Abstract
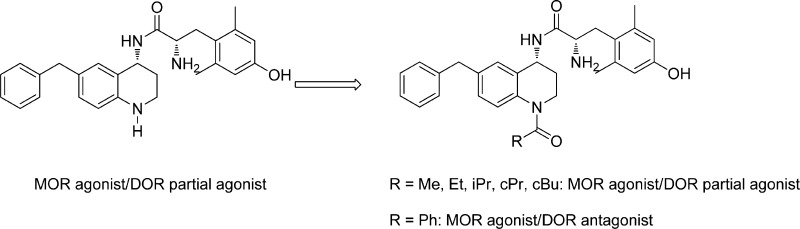
N-Acetylation of the tetrahydroquinoline (THQ) core of a series of μ-opioid receptor (MOR) agonist/δ-opioid receptor (DOR) antagonist ligands increases DOR affinity, resulting in ligands with balanced MOR and DOR affinities. We report a series of N-substituted THQ analogues that incorporate various carbonyl-containing moieties to maintain DOR affinity and define the steric and electronic requirements of the binding pocket across the opioid receptors. 4h produced in vivo antinociception (ip) for 1 h at 10 mg/kg.
Introduction
Mu-opioid receptor (MOR) agonists, such as morphine, are the gold standard in the treatment of moderate to severe pain, however, their therapeutic use is often limited due to serious side effects including tolerance, dependence, respiratory depression, and constipation.1−3 Numerous studies have shown promise for ameliorating MOR-related side effects through simultaneous action at δ-opioid receptors (DOR). DOR agonists have been shown to reduce the development of MOR agonist tolerance and dependence,4,5 presumably due to the ability of DOR agonists to potentiate MOR mediated analgesia.6 DOR agonists have also been shown to block κ-opioid receptor (KOR) mediated antinociception which has possible implications in reversing KOR related addictive behaviors.7 Strong evidence has also accumulated for the potential of DOR antagonists as agents to mitigate MOR-mediated side effects. Recently, there have been multiple reports on the utility and application of mixed-efficacy compounds that simultaneously produce MOR activation while acting as DOR antagonists.8−15 While the mechanism remains unclear, this profile has been shown to elicit the desired analgesia of a MOR agonist but with reduced risk of tolerance and dependence in a number of preclinical models of analgesia.16−18
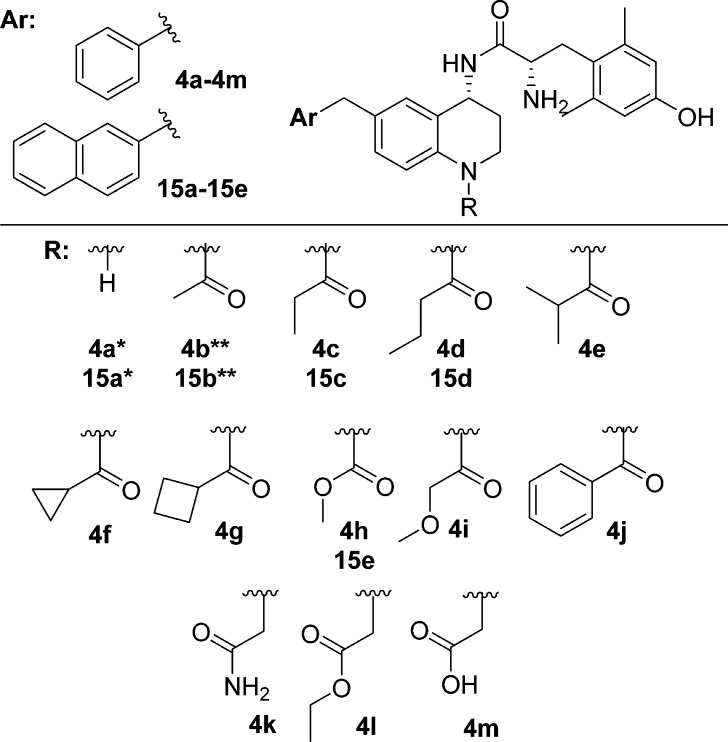
We have previously described a series of peptidomimetics in which key opioid pharmacophore elements were transferred from a peptide scaffold to a tetrahydroquinoline (THQ) core, a smaller, more drug-like scaffold. Expanding upon this initial report, we modified the THQ core in an effort to increase metabolic stability in two ways: through N-acetylation of the THQ nitrogen and through replacement the THQ core with a tetrahydronaphthalene (THN) core.9 Through these modifications, we found that N-acetylation of the THQ core of the mixed-efficacy MOR agonist/DOR antagonist peptidomimetics improves DOR affinity, which results in an overall better balance of affinities at MOR and DOR and provides selectivity over the κ-opioid receptor (KOR).9 Although the optimal balance of MOR affinity and DOR affinity is unknown, we reasoned that similar high affinity profiles at both MOR and DOR was a reasonable goal, as this would ensure that both MOR and DOR character would be similarly represented. In the same report, we proposed that an additional polar contact between the carbonyl of the N-acetyl moiety and Tyr129 of DOR in transmembrane 3 (TM3) helix is responsible for this increase in DOR affinity.9 Analogues in the present structure–activity relationship (SAR) campaign were therefore designed (1) to include a carbonyl moiety in order to maintain the high DOR affinity seen in the initial analogue series and (2) to incorporate various aliphatic, cyclic, aromatic, and heteroatom-containing functionalities (Figure 1) to probe the effect of such modifications on the binding affinity and efficacy profiles not only at DOR but also at MOR and KOR.
Figure 1.
Peptidomimetic scaffold and N-substitutions; *previously published in ref (8), **previously published in ref (9).
Results
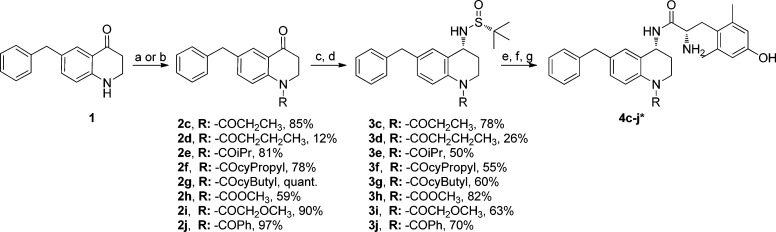
Synthesis of N-Acylated Benzyl Pendant Analogues 4c–4j
Analogues 4a and 4b have been previously reported8,9 and are included here to serve as standards of comparison for the additional benzyl pendant N-substituted series. The majority of the remaining peptidomimetics (4c–4j) reported herein were prepared in four steps starting from the dihydroquinolinone 1, which was prepared as previously reported (Scheme 1).9 Compound 1 was subjected to acylation using either an acid anhydride or acyl chloride to form intermediates 2c–32j. It should be noted that the low yield for the synthesis of 2d was due to the use of pyridine, which was originally included to help catalyze the reaction. However, we found that running the reactions in neat anhydride at temperatures between 90 and 120 °C led to better product conversion, and for the remainder of syntheses, we excluded pyridine in this reaction step. Next, intermediates 2c–2j were treated with (R)-t-butanesulfinamide and Ti(OEt)4 to yield imines in situ, which were reduced with NaBH4 to form the desired R-stereochemistry of intermediates 3c–3j.19−21 The Ellman auxiliary was cleaved using concentrated hydrochloric acid, forming hydrochloride salts,19 which were then coupled to di-Boc protected 2′,6′-dimethyl-l-tyrosine (di-Boc-Dmt) and subsequently deprotected using trifluoroacetic acid (TFA) to yield analogues 4c–4j. As we have previously shown, the Ellman chemistry employed here produces the desired (R)-stereocenter with high enantiomeric excess,11 as evidenced in the current syntheses by the absence of NMR-detectable disateromer after coupling to diBoc-Dmt.
Scheme 1. Synthesis of N-Acylated Benzyl Pendant Analogues 4c–4j.
(a) neat acid anhydride (excess), 100 °C, 24 h (for compunds 2c, 2d); (b) acid chloride (eq), DCM, (for 2e–j); (c) (R)-t-butanesulfinamide (2–3 equiv), THF, Ti(OEt)4 (4–6 equiv), 0 °C, then reflux at 75 °C; (d) NaBH4 (6 equiv), THF, −50 °C to RT, then MeOH, RT; (e) HCl (6 equiv), dioxane, RT; (f) diBoc-Dmt (1.05 equiv), PyBOP (1 equiv), 6Cl-HOBt (1 equiv), DIPEA (10 equiv), DMF, RT; (g) 1:1 TFA:DCM (excess). Note: not all crude final product was purified; as such a yield was not calculated.
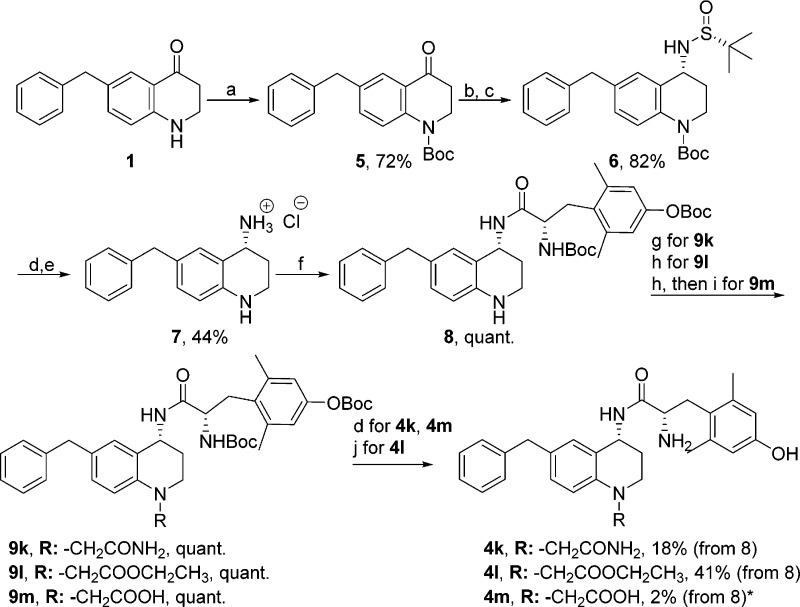
Synthesis of N-Alkylated Benzyl Pendant Analogues 4k–4m
The synthesis of the remaining analogues 4k–4m began by protecting the nitrogen in 1 with a tert-butyloxycarbonyl (Boc) group forming 5 (Scheme 2), as previously described.8 Intermediate 5 was treated with (R)-t-butanesulfinamide and Ti(OEt)4 to yield an imine in situ, which was reduced with NaBH4 to give the desired R-stereochemistry of intermediate 6.19−21 To ensure both the cleavage of the Ellman auxiliary and the removal of the Boc group, 6 was treated first with TFA then with conc HCl in dioxane to ultimately yield the hydrochloride salt 7. Intermediate 7 was coupled to diBoc-Dmt, forming 8. From this intermediate, 8, the syntheses of 4k, 4l, and 4m diverged. The synthesis of 4k was completed by first performing a Finkelstein reaction on 2-chloroacetamide to form 2-iodoacetamide, which was cannulated into a solution containing 8 and N,N-diisopropylethylamine (DIPEA) to give 9k. Intermediate 9k was treated with TFA to remove the Boc groups, yielding 4k. To complete the syntheses of 4l and 4m, ethyl 2-bromoacetate was added to intermediate 8 in the presence of potassium carbonate (K2CO3) to give 9l. Intermediate 9l was then treated with TFA to remove Boc groups and afford compound 4l. Compound 4m was completed by first saponification of the ester of 9l with lithium hydroxide, which was subsequently treated with TFA to remove the Boc groups and yield compound 4m.
Scheme 2. Synthesis of N-Alkylated Benzyl Pendant Analogues 4k–4m.
(a) Boc2O (1.2–2 equiv), DMAP (0.1 equiv), DIPEA (1.2–2 equiv), DCM, 60 °C; (b) (R)-t-butanesulfinamide (2–3 equiv), THF, Ti(OEt)4 (4–6 equiv), 0 °C, then reflux at 75 °C; (c)NaBH4 (6 equiv), THF, −50 °C to RT, then MeOH, RT; (d) 1:1 TFA:DCM (excess); (e) HCl (6 equiv), dioxane, RT; (f) diBoc-Dmt (1.05 equiv), PyBOP (1 equiv), 6Cl-HOBt (1 equiv), DIPEA (10 equiv), DMF, RT; (g) Nal (2 equiv), 2-chloroacetamide (2 equiv), then DIPEA (1.5 equiv); (h) ethyl 2-bromoacetate (10 equiv), K2CO3 (2 equiv), CH3CN, 24 h; (i) LiOH (excess), EtOH, 60 °C, 2.5 h; (j) 3 N HCl (excess), RT. *Yield was higher, but the product was difficult to purify. Therefore, only 1.6 mg of 95% pure product was isolated, which was sufficient for opioid receptor binding and efficacy assays.
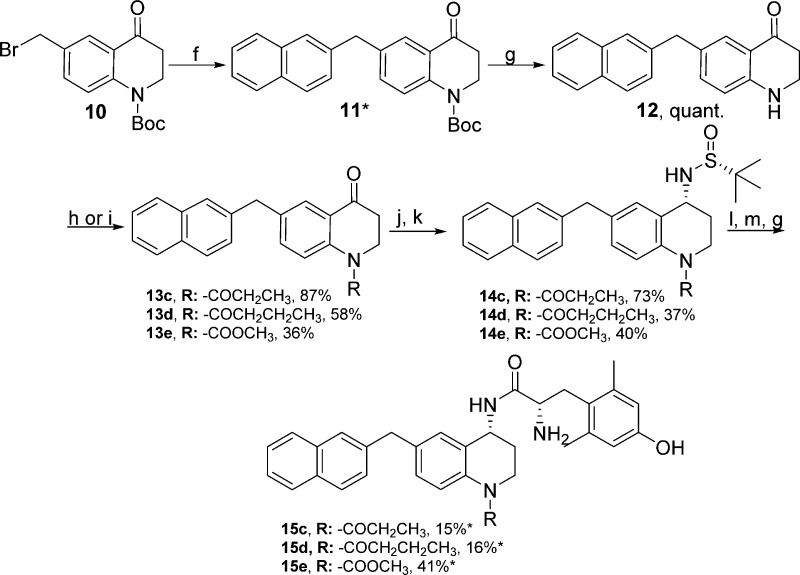
Synthesis of N-Acylated 2-Methylnaphthyl Pendant Analogues 15c–15e
Analogues 15a and 15b have been previously reported8,9 and are included here to serve as standards of comparison for the additional 2-methylnaphthyl pendant N-substituted series. Synthesis of the N-acylated peptidomimetics containing the 2-methylnaphthyl pendant began by forming intermediate 10, which was synthesized as previously described.11 Intermediate 10 was subjected to Suzuki cross-coupling to incorporate the 2-methylnaphthyl moiety and yield 11. Intermediate 11 was treated with TFA to remove the Boc group, forming 12. Intermediate 12 was treated with propionic anhydride to yield 13c, butyric anhydride to yield 13d, or methylchloroformate to yield 13e. Intermediates 13c–13e were treated with (R)-t-butanesulfinamide and Ti(OEt)4 to form a chiral imine in situ, which was reduced with NaBH4 to form the desired R-stereochemistry for intermediate 14c–14e.19−21 The Ellman auxiliary was cleaved using concentrated HCl forming primary amines, which were then coupled to diBoc-Dmt and deprotected to yield compound 15c–15e (Scheme 3).
Scheme 3. Synthesis of N-Acylated 2-Methylnaphthyl Pendant Analogues 15c–15e.
