrow biopsy for previous involvement by lymphoma.
PET/CT imaging
18F-FDG PET imaging and image analysis PET were performed using a dedicated whole-body PET/CT scanner (GE Discovery LS PET/CT system; GE Medical Systems Company, Milwaukee, Wisconsin, USA). All patients fasted for at least 4 h before the PET scan and had blood glucose levels lower than 140 mg/dl at the time of injection. The dose of 18F-FDG injection was 0.15 mCi/kg body weight. The PET/CT scan was initiated 45–60 min (median: 53 min) after the 18F-FDG injection. An unenhanced CT image was obtained using a standardized protocol (120 kV, 160 mA, pitch 0.8 : 1, single cycle rotation time 0.8 s, matrix size 512×512, FOV 500 mm) and a section thickness of 5.0 mm, which was matched to the section thickness of the PET images. Immediately after CT, PET was performed covering the identical axial field. The acquisition time for PET was 4 min per table position and the acquisition mode was 2D. Patients were instructed to breathe shallowly during the acquisition of CT and PET. PET images were reconstructed using the method of ordered subsets expectation maximization and the CT data were used for segmented attenuation correction. The PET reconstruction parameters were as follows: iteration 2, subsets 28, matrix size 128×128, postfiltering 6.0 full-width at half-maximum (mm), loop filtering 4.3 full-width at half-maximum (mm), and Gaussian smoothing 8.0 mm. Coregistered images were displayed using Xeleris software (GE Medical Systems Company).
PET/CT analysis
All PET images and SUV-based assessment of 18F-FDG uptake were analyzed by a consensus of two experienced nuclear medicine physicians who were unaware of clinical data. A PET-positive lesion was defined according to the modified IWG criteria. The SUVmax was defined as the maximum SUV of the hypermetabolic lesion showing the highest 18F-FDG uptake and was calculated on the basis of the attenuation-corrected images, the amount of injected 18F-FDG, and the body weight [SUVmax=maximum activity concentration in the region of interest (ROI) (MBq/kg)/injected dose (MBq)/body weight (kg)].
Immunohistochemistry
Paraffin-embedded sections (thickness, 4 μm) of the tissue specimens were prepared. Immunohistochemical (IHC) staining was performed to analyze the expressions of CD10, MUM-1, Bcl-6, Bcl-2, Ki-67, Glut-1, and Glut-3 in the lymphoma tissues using the Novolink Polymer Detection Systems (Novocastra Laboratories Ltd, Newcastle Upon Tyne, UK) according to the manufacturer’s instructions. The following antibodies were used: CD10 (clone 56C6, ready-to-use; Novocastra Laboratories Ltd), MUM-1 (clone MUM1P 1 : 50; Dako, Glostrup, Denmark), Bcl-6 (clone PG-B6p, 1 : 50; Dako), Bcl-2 (clone 124, 1 : 200; Dako), Ki-67 (clone MIB-1, 1 : 200; Dako), Glut-1 (clone C-terminus, 1 : 50; Merck Millipore, Darmstadt, Germany), and Glut-3 (polyclonal, 1 : 50; Abcam, Cambridge, UK).
Evaluation of the immunostaining was performed as follows: CD10, Bcl-2, Glut-1, and Glut-3 expression was localized to the cell membrane, whereas MUM-1, Bcl-6, and Ki-67 expression was localized to the nucleus. Under a high-power microscope (×400), five visual fields were selected randomly from each section and 100 cells from each field were counted. Then, the proportion of positive cells among these 500 cells was calculated. According to the literature, CD10, MUM-1, Bcl-6, and Bcl-2 were considered positive if expressed by more than 30% of the tumor cells. Patients were assigned to germinal center B-cell-like (GCB) or non-GCB subgroups on the basis of the immunophenotype, as determined using the Hans model 20. According to the percentage of Ki-67-positive cells, patients were divided into a high Ki-67 expression group (% expression> 50%) and a low Ki-67 expression group (% expression≤50%). The semiquantitative immunoreactive score (IRS) method was used to calculate the expressions of the Glut-1 and Glut-3 proteins 21,22. Staining intensity was scored as follows: no staining, 0 points; weak staining, 1 point; moderate staining, 2 points; and strong staining, 3 points. The percentage of positive cells was scored as follows: 0%, 0 points; less than 10%, 1 point; 11–50%, 2 points; 51–80%, 3 points; and more than 80%, 4 points. The final score was obtained by multiplying the level of staining intensity with the percentage of positive cells. Final scores of less than 6 indicated low expression, whereas scores of at least 6 indicated high expression.
Statistical analysis
Progression-free survival (PFS) was defined as the interval between diagnosis and the first detection of disease progression or recurrence, or death caused by factors unrelated to the lymphoma or its treatment. Overall survival (OS) was defined as the interval between diagnosis and death from any cause.
Data were subjected to a normality test using the single-sample Kolmogorov–Smirnov test. The t-test was used to compare the mean SUVmax among groups with various clinical characteristics (sex, age, systemic symptoms, number of extranodal sites, and molecular subtype), biochemical indicators, and molecular markers. Comparisons of SUVmax among groups on the basis of other clinical characteristics (staging, PS score, and IPI and R-IPI risk groups) were performed using one-way analysis of variance analysis. The correlation of SUVmax with biochemical indicators was analyzed using Spearman correlation analysis. Response rates were compared among the groups using the Pearson χ2-test. The receiver operating characteristic (ROC) curve was used to determine the optimal cut-off values of various biochemical indicators and SUVmax. Univariate survival analyses were carried out using the Kaplan–Meier method and the factors were compared using the log-rank test. Multivariate analyses were carried out using the Cox proportional hazard model. All the statistical analyses and graphics were carried out using the SPSS 17.0 software (SPSS Inc., Chicago, Illinois, USA). Differences with a P value of less than 0.05 on a two-tailed test were considered statistically significant.