(f) 2-Naphthalenylboronic acid, Pd(dppf)Cl2, K2CO3, 3:1 acetone:H2O; (g) 1:1 TFA:DCM; (h) neat acid anhydride, reflux, 24 h (for 15c and 15d); (i) MeCOOCl, DCM, RT, 16 h (for 15e); (j) (R)-t-butanesulfinamide, THF, Ti(OEt)4, 0 °C, then reflux at 75 °C; (k) NaBH4, THF, – 50 °C to RT, 3 h, then MeOH, RT; (l) HCl, dioxane, RT, 3 h; (m) diBoc-Dmt, PyBOP, 6Cl-HOBt, DIPEA, DMF, RT. *Not all of the crude compound was purified, so yield was not calculated or appears low.
Opioid Receptor Binding and Efficacy
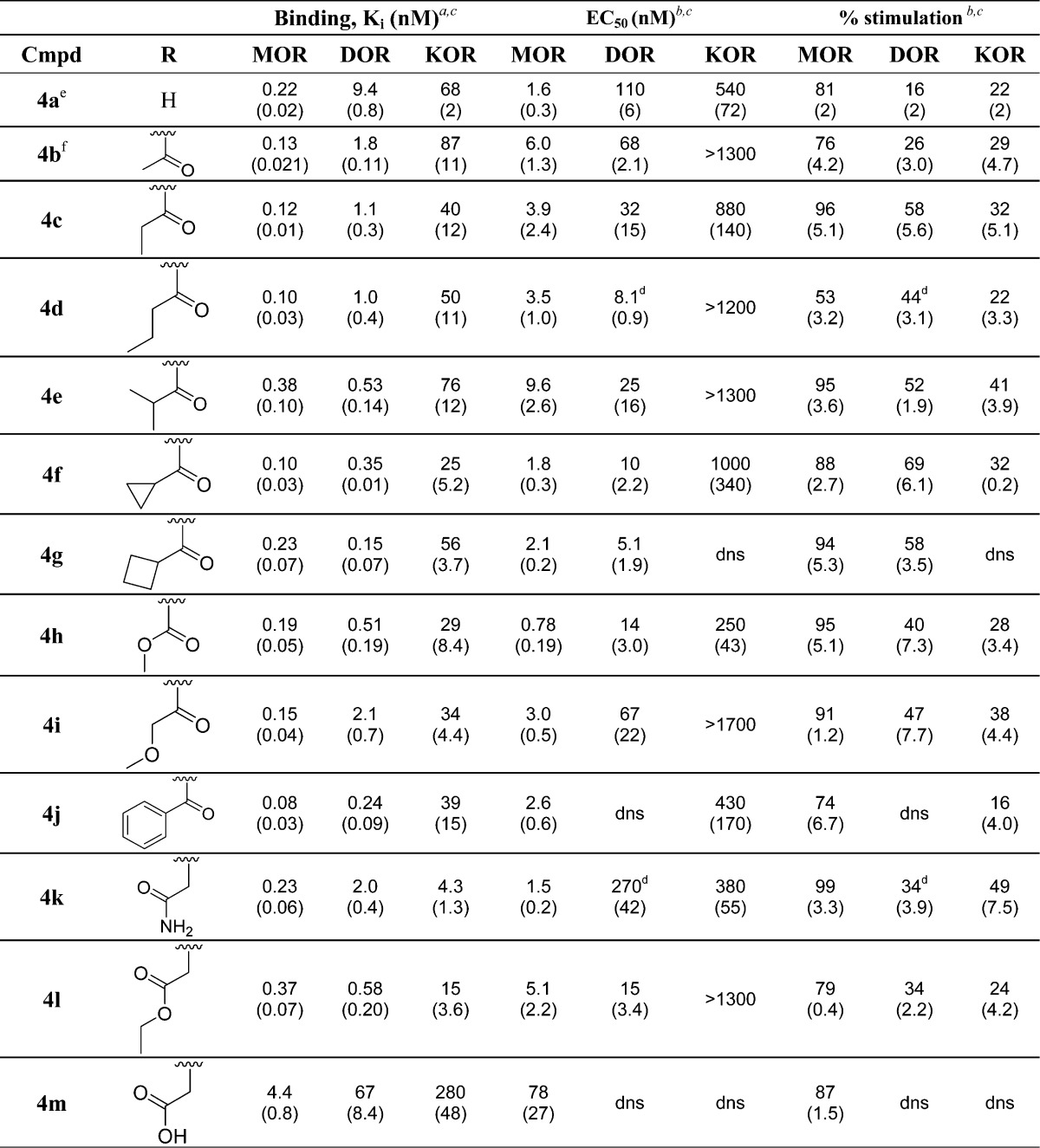
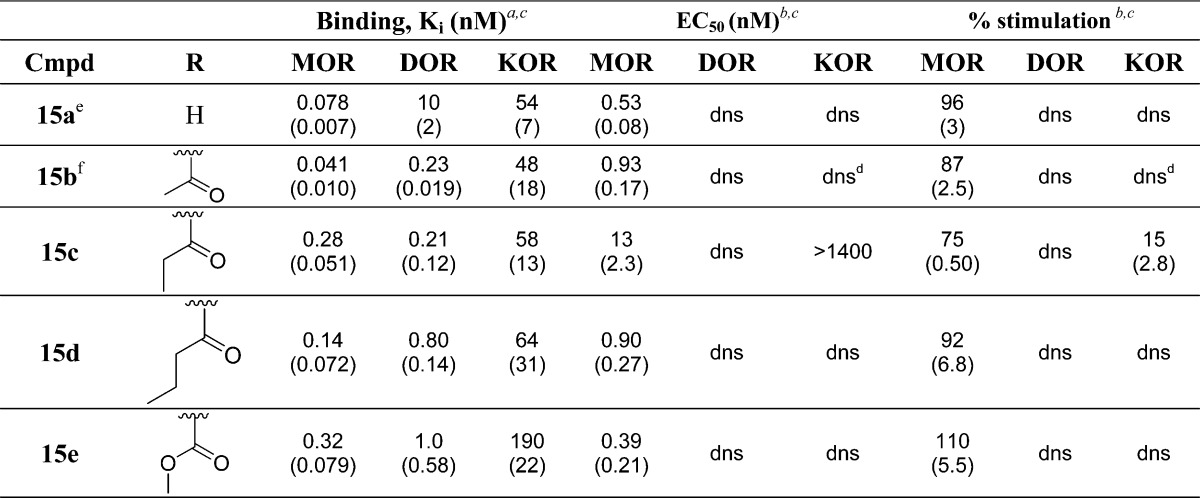
Analogues 4c–4m and 15c–15e were evaluated in in vitro MOR, DOR, and KOR binding and efficacy assays, as previously described22−24 (Tables 1 and 2). Binding affinities (Ki (nM)) were obtained by competitive displacement of radiolabeled [3H]diprenorphine in membrane preparations from C6 cells stably expressing MOR (C6-MOR) or DOR (C6-DOR) or CHO cells stably expressing KOR (CHO-KOR), as previously described22,23 (Table 1). Efficacy data were obtained using agonist-induced stimulation of [35S]GTPγS binding to G protein24 in the same cell preparations as previously mentioned. Efficacy data are represented as percent maximal stimulation relative to standard agonist (DAMGO at MOR, DPDPE at DOR, and U69593 at KOR), and potency (EC50 (nM)) values are calculated as the concentration of drug required to produce half-maximal stimulation (Table 1). Briefly, all analogues in both the benzyl and 2-methylnaphthyl series maintained high affinity binding and efficacy at MOR. Additionally, as predicted, all analogues across both series maintained high binding affinity at DOR, relative to the unsubstituted analogues 4a and 15a, excluding 4m, which displayed reduced affinity across all three receptors. Interestingly, most of the N-substituted analogues in the benzyl pendant series produced increased DOR efficacy (Table 1), creating varying degrees of MOR agonist/DOR agonist ligands in the in vitro assays, while all analogues in the N-substituted 2-methylnaphthyl series displayed minimal or no efficacy at DOR, maintaining the MOR agonist/DOR antagonist profile (Table 2). Lastly, while all analogues maintained selectivity for MOR and DOR over KOR, KOR affinity for many of the N-substituted analogues in the benzyl series increased relative to 4a (Table 1).
Table 1. Binding Affinity and Efficacy Data for Benzyl Pendant Peptidomimetics.
Binding affinities (Ki (nM)) were obtained by competitive displacement of radiolabeled [3H]diprenorphine in membrane preparations.
Efficacy data were obtained using agonist induced stimulation of [35S]GTPγS binding. Efficacy is represented as percent maximal stimulation relative to standard agonist DAMGO (MOR), DPDPE (DOR), or U69,593 (KOR) at 10 μM. Potency (EC50 (nM)) is calculated from the percent maximal stimulation as the concentration of drug required to produce half the maximal stimulation
All values are expressed as the mean with SEM in parentheses for n = 3 independent assays in duplicate, unless otherwise noted.
n = 2 independent assays in duplicate. dns: does not stimulate.
Published in ref (8).
Published in ref (9).
Table 2. Binding Affinity and Efficacy Data for 2-Methylnaphthyl Pendant Peptidomimetics.
Binding affinities (Ki (nM)) were obtained by competitive displacement of radiolabeled [3H]diprenorphine in membrane preparations.
Efficacy data were obtained using agonist induced stimulation of [35S]GTPγS binding. Efficacy is represented as percent maximal stimulation relative to standard agonist DAMGO (MOR), DPDPE (DOR), or U69,593 (KOR) at 10 μM. Potency (EC50 (nM)) is calculated from the percent maximal stimulation as the concentration of drug required to produce half the maximal stimulation
All values are expressed as the mean with SEM in parentheses for n = 3 independent assays in duplicate, unless otherwise noted.
n = 2 independent assays in duplicate. dns: does not stimulate.
Published in ref (8).
Published in ref (9).
In Vivo Warm Water Tail Withdrawal (WWTW) Assay
Although the benzyl pendant N-substituted series displayed varying degrees of stimulation at DOR, all analogues still exhibited both high affinity and efficacy at MOR; consequently, we elected to test select analogues (4e–4k) for in vivo efficacy in the WWTW assay using a cumulative dosing procedure. Additionally, because all new compounds in the 2-methylnaphthyl pendant series displayed the desired MOR agonist/DOR antagonist profile, compounds 15c–15e were also tested for in vivo efficacy in the same assay. Briefly, withdrawal latencies were determined by placing a mouse into a cylindrical plastic restrainer and immersing 2–3 cm of the tail tip into a water bath maintained at 50 °C. The latency to withdrawal or to rapidly flick the tail back and forth was recorded with a maximum cutoff time of 20 s to prevent tissue damage. Acute antinociceptive effects were determined using a cumulative dosing procedure, as previously described.25 Each animal received an intraperitoneal (ip) injection of saline, and then 30 min later, the baseline withdrawal latencies were recorded. Following baseline determinations, three increasing doses (1, 2.2, and 6.8 mg/kg) of a peptidomimetic (dissolved in either deionized water or 1:1:8 solution of ethanol:Alkumuls:deionized water, as was the case for 15c–15e) were given ip at 30 min intervals to provide final doses of 1, 3.2, and 10 mg/kg. Thirty minutes after each injection, the tail withdrawal latency was measured as described above. To determine the duration of antinociceptive action, the tail-withdrawal test was performed at varying times following administration of a peptidomimetic (10 mg/kg, ip).
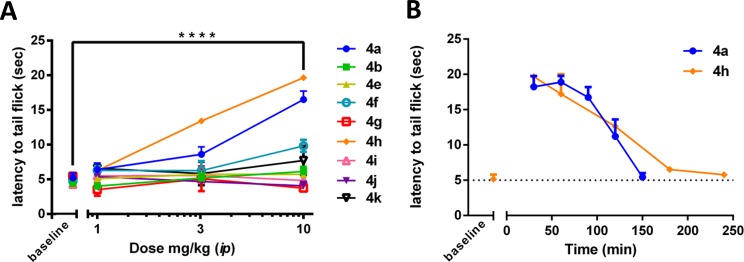
Of all compounds tested in vivo, only two of the novel N-substituted analogues tested displayed in vivo efficacy after ip administration (Figure 2A). Analogue 4f produced partial antinociception with an 11 s latency to tail flick at the 10 mg/kg dosage following ip administration, while 4h produced full antinociception, reaching the 20 s latency to tail flick cutoff time point at the same dosage. Because 4h displayed full antinociception, we performed a time course assay to determine the duration of antinociceptive action. As can be seen in Figure 2B, 4h has a similar duration of action to that of 4a, producing maximal antinociception for approximately 1 h following a 10 mg/kg ip dose.
Figure 2.
(A) Cumulative antinociceptive dose response curves of select benzyl pendant analogues (n = 3 for all analogues) in the mouse WWTW assay following ip administration. Plotted as average ± SEM. ****, p < 0.0001 for 4a and 4h for the 10 mg/kg dose when compared to baseline, p < 0.001 for 4f when compared to baseline (not indicated on graph). (B) Time course of antinociception of 4a and 4h (n = 3) in the mouse WWTW assay following ip administration of 10 mg/kg. Plotted as average ± SEM. Data for 4a from ref (8).
Discussion and Conclusions
All new analogues across both series sets, excluding 4m, maintained high binding affinity at MOR and exhibited an increased DOR binding affinity when compared to the unsubstituted parent compounds 4a and 15a, presumably due to the carbonyl moiety incorporated into each of the N-substitutions, as had been previously observed for 15b.9 The only exception to this trend, 4m, contains a carboxylic acid moiety and displayed significantly decreased affinity across all three opioid receptors. The poor binding affinity of 4m could be potentially due to electrostatic repulsion between its carboxylic acid moiety and a conserved negatively charged residue in TM5 (Asp210 in DOR, Asp223 in KOR, and Glu229 in MOR), which may also be complemented by acidic Glu294 in KOR. On the basis of these initial in vitro results and previously reported computational modeling,9 it appears that a carbonyl-containing moiety is beneficial in maintaining higher binding affinity at DOR while a negatively charged species is not well tolerated.
An additional trend seen in the benzyl series is that increasing the N-acyl chain length or overall bulk increases DOR stimulation, with the exception of 4j, which contains a benzoyl moiety and produces no stimulation at DOR. As seen in Table 1, the unsubstituted compound 4a shows very weak stimulation at DOR (16% maximal stimulation) while the N-acetyl analogue 4b increased maximal stimulation at DOR to 26% and the N-propionyl (4c) and N-butyryl (4d) analogues further increased DOR stimulation to between 40 and 60%. Analogues 4e–4g contain branched or cyclic N-substitutions, and in the case of 4f (containing a cyclopropanecarbonyl moiety) and 4g (containing a cyclobutanecarbonyl moiety), DOR stimulation increased to 69% and 58%, respectively.
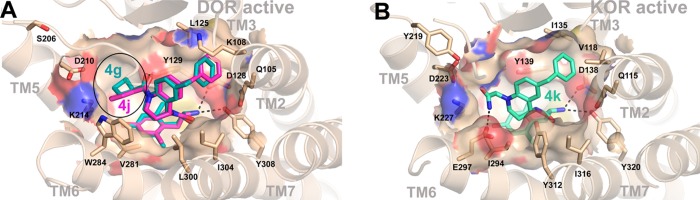
The computational model of 4g in the active conformation of DOR (Figure 3A) demonstrates the presence of a hydrophobic cavity in the ligand binding pocket formed by residues from extracellular loop 2 (Leu200 and Phe202) and TM5 (Thr213 and Val217), which is preserved in MOR and KOR. It can be seen that the cyclobutanecarbonyl moiety extends into this cavity forming favorable hydrophobic interactions. These interactions may contribute to the improved binding affinity of compounds 4c–4f toward the active receptor conformation and to their increased efficacy at DOR. As also seen in Figure 3A, compound 4j has a benzoyl moiety which appears to be too bulky to fit this cavity in the active receptor conformation although it can be easily accommodated in the slightly enlarged ligand pocket of the inactive receptor state.10 Consequently, 4j displays DOR antagonist, rather than DOR agonist activity.
Figure 3.
(A) Structure of 4g (teal) and 4j (magenta) bound in the active conformation of DOR with the area of the binding pocket occupied by the acyl chain circled (black). (B) Structure of 4k (green) bound in the active conformation of KOR with a potential polar contact between E297 and the terminal amide of the ligand which could be responsible for the increased KOR affinity and efficacy.
Similarly, hydrophobic interactions with the nonpolar cavity may also contribute to the improved binding affinity toward KOR of compounds 4c, 4f, 4g, and 4j with long or bulky nonpolar N-substitutions (Ki values ranging from 8 to 56 nM, as compared to Ki = 68 nM of 4a), which act as partial agonists. However, this cannot explain the observed activity of 4k, which has a polar amide group as an N-substitution. Compound 4k not only displays increased affinity at KOR (4.3 vs 68 nM for 4a) but also exhibits significant KOR stimulation (49%). The docking of 4k into the model of the active conformation of KOR (Figure 3B) suggests that formation of a hydrogen bond between N-substituted amide of 4k and Glu297 from TM6 of KOR may be the reason for the improved binding and agonist properties of this compound.
While analogues 4c and 4e–g all represent a subset of analogues with a MOR agonist/DOR agonist in vitro profile, 4g displayed balanced binding affinities (Ki = 0.23 nM at MOR and 0.15 nM at DOR), low nanomolar potency (EC50 = 2.1 nM at MOR and 5.1 nM at DOR), and high efficacy at both MOR and DOR (94% and 58%, respectively), along with weak binding and no efficacy at KOR. While this profile was not our initial focus, several reports have shown that coadministration of a DOR agonist with a MOR agonist can increase both the potency and efficacy of the MOR agonist.4,5 These findings suggest ligands producing a mixed-efficacy MOR agonist/DOR agonist profile could allow for the effective management of pain with a decrease in side effects seen with the administration of only a MOR agonist. Indeed, this has been observed with MMP-2200, a MOR agonist/DOR agonist peptide ligand that produces in vivo activity in mice with reduced side effects.4
Additionally, we have previously shown that 15b, with a 2-methylnaphthyl pendant and an acetyl N-substitution produces in vivo antinociception for greater than 3 h in the WWTW assay.9 Thus, we hypothesized that compounds containing a 2-methylnaphthyl pendant with carbonyl-containing N-substitutions could produce similar, or longer lasting, in vivo activity when compared to 15b. We synthesized a modest set of 2-methylnaphthyl analogues and found that as the acyl chain length increases, the balanced-affinity MOR agonist/DOR antagonist profile seen with 15b remains essentially constant, while KOR affinity slightly decreases, indicating that increased chain length helps provide MOR and DOR selectivity over KOR (Table 2). The high affinity binding and lack of stimulation at DOR corroborates our previous findings that additional hydrophobic bulk in the pendant region of our peptidomimetic series increases affinity to DOR in the inactive conformation while being incompatible with the smaller DOR active state binding site.9 This trend suggests that the differences in DOR efficacy between most of the compounds in the benzyl series (16–70%) and the 2-methylnaphthyl series (0–15%) is due to differences in bulk between these two pendant moieties. While the in vitro profile of the 2-methylnaphthyl analogues is very promising in that all analogues are high affinity, potent MOR agonists and high affinity DOR antagonists, none of the novel analogues in this series (15c–15e) produced antinociception in the WWTW assay.
Finally, as we have seen with other peptidomimetic series,9,11 in vivo activity is not predictable from in vitro profiles and physiochemical parameters. This fact remains true for this series of N-substituted analogues where 4h is the only analogue to emerge that produces full antinociception in the WWTW assay at a 10 mg/kg dose. 4h is a mixed-efficacy peptidomimetic with nearly balanced affinities at MOR and DOR (Ki = 0.19 and 0.51 nM, respectively) and produces potent (EC50 = 0.78), full stimulation at MOR (95%), and moderately potent (EC50 = 14 nM) stimulation at DOR (40%) and selectivity for MOR and DOR over KOR. Further, 4h produces in vivo activity for 1 h, a similar duration of action to 4a. Planned pharmacokinetic and metabolic stability testing of key members of our series are expected to shed light on the as yet unexplained differences in in vivo activity.
In conclusion, we have expanded our opioid peptidomimetic library by incorporating various N-substitutions on our previously published,8 unsubstituted peptidomimetics 4a and 15a after observing that N-acetylation of the THQ nitrogen in 4a and 15a to give 4b and 15b increases DOR affinity.9 All of the N-substitutions in the present study contain a carbonyl moiety, which appears to be beneficial in maintaining high DOR affinity, an important factor in the development of bifunctional MOR/DOR ligands. Through this synthetic campaign, we have also discovered that an increased acyl chain length or additional bulk increases DOR stimulation within the benzyl pendant series while increasing acyl chain length on the 2-methylnaphthyl series did not increase DOR stimulation, suggesting that pendant moieties play an important role in anchoring the ligand to DOR in the inactive conformation. Analogue 4h, which displays high efficacy at MOR and moderate efficacy at DOR and produces full in vivo antinociception in the WWTW assay at a 10 mg/kg dose, may serve as a lead in the development of bifunctional MOR/DOR agonists.
Experimental Section
Chemistry
All reagents and solvents were obtained from commercial sources and used without additional purification. Suzuki couplings were performed on a Discover S-class (CEM) microwave in a closed vessel with maximum power input of 300 W and temperature set at 110 °C for 10–60 min under the standard method from their Synergy software. Flash column chromatography was carried out using P60 silica gel (230–400 mesh) either manually or with a Biotage Isolera instrument. Before flash column chromatograph was performed, crude mixtures were analyzed using thin-layer chromatography using 2:3 EA:hex, 1:1 EA:hex, and/or 3:2 EA:hex. The Rf values of products and impurities were calculated then copied into either the linear gradient or stepwise gradient wizard on the Biotage Isolera instrument. The TLC wizard then determined the optimal purification technique which was used to purify crude mixtures. Purification of final compounds was performed using a Waters semipreparative HPLC with a Vydac protein and peptide C18 reverse phase column, using a linear gradient of 0% solvent B (0.1% TFA in acetonitrile) in solvent A (0.1% TFA in water) to 100% solvent B in solvent A at a rate 1% per minute and monitoring UV absorbance at 230 nm. Purity of synthesized compounds was determined on a Waters Alliance 2690 analytical HPLC instrument and a Vydac protein and peptide C18 reverse phase column, using a linear gradient of 0% solvent B in solvent A to 45%, 70%, or 90% solvent B in solvent A in 45, 70, or 90 min, respectively, and UV absorbance at 230 nm (gradient A). Purities of the final compounds used for testing were ≥95%, unless otherwise stated, as determined by HPLC. 1H NMR and 13C NMR data were obtained on either a 400 or 500 MHz Varian spectrometer using CDCl3 or CD3OD solvents. The identity of each compound was verified by mass spectrometry using an Agilent 6130 LC–MS mass spectrometer in positive mode.
General Procedure A for the Synthesis of N-Acylated THQ Analogues Using an Acid Anhydride
To a flame-dried round-bottom flask under Ar was added the 2,3-dihydroquinolin-4(1H)-one. The reaction vessel was placed back under vacuum for 5 min then excess acid anhydride (propionic or butyric) was added via syringe and the solution stirred for 5 min. The round-bottom flask was flooded with Ar, equipped with a condenser, and placed in oil bath at 90–120 °C. The reaction stirred at reflux for 12–20 h under Ar and was monitored by TLC. Once the reaction was complete, the solvent was removed under reduced pressure, yielding a crude yellow oil which was purified using silica gel chromatograph to obtain the pure product.
General Procedure B for the Synthesis of N-Acylated THQ Analogues Using an Acid Chloride
To a flame-dried round-bottom flask with stir bar under Ar atomosphere was added 2,3-dihydroquinolin-4(1H)-one intermediate (1.0 equiv). The reaction vessel was re-evacuated then flooded with Ar. Anhydrous DCM was added via a syringe and the starting material dissolved. Next, the acid chloride (2.0 equiv) was added via syringe. The reaction was monitored by TLC. Once reaction was complete, solvent was removed under reduced pressure. The crude mixture was purified using column chromatography to yield the pure product.
General Procedure C for the Synthesis of (R,R) THQ Sulfinamides.19−21
To a round-bottom flask already containing dried, desiccated N-substituted dihydroquinolinone intermediate (1.0 equiv) was added (R)-2-methylpropane-2-sulfinamide (3.0 equiv), then the round-bottom flask was placed under vacuum for 10 min. Meanwhile, a reflux condenser was flame-dried under vacuum and then flooded with Ar. Next, anhyd THF (∼20 mL) was added to the reaction vessel containing starting reagents via syringe. The reaction solution allowed to stir under vacuum for ∼5 min and then was flooded with Ar. The round-bottom flask was placed in ice bath and allowed to equilibrate. Next, Ti(OEt)4 (6.0 equiv) was added slowly via syringe. Once addition was complete, the reaction vessel was taken out of the ice bath and placed in an oil bath at 70–75 °C, equipped with condenser, and stirred for 16–48 h under Ar. The reaction was monitored by TLC for loss of ketone. Once sufficient conversion to the tert-butanesulfinyl imine was observed, the reaction vessel was taken out of the oil bath and cooled to ambient temperature. Meanwhile, an additional round-bottom flask containing a stir bar was flame-dried under vacuum, then flooded with Ar, then NaBH4 was added quickly, and then reaction vessel was placed back under vacuum for 5 min. Minimal anhyd THF was added (∼5 mL), and the vessel allowed to stir under vacuum for ∼5 min, then was flooded with Ar. The round-bottom flask was placed in dry ice/xylenes bath and allowed to equilibrate. Contents from the round-bottom flask containing the imine intermediate were transferred to round-bottom flask containing NaBH4 via cannula. Once contents completely added, the reaction was taken out of dry ice/xylenes bath and allowed to warm to room temperature. The reaction stirred at ambient temperature for 2–3 h. Once the reaction was complete, MeOH was added to quench. The solvent was removed under reduced pressure, yielding a solid residue. The residue was resuspended in DCM, solid remained, and the remaining solid was removed by filtration through a cotton plug and the mother liquor was concentrated and purified using silica gel chromatography to yield pure sulfinamide.
General Procedure D for diBoc-Dmt Coupling to Form Final Product
To a round-bottom flask already containing sulfinamide intermediate was added 15–20 mL of dioxane followed by conc HCl (6.0 equiv). The reaction stirred at RT for up to 3 h. Solvent was removed under reduced pressure to yield slightly yellow, clear residue. The residue was resuspended in Et2O. If a white solid precipitated (the HCl salt of the amine): solid was removed via filtration as product without any further purification necessary. If a white solid did not precipitate, but residue remains as film on flask: residue washed with fresh Et2O (3 × 5 mL) and dried without any further purification necessary. The (R)-amine intermediate (1.0 equiv) and diBoc-Dmt (1.0–1.05 equiv) and the coupling reagents PyBOP (1.0 equiv) or HATU (1.0 equiv), HOBt-Cl (1.0 equiv), were dissolved in DMF (10–15 mL) followed by the addition of the and DIPEA (10.0 equiv). The reaction mixture stirred for 18 h at room temperature. After concentration under reduced pressure, the crude residue was dissolved in a 1:1 mixture of DCM and TFA (10 mL) and stirred for 1 h. The mixture was concentrated and purified by semipreparative HPLC, then lyophilized to yield the final compound.
6-Benzyl-2,3-dihydroquinolin-4(1H)-one (2c)
2c was synthesized according to a modified general procedure A using 1 (110 mg, 0.47 mmol, 1.0 equiv) and excess propionic anhydride. After reaction was complete, reaction mixture was cooled to RT, then transferred to a separatory funnel, and EA and NaHCO3 were added and the layers separated. The aqueous layer was extracted with EA, and the combined organic layers washed with brine then dried over MgSO4 and purified using silica gel chromatography to yield the title compound 2c as a clear, colorless oil (120 mg, 85%). 1H NMR (500 MHz, CDCl3) δ 7.86 (d, J = 0.9, 1H), 7.39 (br s, 1H), 7.35 (dd, J = 8.2, 2.2, 1H), 7.29 (t, J = 7.4, 2H), 7.23–7.16 (m, 3H), 4.20 (t, J = 6.2, 2H), 3.98 (s, 2H), 2.75 (t, J = 6.2, 2H), 2.58 (q, J = 7.4, 2H), 1.20 (t, J = 7.4, 3H). 13C NMR (126 MHz, CDCl3) δ 194.27, 173.03, 142.21, 140.13, 138.78, 134.63, 128.90, 128.71, 127.62, 126.49, 126.11, 124.44, 43.88, 41.29, 39.62, 27.98, 9.91.
6-Benzyl-1-butyryl-2,3-dihydroquinolin-4(1H)-one (2d)
2d was synthesized according to a modified general procedure A using 1 (0.18 g, 0.78 mmol, 1.0 equiv) and excess butyric anhydride (5 mL) and pyridine (4 mL). After reaction was complete (24 h), the reaction was quenched with H2O. DCM was added, and the aqueous layer was extracted with DCM. Combined organic extracts were washed with 2 M NaOH and dried with MgSO4. Solvents were filtered and removed under reduced pressure, and the crude residue was purified via silica gel chromatography to yield the title compound 2d as an oil (0.029 g, 12%). 1H NMR (400 MHz, CDCl3) δ 7.87 (t, J = 1.3, 1H), 7.39–7.34 (m, 2H), 7.33–7.25 (m, 2H), 7.25–7.16 (m, 3H), 4.21 (t, J = 6.2, 2H), 3.99 (s, 2H), 2.76 (t, J = 6.2, 2H), 2.54 (t, J = 8.0, 2H), 1.79–1.66 (m, 2H), 0.95 (t, J = 7.4, 3H). 13C NMR (101 MHz, CDCl3) δ 194.37, 172.35, 142.31, 140.18, 138.87, 134.71, 128.99, 128.80, 127.77, 126.58, 126.18, 124.55, 43.97, 41.38, 39.82, 36.57, 19.25, 13.94.
6-Benzyl-1-isobutyryl-2,3-dihydroquinolin-4(1H)-one (2e)
2e was synthesized according to general procedure B starting from 1 (100 mg, 0.42 mmol, 1.0 equiv) to yield the title compound 2e as a clear, colorless oil (105 mg, 80.8%). 1H NMR (500 MHz, CDCl3) δ 7.87 (d, J = 2.0 Hz, 1H), 7.37–7.25 (m, 4H), 7.25–7.17 (m, 3H), 4.21 (t, J = 6.3 Hz, 2H), 3.99 (s, 2H), 3.14 (hept, J = 6.7 Hz, 1H), 2.75 (t, J = 6.2 Hz, 2H), 1.18 (d, J = 6.7 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 194.33, 176.97, 142.29, 140.05, 138.84, 134.59, 128.90, 128.87, 128.68, 127.64, 126.46, 126.15, 124.22, 43.90, 41.25, 39.79, 31.13, 19.88.