Results
Patient characteristics
Of the 162 patients with newly diagnosed DLBCL, 22 were excluded because of the absence of any visible lesions on PET/CT (12 patients with primary gastrointestinal lymphoma, four with primary testicular lymphoma, and six with stage I nodal lymphoma); these patients had undergone tumor resection before the PET/CT examination. Thus, ultimately, 140 patients were enrolled in this study, including 75 men and 65 women, with a median age of 57 years (range, 17–83 years). Patients were followed up until 30 September 2014, with a median follow-up duration of 30 months (range, 4–124 months). The patients’ clinical data are presented in Table 1. Of the patients enrolled, 72.9% (102/140) received treatment with the R-CHOP regimen; the rest received CHOP chemotherapy rather than R-CHOP according to their own decision (mainly because of the poor economic conditions and unrelated to staging or prognosis).
Table 1.
Patient characteristics and relationship between SUVmax and clinical factors in patients with newly diagnosed DLBCL
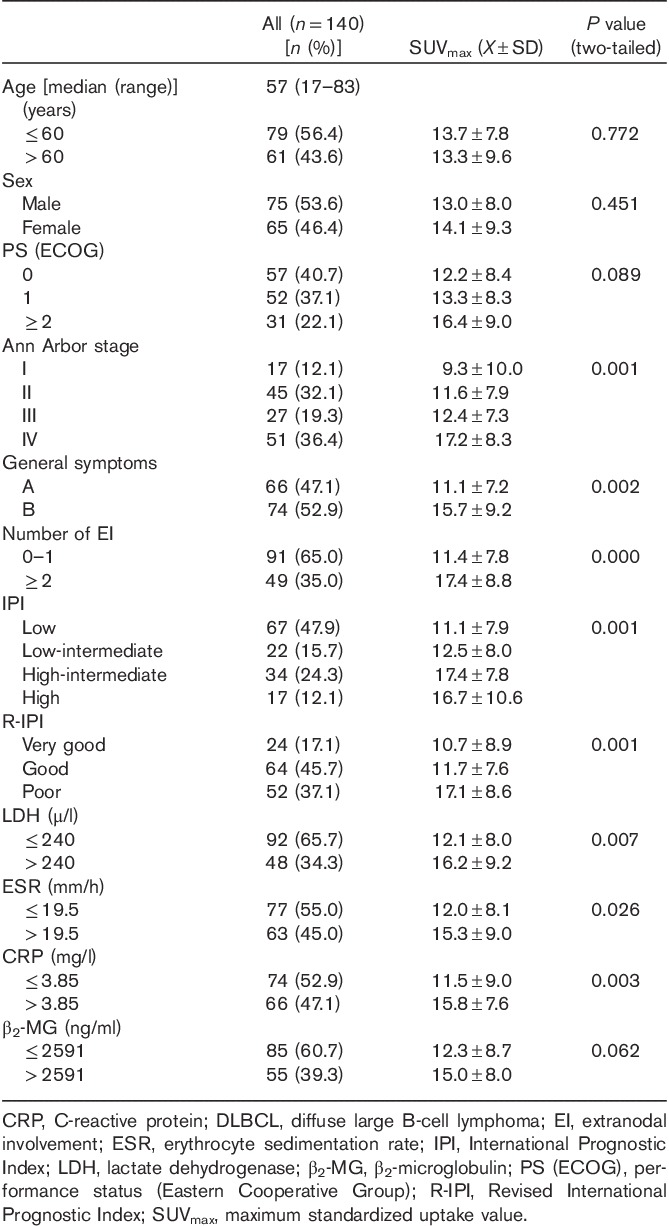
Relationship of pretreatment SUVmax with clinical factors
All the patients enrolled underwent pretreatment PET/CT, which yielded a median SUVmax of 12.2 (range, 1.7–42.7). We determined the relationship of the pretreatment SUVmax with various clinical factors. As shown in Table 1, SUVmax between groups differed significantly with respect to disease stage, presence of B symptoms, number of extranodal sites, and IPI or R-IPI scores, but not for age, sex, or PS.
Relationship of pretreatment SUVmax with biochemical indicators
Comparative and correlation analyses were carried out between the pretreatment SUVmax and each biochemical indicator in different groups. ROC curve analysis showed that the optimal cut-off values of lactate dehydrogenase (LDH), ESR, CRP, and β2-microglobulin level to predict SUVmax were 240 U/l, 19.5 mm/h, 3.85 mg/l, and 2591 ng/ml, respectively. SUVmax differed significantly with respect to LDH, ESR, and CRP levels, but not with β2-microglobulin levels (Table 1). Further correlation analyses showed that the baseline SUVmax was correlated positively with LDH (r=0.312, P=0.000), correlated weakly with ESR (r=0.203, P=0.016) and CRP (r=0.215, P=0.011), and not correlated with β2-microglobulin (r=0.163, P=0.055).
Correlation of pretreatment SUVmax with molecular markers
In this study, IHC detection of the expression of the molecular markers CD10, MUM-1, Bcl-6, Bcl-2, and Ki-67 in lymphoma specimens was performed in 81 patients. The results showed that 17.3% (14/81) of the patients belonged to the GCB subtype and 82.7% (67/81) to the non-GCB subtype according to the Hans model. The pretreatment SUVmax did not differ significantly between the GCB and the non-GCB groups (P=0.655). Bcl-2 expression was detected in 64.2% (52/81) of patients and SUVmax did not differ between the Bcl-2-negative and Bcl-2-positive groups (P=0.608). High Ki-67 expression was present in 77.8% (63/81) patients and the SUVmax in this group was significantly higher than that in the low Ki-67 expression group (15.1±10.0 vs. 9.4±4.9, P=0.001; Table 2).