6-Benzyl-1-(cyclopropanecarbonyl)-2,3-dihydroquinolin-4(1H)-one (2f)
2f was synthesized following a modified general procedure B starting with 1 (0.15 g, 0.65 mmol, 1.0 equiv) and also using Et3N (0.18 mL, 1.29 mmol, 2.0 equiv). The reaction stirred for 3 h, after which time satd NaHCO3 was added. Aqueous layer was extracted with DCM. Combined organic extracts were washed with brine and dried with MgSO4. Solvents were filtered and removed under reduced pressure, and crude residue was purified via silica gel chromatography to yield the title compound 2f as a colorless oil (0.15 g, 78%). 1H NMR (500 MHz, CDCl3) δ 7.88 (d, J = 2.2, 1H), 7.46 (d, J = 8.3, 1H), 7.35 (dd, J = 8.4, 2.2, 1H), 7.31–7.26 (m, 2H), 7.22–7.17 (m, 3H), 4.26 (t, J = 6.3, 2H), 3.98 (s, 2H), 2.76 (t, J = 6.3, 2H), 2.06–1.96 (m, 1H), 1.21–1.15 (m, 2H), 0.91–0.82 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 194.41, 173.00, 142.45, 140.13, 138.53, 134.49, 128.89, 128.71, 127.74, 126.49, 125.85, 124.09, 43.53, 41.28, 39.70, 13.74, 9.77.
6-Benzyl-1-(cyclobutanecarbonyl)-2,3-dihydroquinolin-4(1H)-one (2g)
2g was synthesized following general procdure B starting with 1 (0.15 g, 0.62 mmol, 1.0 equiv). The reaction stirred for 3 h, after which time satd NaHCO3 was added. Aqueous layer was extracted with DCM. Combined organic extracts were washed with brine and dried with MgSO4. Solvents were filtered and removed under reduced pressure, and crude residue was purified via silica gel chromatography to yield the title compound 2g as a white solid (0.20 g, 99%). 1H NMR (500 MHz, CDCl3) δ 7.85 (d, J = 1.3, 1H), 7.48–7.37 (br s, 1H), 7.35 (dd, J = 8.2, 2.2, 1H), 7.29 (t, J = 7.5, 2H), 7.24–7.13 (m, 3H), 4.12 (t, J = 6.2, 2H), 3.98 (s, 2H), 3.52 (p, J = 8.4, 1H), 2.72 (t, J = 6.2, 2H), 2.43 (dq, J = 11.8, 9.2, 2H), 2.13 (q, J = 9.9, 2H), 2.02–1.88 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 194.09, 174.02, 142.14, 140.14, 138.52, 134.63, 128.85, 128.64, 127.49, 126.41, 125.75, 123.84, 43.78, 41.24, 39.57, 37.95, 25.72, 17.83.
Methyl 6-Benzyl-4-oxo-3,4-dihydroquinoline-1(2H)-carboxylate (2h)
2h was synthesized following general procedure B starting from the 1 (150 mg, 0.63 mmol, 1.0 equiv) to yield the title compound 2h as a clear, colorless oil (111 mg, 59.4%). 1H NMR (500 MHz, CDCl3) δ 7.86 (d, J = 2.1 Hz, 1H), 7.71 (d, J = 8.6 Hz, 1H), 7.34 (dd, J = 8.6, 2.2 Hz, 1H), 7.29 (t, J = 7.5 Hz, 2H), 7.23–7.16 (m, 3H), 4.18 (t, J = 6.3 Hz, 2H), 3.97 (s, 2H), 3.84 (s, 3H), 2.76 (t, J = 6.3 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 193.79, 154.23, 141.74, 140.24, 137.32, 134.79, 128.74, 128.51, 127.13, 126.25, 124.79, 123.58, 53.30, 44.40, 41.09, 38.81.
6-Benzyl-1-(2-methoxyacetyl)-2,3-dihydroquinolin-4(1H)-one (2i)
2i was synthesized following general procedure B starting from the 1 (150 mg, 0.63 mmol, 1.0 equiv) to yield the title compound 2i as a white solid (176 mg, 89.8%). 1H NMR (500 MHz, CDCl3) δ 7.87 (d, J = 2.2 Hz, 1H), 7.37 (dd, J = 8.4, 2.2 Hz, 1H), 7.28 (t, J = 7.6 Hz, 2H), 7.23–7.15 (m, 3H), 4.26 (s, 2H), 4.17 (t, J = 6.3 Hz, 2H), 3.98 (s, 2H), 3.45 (s, 3H), 2.78 (t, J = 6.2 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 193.62, 168.07, 141.25, 139.91, 139.02, 134.72, 128.77, 128.58, 128.55, 127.56, 126.37, 125.78, 123.66, 76.74, 71.84, 59.24, 43.95, 41.17, 39.41.
1-Benzoyl-6-benzyl-2,3-dihydroquinolin-4(1H)-one (2j)
2j was synthesized following general procedure B starting from the 1 (100 mg, 0.42 mmol, 1.0 equiv) to yield the title compound 2j as a clear, slightly yellow oil (139 mg, 96.5%). 1H NMR (500 MHz, CDCl3) δ 7.78 (d, J = 2.1 Hz, 1H), 7.42–7.34 (m, 3H), 7.31–7.25 (m, 2H), 7.22–7.17 (m, 2H), 7.14–7.10 (m, 1H), 7.08–7.04 (m, 2H), 7.01 (dd, J = 8.5, 2.2 Hz, 1H), 6.81 (d, J = 8.4 Hz, 1H), 4.21 (t, J = 6.3 Hz, 2H), 3.85 (s, 2H), 2.77 (t, J = 6.3 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 193.73, 170.04, 142.60, 139.99, 138.06, 135.06, 134.31, 130.99, 128.78, 128.58, 128.47, 128.39, 127.38, 126.36, 124.82, 124.63, 45.30, 41.10, 39.50.
(R)-N-((R)-6-Benzyl-1-propionyl-1,2,3,4-tetrahydroquinolin-4-yl)-2-methylpropane-2-sulfinamide (3c)
3c was synthesized following general procedure C using 2c (120 mg, 0.40 mmol, 1.0 equiv), (R)-2-methylpropane-2-sulfinamide (150 mg, 1.2 mmol, 3.0 equiv), and Ti(OEt)4 (0.63 mL, 2.4 mmol, 6.0 equiv) to form the (R)-tert-butanesulfinyl imine intermediate in situ. Once sufficient ketone was converted into imine intermediate (after 17 h), the reaction mixture was transferred via cannula to a round-bottom flask containing NaBH4 (90 mg, 2.4 mmol, 6.0 equiv) and 20 mL of THF in a xylenes dry ice bath; after addition, the solution was stirred at room temperature for 3 h before being quenched with MeOH. Once resultant solid was removed, crude residue was purified using silica gel chromatography to yield the title compound 3c as a clear, colorless oil (120 mg, 78%). 1H NMR (500 MHz, CDCl3) δ 7.69 (d, J = 8.2 Hz, 1H), 7.29–7.21 (m, 2H), 7.20–7.15 (qd, J = 6.5, 5.4, 1.6 Hz, 4H), 7.06 (dd, J = 8.5, 2.3 Hz, 1H), 4.52 (q, J = 3.4 Hz, 1H), 3.97–3.87 (m, 3H), 3.57 (tdd, J = 12.9, 4.2, 1.7 Hz, 1H), 3.32 (bs, 1H), 2.20–2.13 (m, 1H), 1.99–1.90 (m, 1H), 1.50 (s, 9H), 1.19 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 153.43, 140.77, 136.51, 136.42, 128.88, 128.71, 128.55, 128.47, 128.34, 125.97, 123.89, 80.97, 55.52, 50.36, 41.08, 40.00, 29.41, 28.22, 22.48.
(R)-N-((R)-6-Benzyl-1-butyryl-1,2,3,4-tetrahydroquinolin-4-yl)-2-methylpropane-2-sulfinamide (3d)
3d was synthesized following general procedure C using 2d (0.029 g, 0.094 mmol, 1.0 equiv) to yield the title compound 3d as a colorless oil (0.010 g, 26%). 1H NMR (400 MHz, CDCl3) δ 7.33–7.27 (m, 2H), 7.24–7.18 (m, 3H), 7.10 (dd, J = 8.3, 2.1, 1H), 4.53 (t, J = 4.6, 1H), 3.95 (s, 2H), 3.94–3.85 (m, 1H), 3.80–3.68 (m, 1H), 3.34 (br s, 1H), 2.51–2.41 (m, 2H), 2.27–2.15 (m, 1H), 2.08–1.97 (m, 1H), 1.74–1.63 (m, 2H), 1.20 (s, 9H), 0.92 (t, J = 7.4, 3H).
(R)-N-((R)-6-Benzyl-1-isobutyryl-1,2,3,4-tetrahydroquinolin-4-yl)-2-methylpropane-2-sulfinamide (3e)
3e was synthesized following general procedure C using 2e (105 mg, 0.34 mmol, 1.0 equiv) to yield the title compound 3e as a clear, colorless oil (70 mg, 50%). 1H NMR (500 MHz, CDCl3) δ 7.34–7.26 (m, 3H), 7.24–7.19 (m, 3H), 7.10 (dd, J = 8.2, 2.2 Hz, 1H), 4.53 (q, J = 3.9 Hz, 1H), 3.96 (s, 2H), 3.94–3.89 (m, 1H), 3.75–3.67 (m, 1H), 3.30 (bs, 1H), 3.12 (p, J = 6.9 Hz, 1H), 2.24–2.15 (m, 1H), 2.08–1.98 (m, 1H), 1.23–1.16 (s, 9H), 1.13 (dd, J = 9.0, 6.7 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 177.35, 140.53, 138.70, 136.67, 128.92, 128.67, 128.58, 128.54, 126.28, 124.60, 55.76, 51.04, 41.37, 40.00, 31.09, 30.96, 22.55, 20.03, 20.01.
(R)-N-((R)-6-Benzyl-1-(cyclopropanecarbonyl)-1,2,3,4-tetrahydroquinolin-4-yl)-2-methylpropane-2-sulfinamide (3f)
3f was synthesized following general procedure C using 2f (0.16 g, 0.51 mmol, 1.0 equiv) to yield the title compound 3f as a colorless oil (0.12 g, 55%). 1H NMR (400 MHz, CDCl3) δ 7.36 (d, J = 8.2, 1H), 7.32–7.26 (m, 3H), 7.23–7.18 (m, 3H), 7.09 (dd, J = 8.3, 2.0, 1H), 4.55 (q, J = 4.3, 1H), 4.01–3.93 (m, 3H), 3.75 (ddd, J = 12.9, 9.3, 5.6, 1H), 3.33 (d, J = 3.6, 1H), 2.23 (dq, J = 14.9, 5.1, 1H), 2.10–1.89 (m, 3H), 1.20 (s, 9H), 1.14–1.08 (m, 2H), 0.78 (dd, J = 7.9, 2.5, 2H). 13C NMR (101 MHz, CDCl3) δ 173.36, 140.65, 138.53, 136.83, 131.83, 128.98, 128.71, 128.58, 128.51, 126.36, 124.97, 55.85, 51.18, 41.47, 39.85, 30.74, 22.61, 13.65, 9.28.
(R)-N-((R)-6-Benzyl-1-(cyclobutanecarbonyl)-1,2,3,4-tetrahydroquinolin-4-yl)-2-methylpropane-2-sulfinamide (3g)
3g was synthesized following general procedure C using 2g (0.20 g, 0.62 mmol, 1.0 equiv) to yield the title compound 3g as a colorless oil (0.16 g, 60%). 1H NMR (500 MHz, CDCl3) δ 7.31–7.25 (m, 2H), 7.23–7.17 (m, 2H), 7.09 (dd, J = 8.3, 2.1, 1H), 4.51 (q, J = 4.3, 1H), 3.95 (s, 2H), 3.86–3.77 (m, 1H), 3.74–3.63 (m, 1H), 3.50–3.39(m, 2H), 2.44–2.30 (m 2H), 2.19 (dq, J = 14.3, 4.9, 1H), 2.12–1.97 (m, 3H), 1.97–1.82 (m, 2H), 1.21 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 174.44, 140.63, 138.39, 136.58, 128.89, 128.73, 128.56, 128.50, 126.23, 124.20, 55.77, 50.96, 41.37, 39.90, 38.22, 30.72, 25.80, 22.60, 17.84.
Methyl (R)-6-Benzyl-4-(((R)-tert-butylsulfinyl)amino)-3,4-dihydroquinoline-1(2H)-carboxylate (3h)
3h was synthesized following general procedure C using 2h (111 mg, 0.376 mmol, 1.0 equiv) to yield the title compound 3h as a clear, colorless oil (124 mg, 82.1%). 1H NMR (500 MHz, CDCl3) δ 7.73 (d, J = 8.7 Hz, 1H), 7.27 (t, J = 7.8 Hz, 2H), 7.23–7.16 (m, 4H), 7.11–7.06 (m, 1H), 4.54 (q, J = 3.6 Hz, 1H), 4.01 (dt, J = 13.0, 4.6 Hz, 1H), 3.93 (s, 2H), 3.78 (d, J = 1.0 Hz, 3H), 3.64 (ddd, J = 12.9, 11.1, 3.8 Hz, 1H), 3.26 (d, J = 2.7 Hz, 1H), 2.24–2.14 (m, 1H), 2.03–1.94 (m, 1H), 1.20 (d, J = 1.0 Hz, 9H). 13C NMR (126 MHz, CDCl3) δ 154.94, 140.75, 137.11, 136.11, 129.11, 128.79, 128.77, 128.57, 128.44, 126.09, 126.08, 123.68, 55.59, 52.94, 50.24, 41.15, 40.23, 29.43, 22.51.
(R)-N-((R)-6-Benzyl-1-(2-methoxyacetyl)-1,2,3,4-tetrahydroquinolin-4-yl)-2-methylpropane-2-sulfinamide (3i)
3i was synthesized following general procedure C using 2i (176 mg, 0.60 mmol, 1.0 equiv) to yield the title compound 3i as a clear, colorless oil (148 mg, 62.7%). 1H NMR (500 MHz, CDCl3) δ 7.30–7.24 (m, 3H), 7.18 (d, J = 7.6 Hz, 3H), 7.09 (dd, J = 8.3, 2.0 Hz, 1H), 4.52 (q, J = 4.3 Hz, 1H), 4.17 (s, 2H), 3.93 (s, 2H), 3.79 (d, J = 40.2 Hz, 11H), 3.44 (d, J = 3.6 Hz, 1H), 3.39 (s, 3H), 2.20 (dq, J = 14.5, 4.9 Hz, 1H), 2.04 (qd, J = 9.2, 8.7, 5.0 Hz, 1H), 1.20 (s, 35H), 1.17 (s, 10H). 13C NMR (126 MHz, CDCl3) δ 168.45, 140.40, 135.69, 128.97, 128.82, 128.68, 128.50, 128.46, 128.21, 126.20, 126.14, 123.95, 71.69, 59.16, 55.72, 55.22, 50.80, 41.38, 41.29, 39.90, 30.47, 29.60, 22.52, 22.21, 22.07.
(R)-N-((R)-1-Benzoyl-6-benzyl-1,2,3,4-tetrahydroquinolin-4-yl)-2-methylpropane-2-sulfinamide (3j)
3j was synthesized following general procedure C using 2j (139 mg, 0.41 mmol, 1.0 equiv) to yield the title compound 3j as an off-white solid (127 mg, 69.8%). 1H NMR (500 MHz, CDCl3) δ 7.31 (td, J = 8.0, 1.4 Hz, 3H), 7.27–7.15 (m, 5H), 7.14–7.09 (m, 1H), 7.09–7.04 (m, 2H), 6.78 (t, J = 9.0 Hz, 2H), 4.54 (q, J = 3.9 Hz, 1H), 3.93 (dt, J = 12.5, 5.1 Hz, 1H), 3.82 (s, 2H), 3.78–3.68 (m, 1H), 3.32 (d, J = 3.1 Hz, 1H), 2.20 (dq, J = 14.0, 4.7 Hz, 1H), 2.02 (ddt, J = 14.5, 9.9, 5.0 Hz, 1H), 1.13 (d, J = 1.5 Hz, 11H). 13C NMR (126 MHz, CDCl3) δ 170.22, 140.50, 138.06, 136.69, 135.96, 130.33, 129.87, 128.79, 128.68, 128.46, 128.34, 128.32, 128.23, 126.15, 125.31, 60.32, 55.72, 50.61, 41.47, 41.19, 30.24, 22.53, 20.99, 14.15.