Table 2.
Relationship between SUVmax and molecular markers in patients with newly diagnosed DLBCL
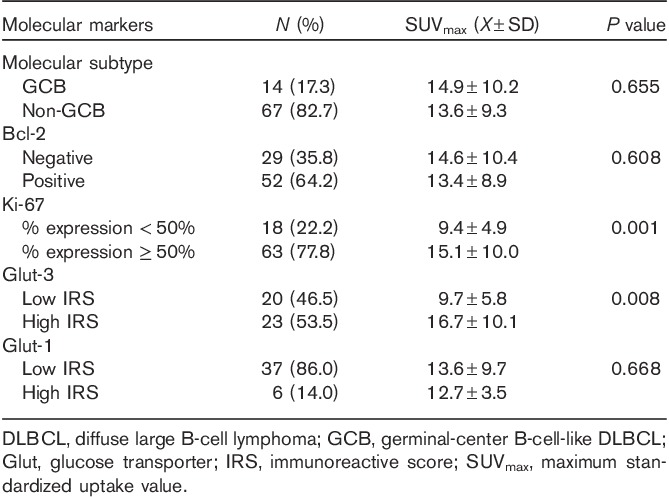
The expressions of the Glut-1 and Glut-3 proteins were assessed in 43 patients. The results showed that the average IRS for Glut-3 expression was 5.4±6.0 (median, 6.0; range, 0–12) and that 53.5% (23/43) of the patients had high Glut-3 expression (IRS≥6). The SUVmax in this group was significantly higher than that in the low Glut-3 expression group (mean±SD, 16.7±10.1 vs. 9.7±5.8, P=0.008). The average IRS for Glut-1 expression was 2.8±2.7 (median, 3.0; range, 0–12) and 14.0% (6/43) of patients had high Glut-1 expression. SUVmax did not show a significant difference between the high and the low Glut-1 expression groups (P=0.668; Table 2).
Determination of the optimal cut-off value of pretreatment SUVmax
The ROC curve showed that the optimal cut-off value of SUVmax to predict disease progression and survival in DLBCL patients was 9.0. The patients were divided into two groups on the basis of this cut-off value: low SUVmax group, 49 patients (pretreatment SUVmax≤9.0), and high SUVmax group, 91 patients (pretreatment SUVmax>9.0).
Correlation of pretreatment SUVmax with treatment response
The treatment response was assessed in all the enrolled patients after the completion of six to eight courses of treatment. The response could be determined in 138 patients. The complete remission rate [complete response (CR)+unconfirmed complete response (Cru)] was 71.7% (76.0% for the R-CHOP group and 60.5% for the CHOP group) and the overall response rate (ORR; CR+CRu+partial response ) was 89.1% (93.0% for the R-CHOP group and 78.9% for the CHOP group).
The CR and ORR rates after immunochemotherapy or chemotherapy were significantly higher in the low SUVmax group (pretreatment SUVmax≤9.0) than in the high SUVmax group (CR: 91.7 vs. 61.1%, P=0.000; ORR: 100 vs. 83.3%, P=0.003). During the study period, 35.2% (32/91) of patients in the high SUVmax group showed disease progression and the median interval between diagnosis and disease progression was 8.0 months (range, 3.0–51.0 months). In 40% (13/32) of these patients, disease progression occurred within 6 months after diagnosis. In the low SUVmax group, the progression rate was 6.12% (3/49), which was significantly lower than the rate in the high SUVmax group (P=0.000). Furthermore, the median interval between diagnosis and disease progression was 17.0 months (range, 14.0–19.0 months).
Correlation of pretreatment SUVmax with survival
In this study, the median follow-up was 30 months, and the median PFS and OS have not yet been reached. The 3-year PFS and OS rates in the entire group were 73.8 and 86.1%, respectively. Significant differences in PFS and OS were found between patients with pretreatment SUVmax above and below the 9.0 cut-off. The 3-year PFS rates in the low and high SUVmax groups were 92.2 and 63.6%, respectively (log-rank test, P=0.000; Fig. 1). The corresponding 3-year OS rates were 100 and 78.3% (log-rank test, P=0.003; Fig. 1).
Fig. 1.
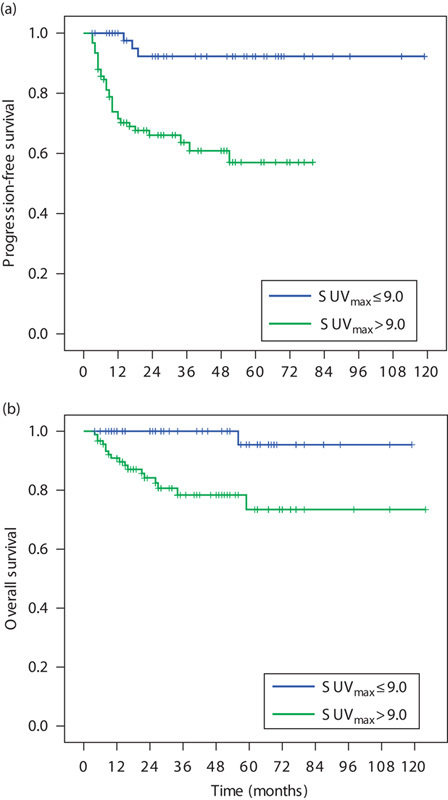
The survival curves of 140 patients receiving first-line R-CHOP or CHOP therapy for newly diagnosed DLBCL. (a) Progression-free survival (PFS) of patients according to SUVmax (SUVmax: ≤9 vs. >9; P=0.000); (b) overall survival (OS) of patients according to SUVmax (SUVmax: ≤9 vs. >9; P=0.003). DLBCL, diffuse large B-cell lymphoma; SUVmax, maximum standard uptake value.
The outcomes according to the R-IPI are presented in Fig. 2. The R-IPI remained predictive in DLBCL patients treated with R-CHOP or CHOP, and it distinguished three separate prognostic groups with 3-year PFS rates ranging from 55.3 to 90.7% (log-rank test, P=0.000) and 3-year OS rates ranging from 74 to 100% (log-rank test, P=0.003). Subgroup analyses were carried out in the different R-IPI risk groups. In the ‘good’ R-IPI subgroup, the PFS did not differ significantly between patients with pretreatment SUVmax values of up to 9.0 and greater than 9.0. However, in the ‘very good’ R-IPI subgroup, the 3-year PFS was significantly better in patients with pretreatment SUVmax values of up to 9.0 than in those with SUVmax values greater than 9.0 (100 vs. 72.9%, P=0.044). Furthermore, in this subgroup, the PFS of two patients with pretreatment SUVmax values of 13.9 and 15.0 was only 5 and 9 months, respectively. Similarly, in the ‘poor’ R-IPI subgroup, a lower pretreatment SUVmax was associated with better survival (3-year PFS: 100 vs. 46.9%, P=0.011). In the ‘poor’ R-IPI subgroup, eight patients with pretreatment SUVmax up to 9.0 currently remain disease-free after a follow-up duration of up to 69 months. In the different R-IPI risk subgroups, the OS did not differ significantly between patients with pretreatment SUVmax values of up to 9.0 and greater than 9.0.
Fig. 2.
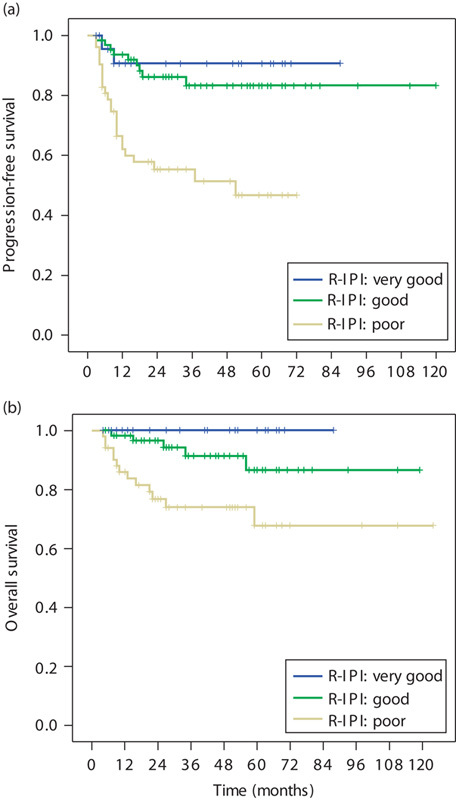
The survival curves of 140 patients receiving first-line R-CHOP or CHOP therapy for newly diagnosed DLBCL. (a) Progression-free survival (PFS) of patients according to R-IPI risk group (P=0.000); (b) overall survival (OS) of patients according to R-IPI risk group (P=0.003). DLBCL, diffuse large B-cell lymphoma; R-IPI, revised International Prognostic Index.
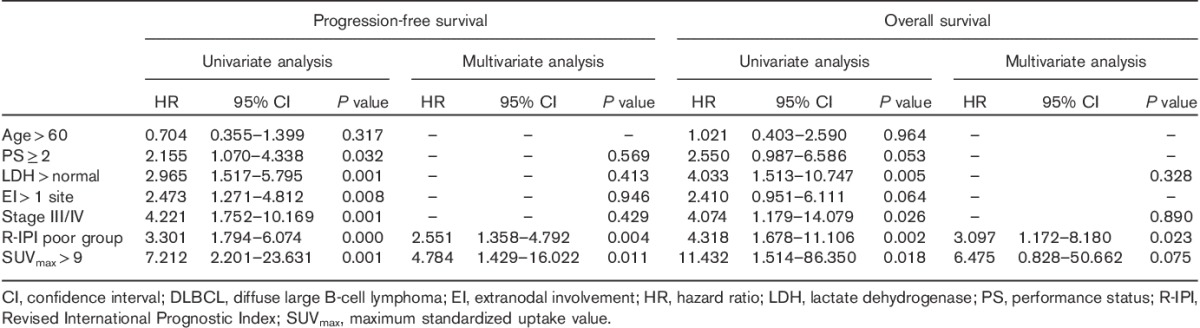
Univariate and multivariate analyses were carried out as follows: five IPI factors (age, PS score, LDH level, number of extranodal sites, and Ann Arbor stage), R-IPI score, and SUVmax were included in the univariate analysis. The results showed that PS score≥2, LDH>normal, number of extranodal sites>1, Ann Arbor stage of III/IV, ‘poor’ R-IPI, and SUVmax>9.0 were unfavorable prognostic factors affecting PFS. Similarly, LDH>normal, Ann Arbor stage of III/IV, ‘poor’ R-IPI, and SUVmax>9.0 predicted low OS. The six variables associated with PFS and four variables associated with OS on univariate analysis were included in a Cox proportional hazard model for multivariate analysis, in which the forward-stepwise method (likelihood ratio) was used. The results showed that R-IPI and SUVmax were independent prognostic factors affecting PFS [R-IPI: P=0.004, hazard ratio (HR)=2.551; SUVmax: P=0.011, HR=4.784], whereas R-IPI was an independent prognostic factor affecting OS (P=0.023, HR=3.097) (Table 3).
Table 3.