(S)-2-Amino-N-((R)-6-benzyl-1,2,3,4-tetrahydroquinolin-4-yl)-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (4a)
4a was synthesized as previously described in ref (8).
(S)-N-((R)-1-Acetyl-6-benzyl-1,2,3,4-tetrahydroquinolin-4-yl)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (4b)
4b was synthesized as previously described in ref (9).
(S)-2-Amino-N-((R)-6-benzyl-1-propionyl-1,2,3,4-tetrahydroquinolin-4-yl)-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (4c)
4c was synthesized following general procedure D using 3c (120 mg, 0.31 mmol, 1.0 equiv) and conc HCl (0.1 mL, 1.0 mmol, 6.0 equiv). After removing solvent, residue was resuspended in Et2O, and solid crashed out. After washing the solid 3× with fresh Et2O, the remaining Et2O was decanted off, yielding a white solid amine hydrochloride salt (27 mg). The (R)-amine intermediate (27 mg, 0.082 mmol, 1.0 equiv) and diBoc-Dmt (33 mg, 0.082 mmol, 1.0 equiv) and the coupling reagents HATU (31 mg, 0.082 mmol, 1.0 equiv) and HOBt-Cl (14 mg, 0.082 mmol, 1.0 equiv) were dissolved in DMF (10–15 mL), followed by the addition of the and DIPEA (14 mL, 0.82 mmol, 10.0 equiv). The reaction mixture stirred for 4 h at room temperature. After concentration under reduced pressure, the crude residue was dissolved in a 1:1 mixture of DCM and TFA (10 mL) and stirred for 1 h. The mixture was concentrated and purified by semipreparative HPLC to yield the title compound as a white solid. Note that not all crude product was purified, so a yield was not calculated. 1H NMR (500 MHz, CD3OD) δ 8.15 (d, J = 8.2, 1H), 7.42 (br s, 1H), 7.27–7.19 (m, 2H), 7.18–7.11 (m, 4H), 7.05 (dd, J = 8.4, 2.1, 1H), 6.51 (s, 2H), 4.96–4.91 (m, 1H), 3.91 (s, 2H), 3.86 (dd, J = 11.5, 5.0, 1H), 3.78 (br s, 1H), 3.26 (dd, J = 13.6, 11.5, 1H), 3.18–3.10 (m, 1H), 3.03 (dd, J = 13.7, 5.1, 1H), 2.58–2.39 (m, 2H), 2.27 (s, 6H), 1.90–1.80 (m, 1H), 1.48–1.39 (m, 1H), 1.11 (t, J = 7.4, 3H). No 13C data acquired. HPLC (gradient A): retention time = 35.6. ESI-MS 486.3 [M + H]+.
(S)-2-Amino-N-((R)-6-benzyl-1-butyryl-1,2,3,4-tetrahydroquinolin-4-yl)-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (4d)
4d was synthesized following general procedure D using 3d (10 mg, 0.024 mmol, 1.0 equiv) with the coupling reagent PyBOP to yield the TFA salt of the title compound, 4d, as a white solid. Note that not all crude product was purified. 1H NMR (500 MHz, CD3OD) δ 7.39 (br s, 1H), 7.27–7.20 (m, 2H), 7.18–7.11 (m, 4H), 7.06 (dd, J = 8.4, 2.1, 1H), 6.50 (s, 2H), 4.93 (t, J = 6.5, 1H), 3.91 (s, 2H), 3.88–3.75 (m, 2H), 3.25 (dd, J = 13.6, 11.5, 1H), 3.15–3.11 (m, 1H), 3.02 (dd, J = 13.7, 5.1, 1H), 2.54–2.39 (m, 2H), 2.27 (s, 6H), 1.91–1.81 (m, 1H), 1.69–1.58 (m, 2H), 1.44–1.40 (m, 1H), 0.93 (t, J = 7.2, 3H). No 13C data acquired. HPLC (gradient A): retention time = 38.5. ESI-MS 500.3 [M + H]+.
(S)-2-Amino-N-((R)-6-benzyl-1-isobutyryl-1,2,3,4-tetrahydroquinolin-4-yl)-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (4e)
4e was synthesized following general procedure D using 3e (70 mg, 0.17 mmol, 1.0 equiv) with the coupling reagent PyBOP to yield the TFA salt of the title compound, 4e, as a white solid (42 mg, 40.7%). Note that not all crude product was purified. 1H NMR (500 MHz, CD3OD) δ 8.18 (d, J = 8.2 Hz, 0H), 7.19 (dt, J = 35.4, 7.7 Hz, 6H), 7.04 (d, J = 8.3 Hz, 3H), 6.51 (s, 2H), 3.92 (d, J = 14.2 Hz, 3H), 3.26 (t, J = 12.6 Hz, 1H), 3.12 (ddt, J = 41.3, 13.4, 5.3 Hz, 4H), 2.27 (s, 7H), 1.86 (dq, J = 12.6, 6.2 Hz, 1H), 1.37 (dd, J = 12.8, 6.4 Hz, 1H), 1.12 (d, J = 6.6 Hz, 5H), 1.05 (d, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 179.32, 169.29, 162.72, 157.37, 142.26, 140.05, 137.53, 129.77, 129.45, 129.20, 127.13, 125.65, 123.29, 116.40, 53.46, 46.89, 42.12, 32.38, 31.92, 31.83, 20.44, 20.17, 19.90. HPLC (gradient A): retention time = 37.8. ESI-MS 500.3 [M + H]+ and 522.3 [M + Na]+.
(S)-2-Amino-N-((R)-6-benzyl-1-(cyclopropanecarbonyl)-1,2,3,4-tetrahydroquinolin-4-yl)-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (4f)
4f was synthesized following general procedure D using 3f (120 mg, 0.28 mmol, 1.0 equiv) with the coupling reagent PyBOP to yield the TFA salt of the title compound, 4f, as a white solid. Note that not all crude product was purified, so a yield was not calculated. 1H NMR (500 MHz, CD3OD) δ 7.39 (d, J = 8.3, 1H), 7.26–7.20 (m, 2H), 7.19–7.12 (m, 4H), 7.07 (dd, J = 8.4, 2.1, 1H), 6.50 (s, 2H), 4.96 (t, J = 6.3, 1H), 3.92 (s, 2H), 3.91–3.80 (m, 2H), 3.29–3.20 (m, 2H), 3.07–2.99 (m, 1H), 2.28 (s, 6H), 1.98–1.92 (m, 1H), 1.89–1.81 (m, 1H), 1.47–1.38 (m, 1H), 1.07–1.01 (m, 1H), 0.99–0.92 (m, 1H), 0.91–0.79 (m, 2H). HPLC (gradient A): retention time = 37.5. ESI-MS 598.3 [M + H]+.
(S)-2-Amino-N-((R)-6-benzyl-1-(cyclobutanecarbonyl)-1,2,3,4-tetrahydroquinolin-4-yl)-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (4g)
4g was synthesized following general procedure D using 3g (160 mg, 0.37 mmol, 1.0 equiv) with the coupling reagent PyBOP to yield the TFA salt of the title compound, 4g, as a white solid. Note that not all crude product was purified, so a yield was not calculated. 1H NMR (500 MHz, CD3OD) δ 7.39 (br s, 1H), 7.26–7.21 (m, 2H), 7.18–7.11 (m, 4H), 7.05 (dd, J = 8.3, 2.1, 1H), 6.50 (s, 2H), 4.98–4.89 (m, 1H), 3.91 (s, 2H), 3.85 (dd, J = 11.5, 5.1, 1H), 3.68 (br s, 1H), 3.50 (p, J = 8.5, 1H), 3.25 (dd, J = 13.6, 11.6, 1H), 3.12–3.01 (m, 2H), 2.35–1.94 (m, 11H), 1.89–1.77 (m, 2H), 1.43–1.32 (m, 1H). HPLC (gradient A): retention time - 39.6. ESI-MS 512.3 [M + H]+.
Methyl (R)-4-((S)-2-Amino-3-(4-hydroxy-2,6-dimethylphenyl)propanamido)-6-benzyl-3,4-dihydroquinoline-1(2H)-carboxylate (4h)
4h was synthesized following general procedure D using 3h (124 mg, 0.31 mmol, 1.0 equiv) and the coupling reagent PyBOP to yield the TFA salt of the title compound, 4h, as a white solid (50 mg, 69.5%). 1H NMR (400 MHz, CD3OD) δ 7.65 (d, J = 8.6 Hz, 1H), 7.26–7.20 (m, 2H), 7.17–7.12 (m, 3H), 7.07 (d, J = 2.2 Hz, 1H), 7.03 (dd, J = 8.8, 1.9 Hz, 1H), 6.50 (s, 2H), 4.97 (t, J = 5.2 Hz, 1H), 3.87 (s, 2H), 3.86–3.81 (m, 1H), 3.75 (d, J = 1.4 Hz, 4H), 3.25 (ddd, J = 13.1, 11.5, 1.4 Hz, 1H), 3.16 (tdd, J = 6.7, 3.8, 1.4 Hz, 12H), 3.05–2.94 (m, 2H), 2.27 (d, J = 1.4 Hz, 7H), 1.90–1.82 (m, 12H), 1.77 (tt, J = 9.7, 4.8 Hz, 1H), 1.54–1.45 (m, 1H). No 13C data acquired. HPLC (gradient A): retention time = 36.6. ESI-MS 488.3 [M + H]+ and 510.3 [M + Na]+.
(S)-2-Amino-N-((R)-6-benzyl-1-(2-methoxyacetyl)-1,2,3,4-tetrahydroquinolin-4-yl)-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (4i)
4i was synthesized following general procedure D using 3i (148 mg, 0.36 mmol, 1.0 equiv) and the coupling reagent PyBOP to yield the TFA salt of the title compound, 4i, as a white solid. Note that not all crude product was purified, so a yield was not calculated. 1H NMR (400 MHz, CD3OD) δ 7.23 (t, J = 7.8 Hz, 2H), 7.14 (dd, J = 9.3, 2.6 Hz, 4H), 7.07 (dd, J = 8.4, 1.9 Hz, 1H), 6.51 (s, 2H), 4.96 (t, J = 5.7 Hz, 1H), 4.25 (dd, J = 14.6, 1.1 Hz, 1H), 4.14 (d, J = 14.5 Hz, 1H), 3.90 (s, 2H), 3.84 (dd, J = 11.5, 5.0 Hz, 1H), 3.40 (s, 3H), 3.25 (t, J = 12.6 Hz, 1H), 3.15 (tdd, J = 6.7, 3.7, 1.2 Hz, 7H), 3.02 (dd, J = 13.7, 5.0 Hz, 1H), 2.27 (s, 6H), 1.88–1.81 (m, 8H), 1.48 (s, 1H). No 13C data acquired. HPLC (gradient A): retention time = 32.5. ESI-MS 502.3 [M + H]+ and 524.3 [M + Na]+.
(S)-2-Amino-N-((R)-1-benzoyl-6-benzyl-1,2,3,4-tetrahydroquinolin-4-yl)-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (4j)
4j was synthesized following general procedure D using 3j (182 mg, 0.41 mmol, 1.0 equiv) and the coupling reagent PyBOP to yield the TFA salt of the title compound, 4j, as a white solid. Note that not all crude product was purified, so a yield was not calculated. 1H NMR (400 MHz, CD3OD) δ 7.47–7.42 (m, 1H), 7.37 (d, J = 6.8 Hz, 4H), 7.25–7.20 (m, 2H), 7.15 (d, J = 7.6 Hz, 1H), 7.11 (d, J = 7.6 Hz, 2H), 6.87–6.76 (m, 2H), 6.49 (s, 2H), 5.04 (t, J = 6.0 Hz, 1H), 3.92–3.81 (m, 4H), 3.25 (d, J = 12.3 Hz, 1H), 3.06 (dd, J = 13.8, 5.1 Hz, 1H), 2.28 (s, 6H), 1.94 (t, J = 10.4 Hz, 1H), 1.48 (dd, J = 13.0, 6.3 Hz, 1H). 13C NMR (126 MHz, CD3OD) δ 172.42, 169.14, 157.49, 142.26, 140.04, 139.67, 137.75, 137.19, 131.74, 130.37, 129.71, 129.50, 129.45, 129.39, 128.99, 127.15, 126.27, 123.18, 116.45, 53.53, 49.00, 46.85, 42.06, 31.98, 31.42, 20.44. HPLC (gradient A): retention time = 39.9. ESI-MS 534.3 [M + H]+ and 556.2 [M + Na]+.
(S)-2-Amino-N-((R)-1-(2-amino-2-oxoethyl)-6-benzyl-1,2,3,4-tetrahydroquinolin-4-yl)-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (4k)
4k was synthesized from 9k by boc-deprotection using 1:1 TFA:DCM. After removal of solvent under reduced pressure, the crude product was purified by semipreparative HPLC and lyophilized to yield the TFA salt of title compound, 9k, as a white powder (2 mg, 18%). HPLC (gradient A): retention time = 29.8. ESI-MS 509.3 [M + H]+. 1H NMR (500 MHz, CD3OD) δ 8.33 (d, J = 7.8 Hz, 1H), 7.19 (t, J = 7.4 Hz, 2H), 7.14–7.07 (m, 3H), 6.92 (d, J = 8.5 Hz, 1H), 6.86 (s, 1H), 6.46 (s, 2H), 6.38 (d, J = 8.5 Hz, 1H), 3.91 (d, J = 17.8 Hz, 1H), 3.80 (dd, J = 11.8, 5.1 Hz, 1H), 3.76 (s, 2H), 3.66–3.59 (m, 1H), 3.25 (t, J = 12.4 Hz, 1H), 3.00 (dd, J = 13.6, 5.1 Hz, 1H), 2.86 (d, J = 12.1 Hz, 1H), 2.38 (t, J = 12.1 Hz, 1H), 1.81 (t, J = 13.1 Hz, 1H), 1.52 (d, J = 13.4 Hz, 1H), 1.29 (s, 1H). 13C NMR (126 MHz, CD3OD) δ 175.88, 157.43, 144.84, 140.04, 131.51, 131.21, 130.86, 129.63, 129.29, 126.85, 123.21, 121.80, 116.48, 112.92, 66.62, 55.53, 53.45, 49.00, 47.37, 47.27, 46.62, 41.77, 31.89, 28.93, 20.46. HPLC (gradient A): retention time 29.8. ESI-MS = 487.3.3 [M + H]+ and 509.3 [M + Na]+.