Univariate and multivariate Cox proportional hazards model analysis for progression-free survival and overall survival of newly diagnosed DLBCL patients
A novel prognostic model on the basis of IPI score and pretreatment SUVmax
A novel prognostic model was established by combining the IPI score and the pretreatment SUVmax value. An absence of any unfavorable IPI factors and a pretreatment SUVmax of up to 9.0 indicated low risk; IPI score of at least 3 and SUVmax greater than 9.0 indicated high risk, and all remaining scenarios indicated moderate risk. This prognostic model was used to carry out a survival analysis. The results showed that the 3-year PFS rates in the low-risk, moderate-risk, and high-risk groups were 100, 86.3, and 46.9% (P=0.000), respectively, whereas the corresponding 3-year OS rates were 100, 92.9, and 62.2% (P=0.000; Fig. 3). This system was included in the Cox proportional hazard model for multivariate analysis with the forward-stepwise method (likelihood ratio). The results showed that the IPI score/SUVmax prognostic model was an independent prognostic factor associated with PFS (P=0.000, HR=5.696, 95% confidence interval: 2.873–11.294) and OS (P=0.000, HR=5.840, 95% CI: 2.173–15.699).
Fig. 3.
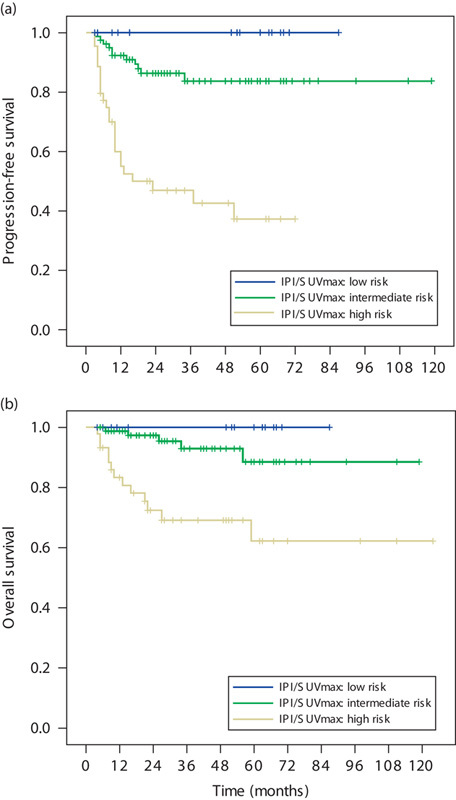
The survival curves of 140 patients receiving first-line R-CHOP or CHOP therapy for newly diagnosed DLBCL. (a) Progression-free survival (PFS) of patients according to novel IPI score/SUVmax prognostic modeling (P=0.000); (b) overall survival (OS) of patients according to novel IPI score/SUVmax prognostic modeling (P=0.000). DLBCL, diffuse large B-cell lymphoma; IPI, International Prognostic Index; SUVmax, maximum standard uptake value.
Discussion
DLBCL is a highly heterogeneous disease, and 30–40% of patients with DLBCL show disease progression or recurrence even after standardized immunochemotherapy 23. Therefore, accurate risk stratification in these patients before treatment is a research hot spot. IPI or R-IPI is still the most commonly used clinical indicator of prognosis in the current rituximab era 24–26, whereas molecular markers that reflect the biological behaviors of tumor cells are typically not used for assessment of prognosis in DLBCL patients. In recent years, some studies have found that gene-expression profiling, miRNA expression patterns, etc., tend to provide predictive information on the molecular biology of DLBCL 27,28. However, these examinations are very expensive and difficult to perform routinely in clinical practice. In contrast, PET/CT has become an essential investigation in patients with newly diagnosed DLBCL and clinicians can easily determine the PET/CT parameter SUVmax, which reflects 18F-FDG uptake. The clinical prognostic value of pretreatment SUVmax has only been reported in a few studies, with small sample sizes and inconsistent results. This study aimed to address this issue and our results indicated that the pretreatment SUVmax was correlated with clinical factors, the molecular markers Ki-67 and Glut-3, and treatment response in DLBCL patients; this parameter was also found to be an important predictor of survival in these patients.
Our study showed that pretreatment SUVmax was correlated significantly with stage, B symptoms, number of extranodal sites, IPI or R-IPI score, LDH, ESR, and CRP. Byun et al. 29 and Hirose et al. 30 have also reported that SUVmax is associated with IPI risk groups. However, the latter study failed to find a correlation of SUVmax with disease stage, B symptoms, and extranodal involvement, as was shown in the present study. The sample size of the study carried out by Hirose and colleagues was relatively small, possibly leading to imbalanced grouping in the subgroup analyses. For example, only 17.6% (12/68) of the patients in their study showed general symptoms or more than two extranodal lesions, which might have introduced a statistical bias. Therefore, studies with large samples are required to confirm our results. The elevated serum LDH level in DLBCL patients reflects the tumor burden and cell turnover, which may account for its correlation with SUVmax.
In our study, pretreatment SUVmax was significantly higher in patients with high Ki-67 expression (77.8% of patients) than in those with low Ki-67 expression. A recent meta-analysis by Deng et al. 8 indicated that SUVmax showed a moderate positive correlation with Ki-67 expression in patients with various cancers, of which the most significant correlation was found in patients with thymic epithelial tumors and gastrointestinal stromal tumors. However, different results have been obtained in studies of lymphoma patients. Chihara et al. 16 studied 24 DLBCL patients and found that the pretreatment SUVmax was correlated weakly with Ki-67 expression, whereas further analyses indicated that Ki-67 expression did not differ significantly between patients with SUVmax of at least 30 and less than 30. Hirose et al. 30 studied 68 DLBCL patients and also failed to find a correlation between SUVmax and Ki-67 expression. However, Watanabe et al. 31 reported a significant correlation between SUVmax at the biopsy site (BSUVmax) and Ki-67 expression in tumor tissues in 36 NHL patients (including 16 DLBCL patients). Similarly, Papajík et al. 7 studied 149 NHL patients (including 78 DLBCL patients) and found that SUVmax was significantly higher in patients with high Ki-67 expression (>60%) than in those with low Ki-67 expression (≤60%). This finding is consistent with our results, suggesting that 18F-FDG uptake is correlated with Ki-67 expression to a certain extent and that pretreatment SUVmax reflects the proliferation activity of lymphoma cells.