Ethyl 2-((R)-4-((S)-2-Amino-3-(4-hydroxy-2,6-dimethylphenyl)propanamido)-6-benzyl-3,4-dihydroquinolin-1(2H)-yl)acetate (4l)
4l was synthesized by treating crude 9l with 3 N HCl to remove the remaining boc-groups and yield the crude product which was purified by semipreparative HPLC and lyophilized to yield the title compound as the TFA salt of 4l, as a white powder (44 mg, 41.1%). 1H NMR (500 MHz, CD3OD) δ 8.17 (d, J = 7.6 Hz, 1H), 7.22–7.17 (m, 2H), 7.14–7.07 (m, 3H), 6.92–6.85 (m, 2H), 6.43 (s, 2H), 6.39 (d, J = 8.4 Hz, 1H), 4.87 (s, 6H), 4.24 (s, 0H), 4.23–4.15 (m, 2H), 3.81 (q, J = 5.3 Hz, 1H), 3.76 (d, J = 8.6 Hz, 2H), 3.31 (s, 2H), 3.25 (dd, J = 13.5, 11.8 Hz, 1H), 3.00 (dd, J = 13.6, 5.0 Hz, 1H), 2.85 (dt, J = 11.8, 4.1 Hz, 1H), 2.42 (td, J = 12.3, 3.2 Hz, 1H), 2.27 (s, 6H), 1.75 (tt, J = 12.8, 4.1 Hz, 1H), 1.57 (dq, J = 13.3, 3.5 Hz, 1H), 1.29 (td, J = 7.1, 1.0 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 173.03, 168.05, 157.42, 144.44, 143.28, 140.00, 131.60, 131.03, 130.84, 129.64, 129.29, 126.84, 123.20, 121.53, 116.47, 112.40, 62.35, 53.49, 53.20, 49.51, 49.34, 49.17, 49.00, 48.83, 48.66, 48.49, 47.56, 46.18, 41.75, 31.87, 28.78, 20.48, 14.51. HPLC (gradient A): retention time = 41.7. ESI-MS 516.3 [M + H]+ and 538.3 [M + Na]+.
2-((R)-4-((S)-2-Amino-3-(4-hydroxy-2,6-dimethylphenyl)propanamido)-6-benzyl-3,4-dihydroquinolin-1(2H)-yl)acetic Acid (4m)
4m was formed by treating the crude residue of 9m with 1:1 TFA:DCM to form crude product which was purified by semipreparative HPLC and lyophilized to yield the TFA salt of the title compound, 4m (1.6 mg, 2.2%). Note that purification was difficult and not all crude product was purified, so a yield was not calculated. HPLC (gradient A): retention time = 26.4. ESI-MS 488.3 [M + H]+ and 510.3 [M + Na]+. No 1H or 13C NMR data acquired. Instead, formation of final product 4m verified by mass spectrometry.
tert-Butyl 6-Benzyl-4-oxo-3,4-dihydroquinoline-1(2H)-carboxylate (5)
5 was synthesized as previously described in ref (8).
tert-Butyl(R)-6-benzyl-4-(((R)-tert-butylsulfinyl)amino)-3,4-dihydroquinoline-1(2H)-carboxylate (6)
6 was synthesized following general procedure C using 5 (1.1 g, 4.8 mmol, 1.0 equiv), (R)-2-methylpropane-2-sulfinamide (175 mg, 1.44 mmol, 3.0 equiv), and Ti(OEt)4 (0.604 mL, 2.88 mmol, 6.0 equiv) to form the (R)-tert-butanesulfinyl imine intermediate in situ. Once sufficient ketone was converted into imine intermediate (after 48 h), the reaction mixture was transferred via cannula to a round-bottom flask containing NaBH4 (109 mg, 2.44 mmol, 6.0 equiv) and 20 mL of THF in a xylenes dry ice bath; after addition, the solution was stirred at room temperature for 3 h before being quenched with MeOH. Once resultant solid was removed, crude residue was purified using silica gel chromatography to yield the title compound 6 as a clear, colorless oil (175 mg, 82.2%). 1H NMR (500 MHz, CDCl3) δ 7.69 (d, J = 8.2 Hz, 1H), 7.29–7.21 (m, 2H), 7.20–7.15 (qd, J = 6.5, 5.4, 1.6 Hz, 4H), 7.06 (dd, J = 8.5, 2.3 Hz, 1H), 4.52 (q, J = 3.4 Hz, 1H), 3.97–3.87 (m, 3H), 3.57 (tdd, J = 12.9, 4.2, 1.7 Hz, 1H), 3.32 (bs, 1H), 2.20–2.13 (m, 1H), 1.99–1.90 (m, 1H), 1.50 (s, 9H), 1.19 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 153.43, 140.77, 136.51, 136.42, 128.88, 128.71, 128.55, 128.47, 128.34, 125.97, 123.89, 80.97, 55.52, 50.36, 41.08, 40.00, 29.41, 28.22, 22.48.
(R)-6-Benzyl-1,2,3,4-tetrahydroquinolin-4-aminium Chloride (7)
7 was synthesized by first treating 6 (400 mg, 0.904 mml, 1.0 equiv) with excess 1:1 TFA:DCM. After 1 h, solvent was removed under reduced pressure. Next, the Ellman auxiliary was cleaved using conc HCl (0.133 mL, 5.43 mmol, 6.0 equiv). After removing solvent, residue was resuspended in Et2O, and solid crashed out. After washing the solid 3× with fresh Et2O, the remaining Et2O was decanted off, yielding the title 7 compound as tan solid (110 mg, 44.4% from 7). 1H NMR (500 MHz, CD3OD) δ 7.68 (d, J = 1.8 Hz, 1H), 7.44 (dd, J = 8.3, 1.8 Hz, 1H), 7.37 (d, J = 8.3 Hz, 1H), 7.31–7.24 (m, 4H), 7.22–7.17 (m, 1H), 4.77 (t, J = 6.0 Hz, 1H), 4.06 (s, 2H), 3.73 (ddd, J = 12.4, 9.0, 3.1 Hz, 1H), 3.65 (ddd, J = 13.0, 7.8, 3.3 Hz, 1H), 2.61 (dddd, J14.8, 9.0, 5.7, 3.3 Hz, 1H), 2.38 (dddd, J = 14.8, 7.8, 6.4, 3.1 Hz, 1H). No 13C NMR data acquired.
tert-Butyl ((S)-1-(((R)-6-Benzyl-1,2,3,4-tetrahydroquinolin-4-yl)amino)-3-(4-((tert-butoxycarbonyl)oxy)-2,6-dimethylphenyl)-1-oxopropan-2-yl)carbamate (8)
8 was synthesized following a modified version of general procedure D using 7 (110 mg, 0.40 mmol, 1.0 equiv). After coupling to diBoc-Dmt, residue was not deprotected with TFA. Instead, the crude residue was purified by semipreparative HPLC and lyophilized to yield the title compound 8 (287 mg, quant). HPLC (gradient A): retention time = 57.0. ESI-MS 630.3 [M + H]+ and ESI-MS 652.3 [M + Na]+. No 1H or 13C NMR data acquired.
tert-Butyl ((S)-1-(((R)-1-(2-Amino-2-oxoethyl)-6-benzyl-1,2,3,4-tetrahydroquinolin-4-yl)amino)-3-(4-((tert-butoxycarbonyl)oxy)-2,6-dimethylphenyl)-1-oxopropan-2-yl)carbamate (9k)
To form 9k from 8, first acetone was degassed for 30 min, then flooded with Ar for an additional 30 min. To a flame-dried round-bottom flask equipped with a stir bar with Ar atmosphere was added sodium iodide (25 mg, 0.16 mmol, 2.0 equiv) and 2-chloroacetamide (15 mg, 0.16 mmol, 2.0 equiv), which was then placed back under vacuum. Next, 10 mL of degassed acetone was added to the reaction vessel via cannula and reaction stirred for 10 min. Meanwhile, to another flame-dried round-bottom flask equipped with a stirbar was added 8, degassed acetone, followed by DIPEA (0.021 mL, 0.12 mmol, 1.5 equiv), and the solution stirred for 10 min. Next, the contents of the round-bottom flask containing the sodium iodide and 2- chloroacetamide was transferred to the flask containing 8 via cannula. The reaction stirred stirred at room temp for 24 h. The next day, the solvent was removed under reduced pressure to yield the crude product (13 mg) that was taken ahead to next step (formation of 4k) without further purification, isolation, or characterization. HPLC (gradient A): retention time = 60.5. ESI-MS 687.3 [M + H]+ and ESI-MS 709.3 [M + Na]+. No 1H or 13C NMR data acquired.
Ethyl 2-((R)-6-Benzyl-4-((S)-2-((tert-butoxycarbonyl)amino)-3-(4-((tert-butoxycarbonyl)oxy)-2,6-dimethylphenyl)propanamido)-3,4-dihydroquinolin-1(2H)-yl)acetate (9l)
To synthesize 91, 8 (214 mg, 0.34 mmol, 1.0 equiv) and K2CO3 (94 mg, 0.68 mmol, 2.0 equiv) were placed in a round-bottom flask and the atmosphere was evacuated then the reaction vessel was flooded with Ar. Anhydrous CH3CN was added via syringe and the solution stirred for 15 min. Next, bromo ethyl acetate (0.377 mL, 3.4 mmol, 10 equiv) was added to the reaction vessel, which was equipped with a condenser and placed in an 80 °C oil bath. After 6 h, no reaction was taking place (reaction was monitored by TLC). Reaction vessel was taken off heat and stirred overnight at RT. After 24 h, the solvent was removed under reduced pressure and crude residue was resuspended in DCM and dI H2O and the layers separated. The organic layer was washed with 5% citric acid solution (1 × 20 mL) and then with brine (1 × 20 mL) and dried over MgSO4 to yield the crude residue of 91. HPLC (gradient A): retention time = 72.4. ESI-MS 716.3 [M + H]+ and ESI-MS 738.3 [M + Na]+. The residue was carried forward (to synthesize 4l and 4m) without further purification, isolation, or characterization. No 1H or 13C NMR data acquired.
2-((R)-6-Benzyl-4-((S)-2-((tert-butoxycarbonyl)amino)-3-(4-((tert-butoxycarbonyl)oxy)-2,6-dimethylphenyl)propanamido)-3,4-dihydroquinolin-1(2H)-yl)acetic Acid (9m)
9m was synthesized by treating crude 9l (107 mg, 0.15 mmol, 1.0 equiv) with LiOH (excess) in EtOH at 60 °C for 2.5 h. Reaction was monitored for loss of starting material by analytical HPLC (gradient A): starting material retention time = 72.4). Once starting material was consumed, solvent was removed under reduced pressure. HPLC (gradient A): retention time = 49.9. The residue was carried forward (4m) without further purification, isolation, or characterization. No 1H or 13C NMR data acquired.
tert-Butyl 6-(Bromomethyl)-4-oxo-3,4-dihydroquinoline-1(2H)-carboxylate (10)
10 was synthesized as previously described in ref (11).
tert-Butyl 6-(Naphthalen-2-ylmethyl)-4-oxo-3,4-dihydroquinoline-1(2H)-carboxylate (11)
11 was synthesized by first degassing a solution of 3:1 acetone:dI H2O for 1 h, then Ar was bubbled through solution for 1 h to ensure removal and displacement of ambient oxygen. Intermediate 10 (1.0 equiv), 2-naphthylboronic acid (2.0 equiv), K2CO3 (3.0 equiv), and Pd(dppf)Cl2 (0.1 equiv) were added to a microwave tube, and the tube was placed under vacuum for 15 min, then flooded with Ar. Roughly 1–2 mL of the 3:1 acetone:dI H2O solution was added to a tube via syringe, then the tube was placed in microwave for 30–60 min with a maximum power of 300 W and a maximum temperature of 100 °C with the “PowerMax” option enabled. Once the microwave reaction was complete, reaction mixture was filtered through a pad of Celite to remove palladium and solvents were removed under reduced pressure to yield a crude brown residue which was purified using silica gel chromatography to obtain the title compound 11 as an off-white solid. Note that not all crude product was purified so yield was not calculated. 1H NMR (500 MHz, CDCl3) δ 7.90 (d, J = 2.2 Hz, 1H), 7.81–7.73 (m, 3H), 7.69 (d, J = 8.7 Hz, 1H), 7.63 (s, 1H), 7.49–7.40 (m, 2H), 7.35 (dt, J = 8.6, 1.8 Hz, 1H), 7.30 (dt, J = 8.4, 1.5 Hz, 1H), 4.16–4.10 (m, 4H), 2.78–2.71 (m, 2H), 1.54 (d, J = 1.3 Hz, 9H). 13C NMR (126 MHz, CDCl3) δ 194.26, 152.75, 142.47, 137.89, 136.72, 134.71, 133.58, 132.13, 128.24, 127.61, 127.54, 127.35, 127.31, 127.25, 127.08, 126.78, 126.05, 125.46, 124.82, 123.87, 82.12, 44.28, 41.33, 38.99, 28.29.
6-(Naphthalen-2-ylmethyl)-2,3-dihydroquinolin-4(1H)-one (12)
12 was synthesized by deprotecting 11 with a 1:1 mixture of TFA:DCM. After stirring at RT for 1 h, solvent was removed under reduced pressure to yield the crude final product, which was then purified using silica gel chromatography to yield the title compound 12 as a yellow solid. Note that not all crude product was purified, so a yield was not calculated. 1H NMR (500 MHz, CDCl3) δ 7.84–7.72 (m, 4H), 7.62 (s, 1H), 7.49–7.38 (m, 2H), 7.33–7.24 (m, 1H), 7.16 (ddd, J = 8.3, 4.8, 1.9 Hz, 1H), 6.65 (ddd, J = 15.4, 8.3, 2.4 Hz, 1H), 4.03 (s, 1H), 3.59–3.53 (m, 2H), 2.77–2.66 (m, 2H), 2.25 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 193.66, 138.64, 136.34, 136.11, 133.56, 132.05, 130.95, 128.11, 127.58, 127.51, 127.40, 127.36, 127.20, 126.87, 125.95, 125.31, 119.67, 119.42, 116.41, 116.22, 42.57, 42.41, 41.14, 38.08.
6-(Naphthalen-2-ylmethyl)-1-propionyl-2,3-dihydroquinolin-4(1H)-one (13c)
13c was synthesized according to general procedure A using 12 (150 mg, 0.52 mmol, 1.0 equiv) and excess propionic anhydride. Once complete, excess anhydride was removed and the crude residue was purified using silica gel chromatography to yield the title compound 13c as a clear, pale-orange oil (156 mg, 87.2%). 1H NMR (500 MHz, CDCl3) δ 7.91 (s, 1H), 7.81–7.73 (m, 3H), 7.63 (s, 1H), 7.47–7.32 (m, 4H), 7.28 (dd, J = 8.4, 1.9 Hz, 1H), 4.22–4.15 (m, 2H), 4.12 (s, 2H), 2.74 (td, J = 6.3, 1.9 Hz, 2H), 2.61–2.52 (m, 2H), 1.21 (t, 3H). 13C NMR (126 MHz, CDCl3) δ 194.08, 172.83, 142.08, 138.43, 137.45, 134.74, 134.52, 133.42, 132.01, 128.23, 127.51, 127.49, 127.41, 127.17, 127.03, 126.03, 125.92, 125.45, 124.30, 123.97, 43.69, 41.27, 39.43, 27.80, 9.71.
1-Butyryl-6-(naphthalen-2-ylmethyl)-2,3-dihydroquinolin-4(1H)-one (13d)
13d was synthesized according to general procedure A using 12 (155 mg, 0.54 mmol, 1.0 equiv) and excess butyric anhydride. Once complete, excess anhydride was removed and the crude residue was purified using silica gel chromatography to yield the title compound 13d as a clear, slightly dark-red oil (112 mg, 58.0%). 1H NMR (500 MHz, CDCl3) δ 7.94 (s, 1H), 7.85–7.76 (m, 3H), 7.66 (s, 1H), 7.51–7.39 (m, 4H), 7.32 (d, J = 8.5 Hz, 1H), 4.20 (t, J = 6.3 Hz, 2H), 4.15 (s, 2H), 2.76 (t, J = 5.9 Hz, 2H), 2.60–2.49 (m, 2H), 1.75 (h, J = 7.7 Hz, 2H), 0.97 (t, J = 7.6 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 194.00, 171.99, 142.10, 138.38, 137.42, 134.69, 134.47, 133.42, 132.01, 128.21, 127.49, 127.47, 127.39, 127.16, 127.02, 126.00, 125.90, 125.42, 124.32, 124.00, 43.71, 41.25, 39.50, 36.28, 18.94, 13.66.