In our study, high Glut-3 expression was detected in 53.5% of the patients. The pretreatment SUVmax was significantly higher in the high Glut-3 expression group than in the low Glut-3 expression group. However, similar results were not obtained in the case of Glut-1 expression. Only a few small studies have investigated the role of Gluts in the mechanism of 18F-FDG uptake in patients with malignant lymphomas, with inconsistent conclusions. In a report involving 1 DLBCL patient, Koga et al. 32 found that 18F-FDG uptake differed with Glut-1 expression in lymphoma lesions. Khandani and colleagues studied 31 lymphoma patients (25 NHL patients, including nine patients with DLBCL, as well as six patients with Hodgkin’s lymphoma) and obtained consistent results. The authors reported that specific Glut-1 expression in lymphoma cells was detected in 45% (14/31) of patients, and the percentage of Glut-1+ cells was significantly and positively correlated with SUVmax. In addition, they reported that Glut-3 expression was detected in only 6.5% (2/31) of patients and was not significantly correlated with SUVmax 33. However, in a study involving 16 patients with B-cell NHL (including 11 DLBCL patients), Shim and colleagues failed to detect Glut-1 expression in lymphoma tissues on IHC, but found moderate-to-strong Glut-3 expression in almost all patients (15/16, positive rate, >60%). However, this study failed to find any correlation between Glut-3 and SUVmax 34. Recently, Hirose et al. 30 carried out studies to investigate BSUVmax and Glut expression, and found that the expressions of Glut-1 and Glut-3 in tumor tissues were closely correlated with the BSUVmax value. Nevertheless, we failed to obtain consistent results with previous studies, which might be attributable to the small sample sizes of the previous studies. There is still a lack of large-scale studies on this topic. From our results, we concluded that Glut-3, but not Glut-1, played a critical role in the mechanism of 18F-FDG uptake in lymphoma cells in DLBCL patients. Currently ongoing studies are focused on investigating Glut protein expression in DLBCL. A recent study by Takahashi et al. 35 focused on Glut mRNA expression and found that the expression of Glut-3 mRNA, but not Glut-1 mRNA, showed a significant positive correlation with preoperative SUVmax in seven patients with primary central nervous system lymphoma (all DLBCL), which verified our conclusion at the gene level.
Our study confirmed that R-IPI still had a clear prognostic value in patients with newly diagnosed DLBCL. More importantly, we found that pretreatment SUVmax was closely associated with treatment response, and univariate and multivariate analyses indicated that pretreatment SUVmax was an independent prognostic factor affecting PFS, especially in patients in the ‘very good’ and ‘poor’ R-IPI risk groups. On the basis of these findings, we developed a novel prognostic model integrating the IPI score and pretreatment SUVmax to improve risk stratification in DLBCL patients. With accurate risk stratification, overtreatment can be avoided in patients with a low risk of progression/recurrence. In patients with a high risk, however, the therapeutic strategy can be adjusted by the addition of new biologic agents to the standardized R-CHOP chemotherapy regimen or by dose intensification to improve outcomes. Our results are consistent with those obtained by Chihara et al. [16] and Miyazaki et al. 17. Both these studies retrospectively analyzed newly diagnosed DLBCL patients who received chemotherapy with rituximab and involved 110 and 50 patients, respectively. Their results showed that pretreatment SUVmax was an important predictor of PFS, and high SUVmax was associated closely with disease progression. Adams et al. 18 recently studied 73 DLBCL patients and obtained results different from ours. Their single-center retrospective study analyzed various parameters obtained during pretreatment PET examination and the prognostic value of the NCCN-IPI, and found no prognostic value of SUVmax for PFS and OS. However, they applied a median of 22 as the cut-off SUVmax value, which differed from the value used in this study. Furthermore, the proportion of stage-III/IV patients in their study was 84.9%, which was significantly higher than that in this study (55.7%). In addition, in their study, 44 (37.6%) of 117 consecutive patients were excluded for various reasons (including incompleteness of data). All these factors may have caused selection bias and had an impact on the statistical results.
The present study may have several limitations. For example, the measurement of SUVmax is influenced by many patient and technical factors, such as blood glucose levels, examination protocols, calibration of the device, spatial resolution, matrix size, applied zoom, voxel volume, reconstruction method, number of iterations, postfiltering, determination of region of interest, partial volume effect, etc. 36. The SUVmax cut-off value may differ among patient populations according to PET/CT scanners and acquisition techniques. This, in part, is likely to be responsible for many of the discrepancies in previously published research. In our study, all patients were scanned using the same PET/CT scanner in a single center according to a standard protocol to maintain reproducibility. Further, the majority of patients (∼70%) were diagnosed with DLBCL by tumor biopsy first and then subjected to a PET/CT scan; thus, the SUVmax in the patients pertained to the remnant lymphoma lesion and might not have been the same as the SUVmax of the entire tumor. In clinical practice, PET/CT is usually performed for disease staging after the diagnosis of lymphoma; prebiopsy PET/CT is not a routine examination method. Currently, only a few studies have compared whole-body SUVmax (WBSUVmax) with that at the biopsy site. Wu et al. 37 found that WBSUVmax was higher than BSUVmax in 15 DLBCL patients (19.6±7.7 vs. 16.6±5.8, P<0.01), suggesting that tumor biopsy is not likely to affect the WBSUVmax value. Therefore, we believe that it is appropriate to replace BSUVmax with WBSUVmax to analyze the correlation with clinicopathologic factors and its prognostic value. Finally, because of the retrospective nature of the present study, large-scale prospective studies are needed to confirm the prognostic value of the pretreatment PET/CT quantization parameters on the basis of the findings obtained from the present retrospective study.
Conclusion
Pretreatment SUVmax in newly diagnosed DLBCL patients is closely associated with IPI and other clinical factors, the expressions of the molecular markers Ki-67 and Glut-3, as well as the clinical response. Both R-IPI and SUVmax are independent prognostic factors affecting PFS in these patients. Furthermore, the novel prognostic model on the basis of IPI scores and pretreatment SUVmax proposed in this study may have significant clinical value for risk stratification in patients with newly diagnosed DLBCL.
Supplementary Material
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.nuclearmedicinecomm.com).
Acknowledgements
This study was funded in part by the Project of Further Accelerating Development of Traditional Chinese Medicine in Shanghai (grant number ZY3-CCCX-3–3037).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Sun J, Yang Q, Lu Z, He M, Gao L, Zhu M, et al. Distribution of lymphoid neoplasms in China: analysis of 4,638 cases according to the World Health Organization classification. Am J Clin Pathol 2012; 138:429–434. [DOI] [PubMed] [Google Scholar]
- 3.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25:579–586. [DOI] [PubMed] [Google Scholar]
- 4.Casasnovas RO, Meignan M, Berriolo-Riedinger A, Bardet S, Julian A, Thieblemont C, et al. SUVmax reduction improves early prognosis value of interim positron emission tomography scans in diffuse large B-cell lymphoma. Blood 2011; 118:37–43. [DOI] [PubMed] [Google Scholar]
- 5.Nols N, Mounier N, Bouazza S, Lhommel R, Costantini S, Vander Borght T, et al. Quantitative and qualitative analysis of metabolic response at interim positron emission tomography scan combined with International Prognostic Index is highly predictive of outcome in diffuse large B-cell lymphoma. Leuk Lymphoma 2014; 55:773–780. [DOI] [PubMed] [Google Scholar]
- 6.Ngeow JY, Quek RH, Ng DC, Hee SW, Tao M, Lim LC, et al. High SUV uptake on FDG-PET/CT predicts for an aggressive B-cell lymphoma in a prospective study of primary FDG-PET/CT staging in lymphoma. Ann Oncol 2009; 20:1543–1547. [DOI] [PubMed] [Google Scholar]
- 7.Papajík T, Mysliveček M, Sedová Z, Buriánková E, Procházka V, Koranda P, et al. Standardised uptake value of 18F-FDG on staging PET/CT in newly diagnosed patients with different subtypes of non-Hodgkin’s lymphoma. Eur J Haematol 2011; 86:32–37. [DOI] [PubMed] [Google Scholar]
- 8.Deng SM, Zhang W, Zhang B, Chen YY, Li JH, Wu YW. Correlation between the uptake of 18F-fluorodeoxyglucose (18F-FDG) and the expression of proliferation-associated antigen Ki-67 in cancer patients: a meta-analysis. PLoS One 2015; 10:e0129028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Geus-Oei LF, van Krieken JH, Aliredjo RP, Krabbe PF, Frielink C, Verhagen AF, et al. Biological correlates of FDG uptake in non-small cell lung cancer. Lung Cancer 2007; 55:79–87. [DOI] [PubMed] [Google Scholar]
- 10.Kaida H, Hiromatsu Y, Kurata S, Kawahara A, Hattori S, Taira T, et al. Relationship between clinicopathological factors and fluorine-18-fluorodeoxyglucose uptake in patients with papillary thyroid cancer. Nucl Med Commun 2011; 32:690–698. [DOI] [PubMed] [Google Scholar]
- 11.Sepesi B, Raymond DP, Polomsky M, Watson TJ, Litle VR, Jones CE, et al. Does the value of PET-CT extend beyond pretreatment staging? An analysis of survival in surgical patients with esophageal cancer. J Gastrointest Surg 2009; 13:2121–2127. [DOI] [PubMed] [Google Scholar]
- 12.van Baardwijk A, Dooms C, van Suylen RJ, Verbeken E, Hochstenbag M, Dehing-Oberije C, et al. The maximum uptake of (18)F-deoxyglucose on positron emission tomography scan correlates with survival, hypoxia inducible factor-1alpha and GLUT-1 in non-small cell lung cancer. Eur J Cancer 2007; 43:1392–1398. [DOI] [PubMed] [Google Scholar]
- 13.de Geus-Oei LF, Wiering B, Krabbe PF, Ruers TJ, Punt CJ, Oyen WJ. FDG-PET for prediction of survival of patients with metastatic colorectal carcinoma. Ann Oncol 2006; 17:1650–1655. [DOI] [PubMed] [Google Scholar]
- 14.Vu CC, Matthews R, Kim B, Franceschi D, Bilfinger TV, Moore WH. Prognostic value of metabolic tumor volume and total lesion glycolysis from18F-FDG PET/CT in patients undergoing stereotactic body radiation therapy for stage I non-small-cell lung cancer. Nucl Med Commun 2013; 34:959–963. [DOI] [PubMed] [Google Scholar]
- 15.Brown C, Howes B, Jamieson GG, Bartholomeusz D, Zingg U, Sullivan TR, Thompson SK. Accuracy of PET-CT in predicting survival in patients with esophageal cancer. World J Surg 2012; 36:1089–1095. [DOI] [PubMed] [Google Scholar]
- 16.Chihara D, Oki Y, Onoda H, Taji H, Yamamoto K, Tamaki T, Morishima Y. High maximum standard uptake value (SUVmax) on PET scan is associated with shorter survival in patients with diffuse large B cell lymphoma. Int J Hematol 2011; 93:502–508. [DOI] [PubMed] [Google Scholar]