Methyl 6-(Naphthalen-2-ylmethyl)-4-oxo-3,4-dihydroquinoline-1(2H)-carboxylate (13e)
13e was synthesized according to general procedure B using 12 (150 mg, 0.52 mmol, 1.0 equiv) and methylchloroformate (0.200 mL, 2.3 mmol, 2.3 equiv). The reaction stirred overnight; once complete, solvent was removed and the crude residue was purified using silica gel chromatography to yield the title compound 109 as a clear oil (65 mg, 36.1%). Starting material was recovered but not included in final yield calculation. Additionally, not all product was purified as the Rf values for the starting material and product were similar and separation was difficult. 1H NMR (500 MHz, CDCl3) δ 7.92 (d, J = 2.2 Hz, 1H), 7.82–7.74 (m, 3H), 7.72 (d, J = 8.6 Hz, 1H), 7.64 (s, 1H), 7.48–7.41 (m, 2H), 7.38 (dd, J = 8.6, 2.3 Hz, 1H), 7.30 (dd, J = 8.4, 1.8 Hz, 1H), 4.18 (t, J = 6.3 Hz, 2H), 4.13 (s, 2H), 3.84 (s, 3H), 2.77 (t, J = 6.3 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 193.81, 154.23, 141.81, 137.73, 137.18, 134.88, 133.49, 132.05, 128.21, 127.55, 127.47, 127.26, 127.23, 127.03, 126.01, 125.42, 124.81, 123.63, 53.32, 44.39, 41.26, 38.81.
(R)-2-Methyl-N-((R)-6-(naphthalen-2-ylmethyl)-1-propionyl-1,2,3,4-tetrahydroquinolin-4-yl)propane-2-sulfinamide (14c)
14c was synthesized according to general procedure C using 13c (156 mg, 0.45 mmol, 1.0 equiv), (R)-2-methylpropane-2-sulfinamide (165 mg, 1.36 mmol, 3.0 equiv), and Ti(OEt)4 (0.57 mL, 2.7 mmol, 6.0 equiv) to form the (R)-tert-butanesulfinyl imine intermediate in situ. Once sufficient ketone was converted into imine intermediate (after 48 h), the reaction mixture was transferred via cannula to a round-bottom flask containing NaBH4 (103 mg, 2.7 mmol, 6.0 equiv) and 20 mL of THF in a xylenes dry ice bath, after addition, the solution was stirred at room temperature for 3 h before being quenched with MeOH. Once resultant solid was removed, crude residue was purified using silica gel chromatography to yield the title compound 14c as a clear, colorless oil (148 mg, 72.5%). No 1H or 13C NMR data acquired. Instead, product taken ahead to next reaction (formation of 15c) without any further isolation, purification, or characterization.
(R)-N-((R)-1-Butyryl-6-(naphthalen-2-ylmethyl)-1,2,3,4-tetrahydroquinolin-4-yl)-2-methylpropane-2-sulfinamide (14d)
14d was synthesized according to general procedure C using 13d (112 mg, 0.31 mmol, 1.0 equiv), (R)-2-methylpropane-2-sulfinamide (114 mg, 0.94 mmol, 3.0 equiv), and Ti(OEt)4 (0.39 mL, 1.9 mmol, 6.0 equiv) to form the (R)-tert-butanesulfinyl imine intermediate in situ. Once sufficient ketone was converted into imine intermediate (after 48 h), the reaction mixture was transferred via cannula to a round-bottom flask containing NaBH4 (71 mg, 1.9 mmol, 6.0 equiv) and 20 mL of THF in a xylenes a dry ice bath; after addition, the solution was stirred at room temperature for 3 h before being quenched with MeOH. Once resultant solid was removed, crude residue was purified using silica gel chromatography to yield the title compound 14d as a clear, colorless oil (54 mg, 37.2%). 1H NMR (500 MHz, CDCl3) δ 7.78 (q, J = 7.7 Hz, 3H), 7.66 (s, 1H), 7.44 (pd, J = 7.1, 3.4 Hz, 2H), 7.33 (dd, J = 6.4, 2.1 Hz, 2H), 7.14 (dt, J = 8.2, 2.0 Hz, 1H), 4.52 (t, J = 4.4 Hz, 1H), 4.11 (s, 2H), 3.89 (dt, J = 12.2, 5.5 Hz, 1H), 3.74 (ddd, J = 13.6, 9.8, 6.2 Hz, 1H), 3.32 (d, J = 3.3 Hz, 1H), 2.52–2.39 (m, 2H), 2.20 (dh, J = 15.3, 7.4, 5.6 Hz, 1H), 2.04 (dq, J = 12.7, 4.6, 3.9 Hz, 1H), 1.74–1.61 (m, 2H), 1.17 (d, J = 1.9 Hz, 9H), 0.91 (t, J = 7.5 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 187.72, 172.68, 138.03, 136.64, 133.57, 132.10, 128.79, 128.72, 128.57, 128.21, 127.58, 127.52, 127.46, 127.10, 126.03, 125.43, 124.85, 124.60, 55.73, 50.96, 41.52, 39.95, 36.69, 30.68, 22.51, 19.14, 13.88.
Methyl (R)-4-(((R)-tert-Butylsulfinyl)amino)-6-(naphthalen-2-ylmethyl)-3,4-dihydroquinoline-1(2H)-carboxylate (14e)
14e was synthesized according to general procedure C using 13e (65 mg, 0.19 mmol, 1.0 equiv), (R)-2-methylpropane-2-sulfinamide (68 mg, 0.57 mmol, 3.0 equiv), and Ti(OEt)4 (0.24 mL, 1.1 mmol, 6.0 equiv) to form the (R)-tert-butanesulfinyl imine intermediate in situ. Once sufficient ketone was converted into imine intermediate (after 48 h), the reaction mixture was transferred via cannula to a round-bottom flask containing NaBH4 (43 mg, 1.1 mmol, 6.0 equiv) and 20 mL of THF in a xylenes dry ice bath, after addition, the solution was stirred at room temperature for 3 h before being quenched with MeOH. Once resultant solid was removed, crude residue was purified using silica gel chromatography to yield the title compound 14e as a clear, colorless oil (34 mg, 40.0%). 1H NMR (500 MHz, CDCl3) δ 7.81–7.71 (m, 4H), 7.64 (d, J = 5.2 Hz, 1H), 7.48–7.39 (m, 2H), 7.34–7.26 (m, 2H), 7.13 (ddd, J = 8.5, 6.0, 2.2 Hz, 1H), 4.55 (h, J = 3.7 Hz, 1H), 4.09 (s, 2H), 4.05–3.96 (m, 1H), 3.78 (s, 3H), 3.69–3.59 (m, 1H), 3.33 (q, J = 2.7 Hz, 1H), 2.18 (ddt, J = 12.8, 10.4, 4.3 Hz, 1H), 2.06–1.93 (m, 1H), 1.18 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 154.94, 138.25, 136.95, 136.16, 133.53, 132.02, 129.23, 128.84, 128.65, 128.08, 127.52, 127.48, 127.43, 126.96, 125.92, 125.30, 123.70, 55.60, 52.95, 50.35, 41.33, 40.28, 29.49, 22.49.
(S)-2-Amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-((R)-6-(naphthalen-2-ylmethyl)-1,2,3,4-tetrahydroquinolin-4-yl)propanamide (15a)
15a was synthesized as previously described in ref (8).
(S)-N-((R)-1-Acetyl-6-(naphthalen-2-ylmethyl)-1,2,3,4-tetrahydroquinolin-4-yl)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (15b)
15b was synthesized as previously described in ref (9).
(S)-2-Amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-((R)-6-(naphthalen-2-ylmethyl)-1-propionyl-1,2,3,4-tetrahydroquinolin-4-yl)propanamide (15c)
15c was synthesized following general procedure D using 14c (148 mg, 0.33 mmol, 1.0 equiv) and conc HCl (6 drops). After removing solvent, residue was resuspended in Et2O and solid crashed out. After washing the solid 3× with fresh Et2O, the remaining Et2O was decanted off, yielding a white solid amine hydrochloride salt (126 mg). The synthesis was completed by following general procedure F with newly formed (R) amine intermediate (60 mg, 0.16 mmol, 1.0 equiv) to yield crude product which was purified by semipreparative HPLC and lyophilized to yield the title compound 15c as a TFA salt (15 mg, 14.7%). Note that not all crude product was purified. 1H NMR (500 MHz, CDCl3) δ 7.76 (ddd, J = 21.9, 8.2, 3.1 Hz, 3H), 7.60 (d, J = 3.2 Hz, 1H), 7.47–7.37 (m, 2H), 7.28 (dt, J = 8.6, 2.2 Hz, 1H), 7.19 (d, J = 3.5 Hz, 1H), 7.15–7.09 (m, 1H), 6.51 (d, J = 3.4 Hz, 2H), 4.93 (p, J = 5.2 Hz, 1H), 4.08 (d, J = 3.4 Hz, 2H), 3.86 (dt, J = 11.2, 4.3 Hz, 1H), 3.78 (s, 1H), 3.24 (td, J = 12.6, 11.2, 3.5 Hz, 1H), 3.15 (s, 1H), 3.03 (dt, J = 13.9, 4.5 Hz, 1H), 2.58–2.41 (m, 2H), 2.26 (d, J = 3.6 Hz, 6H), 1.85 (dtd, J = 13.5, 8.7, 4.7 Hz, 1H), 1.45 (s, 1H), 1.11 (td, J = 7.1, 3.2 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 175.85, 169.20, 157.47, 140.07, 139.86, 137.62, 135.07, 133.59, 129.47, 129.07, 128.58, 128.46, 128.38, 127.87, 127.09, 126.46, 125.87, 123.24, 116.45, 53.49, 47.11, 42.27, 31.95, 31.29, 28.92, 20.42, 10.06. HPLC (gradient A): retention time = 41.3. ESI-MS 536.3 [M + H]+ and 558.3 [M + Na]+.
(S)-2-Amino-N-((R)-1-butyryl-6-(naphthalen-2-ylmethyl)-1,2,3,4-tetrahydroquinolin-4-yl)-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (15d)
15d was synthesized following general procedure D using 14d (34 mg, 0.076 mmol, 1.0 equiv) and conc HCl (3 drops). After removing solvent, residue was resuspended in Et2O and solid crashed out. After washing the solid 3× with fresh Et2O, the remaining Et2O was decanted off, yielding a white solid amine hydrochloride salt (46 mg). The synthesis was completed by following general procedure F with newly formed (R) amine intermediate (46 mg, 0.12 mmol, 1.0 equiv) to yield crude product which was purified by semipreparative HPLC and lyophilized to yield the title compound 15d as a TFA salt (12 mg, 15.6%). Note that not all crude product was purified. No 1H or 13C data acquired. Instead, final product was verified only via mass spectrometry. HPLC (gradient A): retention time = 44.0. ESI-MS 550.3 [M + H]+ and 572.3 [M + Na]+.
Methyl (R)-4-((S)-2-Amino-3-(4-hydroxy-2,6-dimethylphenyl)propanamido)-6-(naphthalen-2-ylmethyl)-3,4-dihydroquinoline-1(2H)-carboxylate (15e)
15e was synthesized following general procedure D using 14e (34 mg, 0.076 mmol, 1.0 equiv) and conc HCl (3 drops). After removing solvent, residue was resuspended in Et2O and solid crashed out. After washing the solid 3× with fresh Et2O, the remaining Et2O was decanted off, yielding a white solid amine hydrochloride salt (19 mg). The synthesis was completed by following general procedure F with newly formed (R) amine intermediate (19 mg, 0.0.16 mmol, 1.0 equiv) to yield crude product which was purified by semipreparative HPLC and lyophilized to yield the title compound 15e as a TFA salt (13 mg, 40.6%). Note that not all crude product was purified. 1H NMR (500 MHz, CD3OD) δ 7.78 (d, J = 7.9 Hz, 1H), 7.73 (dd, J = 8.3, 2.9 Hz, 2H), 7.67 (d, J = 8.5 Hz, 1H), 7.59 (s, 1H), 7.42 (dt, J = 8.9, 6.2 Hz, 2H), 7.27 (dt, J = 8.4, 1.7 Hz, 1H), 7.12 (s, 1H), 7.09 (dt, J = 8.6, 1.8 Hz, 1H), 6.50 (s, 2H), 4.96 (t, J = 5.1 Hz, 1H), 4.04 (s, 2H), 3.87–3.80 (m, 1H), 3.75 (d, J = 1.5 Hz, 4H), 3.28–3.19 (m, 1H), 3.00 (dt, J = 12.6, 8.3 Hz, 2H), 2.26 (d, J = 1.5 Hz, 7H), 1.78 (ddt, J = 14.7, 9.9, 4.7 Hz, 1H), 1.50 (ddd, J = 13.4, 8.6, 4.8 Hz, 1H). 13C NMR (126 MHz, CD3OD) δ 168.96, 157.48, 156.61, 139.99, 138.03, 137.48, 135.07, 133.57, 130.31, 129.72, 129.02, 128.78, 128.57, 128.46, 128.39, 127.80, 127.05, 126.41, 124.63, 123.19, 116.43, 111.40, 53.54, 53.41, 47.06, 42.32, 42.14, 31.92, 30.30, 20.44. HPLC (gradient A): retention time = 42.7. ESI-MS 538.3 [M + H]+ and 560.3 [M + Na]+.
In Vitro Pharmacology
Cell Lines and Membrane Preparations
All tissue culture reagents were purchased from Gibco Life Sciences (Grand Island, NY, U.S.). C6-rat glioma cells stably transfected with a rat μ (C6-MOR) or rat δ (C6-DOR) opioid receptor22 and Chinese hamster ovary (CHO) cells stably expressing a human κ (CHO-KOR) opioid receptor23 were used for all in vitro assays. Cells were grown to confluence at 37 °C in 5% CO2 in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum and 5% penicillin/streptomycin. Membranes were prepared by washing confluent cells three times with ice-cold phosphate buffered saline (0.9% NaCl, 0.61 mM Na2HPO4, 0.38 mM KH2PO4, pH 7.4). Cells were detached from the plates by incubation in warm harvesting buffer (20 mM HEPES, 150 mM NaCl, 0.68 mM EDTA, pH 7.4) and pelleted by centrifugation at 1600 rpm for 3 min. The cell pellet was suspended in ice-cold 50 mM Tris-HCl buffer, pH 7.4, and homogenized with a Tissue Tearor (Biospec Products, Inc., Bartlesville, OK, U.S.) for 20 s. The homogenate was centrifuged at 15000 rpm for 20 min at 4 °C. The pellet was rehomogenized in 50 mM Tris-HCl with a Tissue Tearor for 10 s, followed by recentrifugation. The final pellet was resuspended in 50 mM Tris-HCl and frozen in aliquots at 80 °C. Protein concentration was determined via a BCA protein assay (Thermo Scientific Pierce, Waltham, MA, U.S.) using bovine serum albumin as the standard.
Radioligand Binding Assays
Radiolabeled compounds were purchased from PerkinElmer (Waltham, MA, U.S.). Opioid ligand binding assays were performed by competitive displacement of 0.2 nM [3H]diprenorphine (250 μCi, 1.85 TBq/mmol) by the peptidomimetic from membrane preparations containing opioid receptors as described above. The assay mixture, containing membranes (20 μg protein/tube) in 50 mM Tris-HCl buffer (pH 7.4), [3H]diprenorphine, and various concentrations of test peptidomimetic, was incubated at room temperature for 1 h to allow binding to reach equilibrium. The samples were rapidly filtered through Whatman GF/C filters using a Brandel harvester (Brandel, Gaithersburg, MD, U.S.) and washed five times with 50 mM Tris-HCl buffer. Bound radioactivity on dried filters was determined by liquid scintillation counting, after saturation with EcoLume liquid scintillation cocktail, in a Wallac 1450 MicroBeta (PerkinElmer, Waltham, MA, U.S.). Nonspecific binding was determined using 10 μM naloxone. The results presented are the mean ± standard error (SE\M) from at least three separate assays performed in duplicate. Ki (nM) values were calculated using nonlinear regression analysis to fit a logistic equation to the competition data using GraphPad Prism, version 6.0c, for Mac OS X (GraphPad Software Inc., La Jolla, CA).