- 17.Miyazaki Y, Nawa Y, Miyagawa M, Kohashi S, Nakase K, Yasukawa M, Hara M. Maximum standard uptake value of 18F-fluorodeoxyglucose positron emission tomography is a prognostic factor for progression-free survival of newly diagnosed patients with diffuse large B cell lymphoma. Ann Hematol 2013; 92:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams HJ, de Klerk JM, Fijnheer R, Heggelman BG, Dubois SV, Nievelstein RA, Kwee TC. Prognostic superiority of the National Comprehensive Cancer Network International Prognostic Index over pretreatment whole-body volumetric-metabolic FDG-PET/CT metrics in diffuse large B-cell lymphoma. Eur J Haematol 2015; 94:532–539. [DOI] [PubMed] [Google Scholar]
- 19.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. World Health Organization Classification of Tumors of hematopoietic and lymphoid tissues[M], 4th ed. Lyon, France: IARC Press; 2008. [Google Scholar]
- 20.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004; 103:275–282. [DOI] [PubMed] [Google Scholar]
- 21.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987; 8:138–140. [PubMed] [Google Scholar]
- 22.van de Nes JA, Griewank KG, Schmid KW, Grabellus F. Immunocytochemical analysis of glucose transporter protein-1 (GLUT-1) in typical, brain invasive, atypical and anaplastic meningioma. Neuropathology 2015; 35:24–36. [DOI] [PubMed] [Google Scholar]
- 23.Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 2010; 116:2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med 1993; 329:987–994. [DOI] [PubMed] [Google Scholar]
- 25.Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 2007; 109:1857–1861. [DOI] [PubMed] [Google Scholar]
- 26.Huang HH, Xiao F, Chen FY, Wang T, Li JM, Wang JM, et al. Reassessment of the prognostic value of the International Prognostic Index and the revised International Prognostic Index in patients with diffuse large B-cell lymphoma: a multicentre study. Exp Ther Med 2012; 4:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutiérrez-García G, Cardesa-Salzmann T, Climent F, González-Barca E, Mercadal S, Mate JL, et al. Gene-expression profiling and not immunophenotypic algorithms predicts prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Blood 2011; 117:4836–4843. [DOI] [PubMed] [Google Scholar]
- 28.Iqbal J, Shen Y, Huang X, Liu Y, Wake L, Liu C, et al. Global microRNA expression profiling uncovers molecular markers for classification and prognosis in aggressive B-cell lymphoma. Blood 2015; 125:1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byun BH, Na, Cheon GJ, Kang HJ, Kim KM, Lee SS, et al. Clinical significance of 18F-FDG uptake by primary sites in patients with diffuse large B cell lymphoma in the head and neck: a pilot study. Ann Nucl Med 2008; 22:645–651. [DOI] [PubMed] [Google Scholar]
- 30.Hirose Y, Suefuji H, Kaida H, Hayakawa M, Hattori S, Kurata S, et al. Relationship between 2-deoxy-2-[(18)F]-fluoro-d-glucose uptake and clinicopathological factors in patients with diffuse large B-cell lymphoma. Leuk Lymphoma 2014; 55:520–525. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe R, Tomita N, Takeuchi K, Sakata S, Tateishi U, Tanaka M, et al. SUVmax in FDG-PET at the biopsy site correlates with the proliferation potential of tumor cells in non-Hodgkin lymphoma. Leuk Lymphoma 2010; 51:279–283. [DOI] [PubMed] [Google Scholar]
- 32.Koga H, Matsuo Y, Sasaki M, Nakagawa M, Kaneko K, Hayashi K, et al. Differential FDG accumulation associated with GLUT-1 expression in a patient with lymphoma. Ann Nucl Med 2003; 17:327–331. [DOI] [PubMed] [Google Scholar]
- 33.Khandani AH, Dunphy CH, Meteesatien P, Dufault DL, Ivanovic M, Shea TC. Glut1 and Glut3 expression in lymphoma and their association with tumor intensity on 18F-fluorodeoxyglucose positron emission tomography. Nucl Med Commun 2009; 30:594–601. [DOI] [PubMed] [Google Scholar]
- 34.Shim HK, Lee WW, Park SY, Kim H, So Y, Kim SE. Expressions of glucose transporter types 1 and 3 and hexokinase-II in diffuse large B-cell lymphoma and other B-cell non-Hodgkin’s lymphomas. Nucl Med Biol 2009; 36:191–197. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi Y, Akahane T, Yamamoto D, Nakamura H, Sawa H, Nitta K, et al. Correlation between positron emission tomography findings and glucose transporter 1, 3 and L-type amino acid transporter 1 mRNA expression in primary central nervous system lymphomas. Mol Clin Oncol 2014; 2:525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wahl RL. Wahl RL. Principles of cancer imaging with fluorodeoxyglucose. Principles and practice of positron emissiontomography. Philadelphia: Lippincott Williams and Wilkins; 2002. 100–110. [Google Scholar]
- 37.Wu X, Pertovaara H, Korkola P, Vornanen M, Eskola H, Kellokumpu-Lehtinen PL. Glucose metabolism correlated with cellular proliferation in diffuse large B-cell lymphoma. Leuk Lymphoma 2012; 53:400–405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.nuclearmedicinecomm.com).