Stimulation of [35S]GTPγS Binding
Agonist stimulation of [35S]guanosine 5′-O-[γ-thio]triphosphate ([355S]GTPγS, 1250 Ci, 46.2 TBq/mmol) binding to G-protein was measured as described previously.24 Briefly, membranes (10–20 μg of protein/tube) were incubated 1 h at 25 °C in GTPγS buffer (50 mM Tris-HCl, 100 mM NaCl, 5 mM MgCl2, pH 7.4) containing 0.1 nM [35S]GTPγS, 30 μM guanosine diphosphate (GDP), and varying concentrations of test peptidomimetic. G-Protein activation following receptor stimulation of [35S]GTPγS (% stimulation) with peptidomimetic was compared with 10 μM of the standard compounds [d-Ala2,N-MePhe4,Gly-ol]enkephalin (DAMGO) at MOR, d-Pen2,5-enkephalin (DPDPE) at DOR, or U69,593 at KOR. The reaction was terminated by vacuum filtration of GF/C filters that were washed 10 times with GTPγS buffer. Bound radioactivity was measured as described above. The results are presented as the mean ± standard error (SEM) from at least three separate assays performed in duplicate; potency (EC50 (nM)) and % stimulation were determined using nonlinear regression analysis with GraphPad Prism, as above.
In Vivo Pharmacology
Animals
Adult male C57BL/6 mice weighing between 20 and 30 g at 8–16 weeks old purchased from Harlan (Indianapolis, IN). Mice were group-housed and had free access to food and water at all times. Experiments were conducted in the housing room, which was maintained on a 12 h light/dark cycle (with lights on at 0700); experiments were conducted during the light cycle. Each mouse was used only once. Studies were performed in accordance with the University of Michigan Committee on the Use and Care of Animals and the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011 publication).
Computational Modeling
Three-dimensional (3D) models of opioid receptors in the inactive conformation were produced as previously described26 using X-ray structures of the mouse MOR (PDB ID: 4dkl),27 the human DOR (PDB ID: 4n6h),28 and the human KOR (PDB ID: 4djh)29 as structural templates. The recently obtained crystal structure of mouse MOR in the active conformation (PDB ID: 5c1m)30 was used as a template for homology modeling of active conformations of DOR and KOR. Structures of receptor loops in the active state were kept similar to those in the crystal structures of corresponding receptors in the inactive state. N-Termini of DOR (residues 33–45) and KOR (residues 45–57) were modeled using the structure of MOR N-terminus in the active conformation with a few adjustments that transformed the central bulge (residues 53–57) into the extended conformation of the polypeptide chain. Such modifications increased the size of the binding pocket and allowed docking of relatively bulky tetra- and pentapeptides and peptidomimetics without hindrances with the N-terminus. Structures of peptidomimetic ligands were generated using the 3D-Builder Application of QUANTA (Accelrys, Inc.), followed by Conformational Search included in the program package. Ligand conformations that demonstrated the best superposition of aromatic substituents of the THQ core with the pharmacophore elements (Tyr1 and Phe3) of receptor-bound conformations of cyclic tetrapeptides31,32 were selected and minimized with CHARMm implemented in QUANTA (Adopted-Basis Newton–Raphson method, 100 steps, ε = 10). Low energy conformations (within 2 kcal/mol) were manually positioned inside the receptor binding cavity to reproduce the binding modes of cyclic tetrapeptides. The docking pose of each ligand was subsequently refined using the solid docking module of QUANTA. The receptor–ligand complexes were then minimized with CHARMm (Adopted-Basis Newton–Raphson method, 30 steps, ε = 10). Models of opioid ligand–receptor complexes are available upon request.
Acknowledgments
This study was supported by NIH grant DA003910 (H.I.M, E.M.J and J.R.T). J.P.A. was supported by the Substance Abuse Interdisciplinary Training Program administered by NIDA, 5 T32 DA007267, and N.W.G. by the Pharmacological Sciences Training Program, T32-GM007767. Tyler Trask, Evan Schramm, and Aaron Chadderdon are thanked for additional in vitro studies.
Glossary
Abbreviations Used
- MOR
μ opioid receptor
- DOR
δ opioid receptor
- KOR
κ opioid receptor
- THQ
tetrahydroquinoline
- THN
tetrahydronaphthalene
- CHO
Chinese hamster ovary
- GTPγS
guanosine 5′-O-[γ-thio]triphosphate
- WWTW
warm water tail withdrawal
- TM
transmembrane
- Boc
tert-butyldicarbonate
- DIPEA
N,N- diisopropylethylamine
- DCM
dichloromethane
- EA
ethyl acetate
- hex
hexanes
- TfOH
trifluoromethanesulfonic acid
- PyBOP
benzotriazol-1-yloxytripyrrolidinophosphonium hexafluorophosphate
- HATU
1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate HOBt-Cl, 6-chlorohydroxybenzotriazole
- diBoc-Dmt
tert-butyloxycarbonyl-protected-l-2,6-dimethyltyrosine
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.6b00308.
Molecular formula strings (CSV)
The authors declare no competing financial interest.
Supplementary Material
References
- Schiller P. W. Bi- or multifunctional opioid peptide drugs. Life Sci. 2010, 86, 598–603. 10.1016/j.lfs.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli A.; Bolognesi M. L.; Minarini A.; Rosini M.; Tumiatti V.; Recanatini M.; Melchiorre C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008, 51, 347–372. 10.1021/jm7009364. [DOI] [PubMed] [Google Scholar]
- Morphy R.; Rankovic Z. Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 2005, 48, 6523–6543. 10.1021/jm058225d. [DOI] [PubMed] [Google Scholar]
- Lowery J. J.; Raymond T. J.; Giuvelis D.; Bidlack J. M.; Polt R.; Bilsky E. J. In vivo characterization of MMP-2200, a mixed delta/mu opioid agonist, in mice. J. Pharmacol. Exp. Ther. 2011, 336, 767–778. 10.1124/jpet.110.172866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthan S. Opioid ligands with mixed mu/delta opioid receptor interactions: an emerging approach to novel analgesics. AAPS J. 2006, 8, E118–E125. 10.1208/aapsj080114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q.; Mosberg H. I.; Porreca F. Modulation of the potency and efficacy of mu-mediated antinociception by delta agonists in the mouse. J. Pharmacol. Exp. Ther. 1990, 254, 683–689. [PubMed] [Google Scholar]
- Taylor A. M. W.; Roberts K. W.; Pradhan A. A.; Akbari H. A.; Walwyn W.; Lutfy K.; Carroll F. I.; Cahill C. M.; Evans C. J. Anti-nociception mediated by a κ opioid receptor agonist is blocked by a δ receptor agonist. Br. J. Pharmacol. 2015, 172, 691–703. 10.1111/bph.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosberg H. I.; Yeomans L.; Harland A. A.; Bender A. M.; Sobczyk-Kojiro K.; Anand J. P.; Clark M. J.; Jutkiewicz E. M.; Traynor J. R. Opioid peptidomimetics: leads for the design of bioavailable mixed efficacy μ opioid receptor (MOR) agonist/δ opioid receptor (DOR) antagonist ligands. J. Med. Chem. 2013, 56, 2139–2149. 10.1021/jm400050y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland A. A.; Yeomans L.; Griggs N. W.; Anand J. P.; Pogozheva I. D.; Jutkiewicz E. M.; Traynor J. R.; Mosberg H. I. Further optimization and evaluation of bioavailable, mixed-efficacy mu-opioid receptor (MOR) agonists/delta-opioid Receptor (DOR) antagonists: balancing MOR and DOR affinities. J. Med. Chem. 2015, 58, 8952–8969. 10.1021/acs.jmedchem.5b01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand J. P.; Purington L. C.; Pogozheva I. D.; Traynor J. R.; Mosberg H. I. Modulation of opioid receptor ligand affinity and efficacy using active and inactive state receptor models. Chem. Biol. Drug Des. 2012, 80, 763–770. 10.1111/cbdd.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A. M.; Griggs N. W.; Anand J. P.; Traynor J. R.; Jutkiewicz E. M.; Mosberg H. I. Asymmetric synthesis and in vitro and in vivo activity of tetrahydroquinolines featuring a diverse set of polar substitutions at the 6 position as mixed-efficacy μ opioid receptor/δ opioid receptor ligands. ACS Chem. Neurosci. 2015, 6, 1428–1435. 10.1021/acschemneuro.5b00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosberg H. I.; Yeomans L.; Anand J. P.; Porter V.; Sobczyk-Kojiro K.; Traynor J. R.; Jutkiewicz E. M. Development of a bioavailable μ opioid receptor (MOPr) agonist, δ opioid receptor (DOPr) antagonist peptide that evokes antinociception without development of acute tolerance. J. Med. Chem. 2014, 57, 3148–3153. 10.1021/jm5002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. L.; Bartlett J. L.; Ananthan S.; Bilsky E. J. In vivo pharmacological characterization of SoRI 9409, a nonpeptidic opioid mu-agonist/delta-antagonist that produces limited antinociceptive tolerance and attenuates morphine physical dependence. J. Pharmacol. Exp. Ther. 2001, 297, 597–605. [PubMed] [Google Scholar]
- Fujita W.; Gomes I.; Dove L. S.; Prohaska D.; McIntyre G.; Devi L. A. Molecular characterization of eluxadoline as a potential ligand targeting mu-delta opioid receptor heteromers. Biochem. Pharmacol. 2014, 92, 448–456. 10.1016/j.bcp.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove L. S.; Lembo A.; Randall C. W.; Fogel R.; Andrae D.; Davenport J. M.; McIntyre G.; Almenoff J. S.; Covington P. S. Eluxadoline benefits patients with irritable bowel syndrome with diarrhea in a phase 2 study. Gastroenterology 2013, 145, 329–338. 10.1053/j.gastro.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Abdelhamid E. E.; Sultana M.; Portoghese P. S.; Takemori A. E. Selective blockage of the delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J. Pharmacol. Exp. Ther. 1991, 258, 299–303. [PubMed] [Google Scholar]
- Fundytus M. E.; Schiller P. W.; Shapiro M.; Weltrowska G.; Coderre T. J. Attenuation of morphine tolerance and dependence with the highly selective delta opioid receptor antagonist TIPP(psi). Eur. J. Pharmacol. 1995, 286, 105–108. 10.1016/0014-2999(95)00554-X. [DOI] [PubMed] [Google Scholar]
- Hepburn M. J.; Little P. J.; Gringas J.; Khun C. M. Differential effects of naltrindole on morphine-induced tolerance and physical dependence in rats. J. Pharmacol. Exp. Ther. 1997, 281, 1350–1356. [PubMed] [Google Scholar]
- Tanuwidjaja J.; Peltier H. M.; Ellman J. A. One-pot asymmetric synthesis of either diastereomer of tert-butanesulfinyl-protected amines from ketones. J. Org. Chem. 2007, 72, 626–629. 10.1021/jo0616512. [DOI] [PubMed] [Google Scholar]
- Borg G.; Cogan D. A.; Ellman J. A. One-pot asymmetric synthesis of tert-butanesulfinyl-protected amines from ketones by the in situ reduction of tert-butanesulfinyl ketimines. Tetrahedron Lett. 1999, 40, 6709–6712. 10.1016/S0040-4039(99)01351-9. [DOI] [Google Scholar]
- Colyer J. T.; Andersen N. G.; Tedrow J. S.; Soukup T. S.; Faul M. M. Reversal of diastereofacial selectivity in hydride reductions of N-tert-butanesulfinyl imines. J. Org. Chem. 2006, 71, 6859–6862. 10.1021/jo0609834. [DOI] [PubMed] [Google Scholar]
- Lee K. O.; Akil H.; Woods J. H.; Traynor J. R. Differential binding properties of oripavines at cloned mu- and delta-opioid receptors. Eur. J. Pharmacol. 1999, 378, 323–330. 10.1016/S0014-2999(99)00460-4. [DOI] [PubMed] [Google Scholar]
- Husbands S. M.; Neilan C. L.; Broadbear J.; Grundt P.; Breeden S.; Aceto M. D.; Woods J. H.; Lewis J. W.; Traynor J. R. BU74, a complex oripavine derivative with potent kappa opioid receptor agonism and delayed opioid antagonism. Eur. J. Pharmacol. 2005, 509, 117–125. 10.1016/j.ejphar.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Traynor J. R.; Nahorski S. R. Modulation by mu-opioid agonists of guanosine-5′-O(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SHY5Y cells. J. Mol. Pharmacol. 1995, 47, 848–854. [DOI] [PubMed] [Google Scholar]
- Lamberts J. T.; Jutkiewicz E. M.; Mortensen R. M.; Traynor J. R. Mu-opioid receptor coupling to Gα(o) plays an important role in opioid antinociception. Neuropsychopharmacology 2011, 36, 2041–2053. 10.1038/npp.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A.; Taylor L. P.; Fine J. L.; Thompson R. C.; Hoversten M. T.; Mosberg H. I.; Watson S. J.; Akil H. Key residues defining the μ-opioid receptor binding pocket: a site-directed mutagensis study. J. Neurochem. 1997, 68, 344–353. 10.1046/j.1471-4159.1997.68010344.x. [DOI] [PubMed] [Google Scholar]
- Manglik A.; Kruse A. C.; Kobilka T. S.; Thian F. S.; Mathiesen J. M.; Sunahara R. K.; Pardo L.; Weis W. I.; Kobilka B. K.; Granier S. Crystal structure of the μ-opioid receptor bound to a morphinan antagonist. Nature 2012, 485, 321–326. 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenalti G.; Giguere P. M.; Katritch V.; Huang X.-P.; Thompson A. A.; Cherezov V.; Roth B. L.; Stevens R.C. Molecular control of d-opioid receptor signaling. Nature 2014, 506, 191–196. 10.1038/nature12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.; Wacker D.; Mileni M.; Katritch V.; Han G. W.; Vardy E.; Liu W.; Thompson A. A.; Huang X.-P.; Carroll F. I.; Mascarella S. W.; Westkaemper R. B.; Mosier P. D.; Roth B. L.; Cherezov V.; Stevens R. C. Structure of the human κ-opioid receptor in complex with JDTic. Nature 2012, 485, 327–332. 10.1038/nature10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.; Manglik A.; Venkatakrishnan A. J.; Laeremans T.; Feinberg E. N.; Sanborn A. L.; Kato H. E.; Livingston K. E.; Thorsen T. S.; Kling R. C.; Granier S.; Gmeiner P.; Husbands S. M.; Traynor J. R.; Weis W.I.; Steyaert J.; Dror R. O.; Kobilka B. K. Structural insights into m-opioid receptor activation. Nature 2015, 524, 315–321. 10.1038/nature14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler C. B.; Pogozheva I. D.; Lomize A. L.; LeVine H.; Mosberg H. I. Complex of an active μ-opioid receptor with a cyclic peptide agonist modeled from experimental constraints. Biochemistry 2004, 43, 15796–15810. 10.1021/bi048413q. [DOI] [PubMed] [Google Scholar]
- Pogozheva I. D.; Przydzial M. J.; Mosberg H. I. Homology modeling of opioid receptor-ligand complexes using experimental constraints. AAPS J. 2005, 7, E434–448. 10.1208/aapsj070243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.